Abstract
Mannheimia haemolytica is an important member of the bovine respiratory disease complex, which is characterized by abundant neutrophil infiltration into the alveoli and fibrin deposition. Recently several authors have reported that human neutrophils release neutrophil extracellular traps (NETs), which are protein-studded DNA matrices capable of trapping and killing pathogens. Here, we demonstrate that the leukotoxin (LKT) of M. haemolytica causes NET formation by bovine neutrophils in a CD18-dependent manner. Using an unacylated, noncytotoxic pro-LKT produced by an ΔlktC mutant of M. haemolytica, we show that binding of unacylated pro-LKT stimulates NET formation despite a lack of cytotoxicity. Inhibition of LKT binding to the CD18 chain of lymphocyte function-associated antigen 1 (LFA-1) on bovine neutrophils reduced NET formation in response to LKT or M. haemolytica cells. Further investigation revealed that NETs formed in response to M. haemolytica are capable of trapping and killing a portion of the bacterial cells. NET formation was confirmed by confocal microscopy and by scanning and transmission electron microscopy. Prior exposure of bovine neutrophils to LKT enhanced subsequent trapping and killing of M. haemolytica cells in bovine NETs. Understanding NET formation in response to M. haemolytica and its LKT provides a new perspective on how neutrophils contribute to the pathogenesis of bovine respiratory disease.
Mannheimia haemolytica is a member of the bovine respiratory disease complex (BRD), causing a severe fibrinous pleuropneumonia sometimes referred to as shipping fever. The pneumonia is characterized by intense neutrophil infiltration in alveoli, intra-alveolar hemorrhage, fibrin deposition, and consolidation of the lungs (42, 56). The importance of neutrophils in the production of inflammatory mediators, recruitment of other leukocytes, and lung damage (17, 56, 67, 74) was demonstrated in calves that were depleted of neutrophils before challenge with M. haemolytica (10, 56). Neutrophil-depleted calves displayed less lung pathology than did control calves infected with M. haemolytica (10, 56). From these data, it is clear that neutrophils are a key player in the pathology of bovine pleuropneumonia; however, the mechanisms by which they contribute to host defense and tissue destruction are not clearly defined.
The most important virulence factor for M. haemolytica is its leukotoxin (LKT), a 104-kDa exotoxin produced during logarithmic-phase growth (18, 32). LKT is a member of the repeats-in-toxin (RTX) toxin family of exoproteins produced by a wide variety of Gram-negative bacteria, including Escherichia coli, Actinobacillus pleuoropneumoniae, and Aggregatibacter actinomycetemcomitans (70). RTX toxins are characterized by a C-terminal glycine-rich nonapeptide repeat region (-G-G-X-G-X-D-X-U-X, where U is a hydrophobic residue) that binds calcium (Ca2+). The latter is required for membrane binding and cytotoxicity (30, 70). RTX toxins can insert into the plasma membrane of target cells, causing lysis and necrotic cell death (30, 70). The N-terminal domain contains amphipathic and hydrophobic domains believed to be required for pore stabilization and formation, respectively (70). More recently, it was shown that LKT also causes apoptosis via a caspase 9-dependent pathway and that LKT is internalized and transported via the cytoskeleton to mitochondria (4-6).
The leukotoxin operon contains the genes lktC, lktA, lktB, and lktD (36, 37, 58). lktA encodes the inactive pro-LKT protein that is not cytotoxic until acylated (62) by the transacylase encoded by lktC. lktB and lktD encode proteins responsible for leader sequence-independent secretion of LKT from the bacterial cell (36, 37, 58). The acylated LKT then binds the CD18 chain of the β2-integrin lymphocyte function-associated antigen 1 (LFA-1) (3, 21-26, 33, 40, 41, 44, 55, 63) on ruminant leukocytes. LKT binding to amino acids 5 to 17 of the signal sequence of CD18 is required for cell death and restricts cytotoxicity to ruminant leukocytes, because the signal sequence for CD18 is not present on mature leukocytes from other mammalian species (55). Other investigators have shown that both the pro- form and mature LKT are capable of binding CD18, although the pro-LKT does not cause cytotoxicity (62). No biological role has been assigned to the pro- form of LKT.
Recently, several authors have shown that human neutrophils are able to undergo a form of cell death, called NETosis, that is distinct from apoptosis and necrosis (12, 13, 31, 51, 69). NETosis is defined as the release of nuclear DNA from an activated neutrophil into the extracellular environment, with little concomitant release of lactate dehydrogenase (LDH) (12). The extracellular DNA and associated proteins (e.g., histones) released by activated neutrophils have been termed neutrophil extracellular traps (NETs) (12). There are four steps leading to NET formation. These are neutrophil activation, nuclear envelope degradation, mixing of nuclear DNA with cytosolic proteins, and extrusion of the DNA-protein mixture from the cell (31). Treatment of human neutrophils with interleukin-8 (IL-8), phorbol 12-myristate 13-acetate (PMA), or lipopolysaccharide (LPS) causes NET formation (12, 31, 69). NET formation also occurs in response to prokaryotic and eukaryotic pathogens (12, 35, 64). To date, no bacterial exotoxin has been shown to cause NET formation.
NETs are composed of extracellular DNA that is studded with antimicrobial proteins. The latter include nuclear histones and primary, secondary, and tertiary granular components such as neutrophil elastase, myeloperoxidase, lactoferrin, and gelatinase (51, 69). When neutrophils become activated and commit to NET formation, they also are capable of trapping and killing pathogens. To date, NETs have been shown to kill a variety of Gram-negative and Gram-positive bacteria, fungi, and protozoans (2, 7-9, 12, 13, 15, 19, 20, 27, 28, 31, 34, 35, 43, 50-53, 59, 64, 67, 70). Here, we examine if M. haemolytica and its LKT cause NET formation by bovine neutrophils and whether NETs are capable of trapping and killing M. haemolytica cells in vitro.
MATERIALS AND METHODS
Neutrophil preparation.
Whole blood was taken by venipuncture from healthy, Holstein cows housed at the University of Wisconsin—Madison Dairy Cattle Center using 0.38% (vol/vol) sodium citrate as an anticoagulant. Blood was centrifuged at 1,000 × g for 15 min, and the plasma and buffy coat were removed. Red blood cells (RBCs) were lysed in a 1:3 dilution of lysis buffer (150 mM ammonium chloride, 10 mM Tris [pH 7.5]) while rotating at 8 rpm. Bovine neutrophils (bPMNs) were pelleted at 1,000 × g and washed four times with Hanks’ balanced salt solution (HBSS; Cellgro, Manassas, VA). Cells were resuspended in serum- and phenol red-free RPMI 1640 medium (Cellgro) and examined by light microscopy. Cells with a purity of >98% bPMNs as determined by visual inspection and >99% viability as determined by trypan blue staining using light microscopy were deemed acceptable for further use.
Bovine serum was obtained by venipuncture from healthy donor cows. The blood was allowed to clot for 30 min at room temperature and centrifuged at 1,000 × g, and the serum was removed and stored at −20°C until use.
Bacteria.
Mannheimia haemolytica A1 (obtained from a bovine pneumonic lung) and an M. haemolytica ΔlktC mutant strain derived from it were both gifts from S. Highlander (Houston, TX). The M. haemolytica ΔlktA strain and its parental strain M. haemolytica A1 isolate D153 (a plasmid-negative strain isolated from the lung of a steer that died of pneumonic mannheimiosis) were obtained from R. Briggs (Ames, IA). Although the ΔlktA strain was constructed from a different parental strain, we confirmed that the two parental strains had similar abilities to cause NET formation. All strains were grown in brain heart infusion (BHI) broth without shaking at 37°C for 10 h. Bacterial cells were then pelleted at 3,750 × g, washed three times in phosphate-buffered saline (PBS), and resuspended in RPMI medium to an optical density of 0.7, which corresponds to 5 × 109 CFU/ml. The number of CFU in each broth culture was extrapolated from growth curves performed in our laboratory and was confirmed by dilution plating on tryptic soy agar (TSA) with 5% sheep red blood cell (RBC) plates (Becton Dickinson, Franklin Lakes, NJ) to enumerate CFU.
Production and purification of LKT.
LKT, pro-LKT, and ΔLKT were prepared from Mannheimia haemolytica A1, an M. haemolytica ΔlktC mutant strain (SH1562) (29), and an M. haemolytica ΔlktA strain, respectively (62). Toxins were produced and purified as described previously (6). Briefly, bacteria were pelleted from 12-h cultures by centrifugation at 3,750 × g for 10 min. The pelleted cells were resuspended in RPMI 1640 medium and incubated for 6 h at 37°C until they reached logarithmic growth phase. Bacterial cells were centrifuged at 7,500 × g for 30 min at 4°C. The supernatant was removed, filtered (0.2 μm), and concentrated using an Amicon filtration system with a 100-kDa-molecular-mass-cutoff membrane (Millipore, Billerica, MA). The LKT was stored at −70°C with 20% (vol/vol) glycerol until used in an experiment. One unit of LKT is defined as the greatest dilution of toxin that killed 50% of target cells (106 BL-3 cells in 200 μl RPMI medium) in 2 h at 37°C as determined with the cellTiter 96 Aqueous One solution cell proliferation assay (Promega, Madison, WI). Pro-LKT and ΔLKT preparations did not exhibit cytotoxicity in this assay. Protein concentrations were determined using a bicinchoninic acid (BCA) assay (Pierce, Rockford, IL).
Quantification of extracellular DNA.
Neutrophil extracellular DNA was quantified using a modified technique described by Fuchs et al. (31). Briefly, neutrophils were incubated for the indicated times with various stimuli and then pelleted at 500 × g for 3 min. The supernatant was removed, and micrococcal nuclease buffer with 0.1 U/μl micrococcal nuclease was added (New England Biolabs, Ipswich, MA) and incubated for 30 min at 37°C (as described by the manufacturer). A 1:200 dilution of PicoGreen (Invitrogen, Carlsbad, CA) in 10 mM Tris base buffered with 1 mM EDTA was added to an equal volume of the nuclease-treated PMN mixture. Fluorescence was determined at an excitation wavelength of 484 nm and an emission wavelength of 520 nm using an automated plate reader (DTX 800 Multimode detector; Beckman Coulter, Brea, CA). NET production was quantified as fold increase, where the fluorescence units for treated neutrophils were divided by the fluorescence units for untreated control neutrophils.
Reagents.
DNase I (source, bovine pancreas), PMA, E. coli lipopolysaccharide (LPS), and cytochalasin D (Cyto D) were purchased from Sigma-Aldrich (St. Louis, MO). The NADPH oxidase inhibitor diphenyleneiodonium chloride (DPI) was purchased from Calbiochem/EMD (Darmstadt, Germany). The caspase family inhibitor Z-VAD-FMK (fluoromethylketone) was purchased from Alexis Biochemicals (Plymouth Meeting, PA). The anti-CD18 antibody (BAQ20A) was purchased from VMRD (Pullman, WA). M. haemolytica A1 LPS was kindly provided by D. McClenahan (Cedar Falls, IA) (46). The anti-LKT antibody (MM601) was kindly provided by S. Srikumaran (Pullman, WA). Mammalian protein extraction reagent (MPER) was purchased from Pierce and used to make bPMN lysates. LDH release was determined using the CytoTox 96 nonradioactive cytotoxicity assay as described by the manufacturer (Promega).
Western blotting.
bPMNs (107) were incubated for 1 h at 37°C with 2 U of LKT, heat-inactivated (100°C for 30 min) LKT, pro-LKT, ΔLKT, or RPMI 1640 (vehicle) while being rotated at 8 rpm. The bPMNs were washed five times with PBS and resuspended in MPER buffer (Pierce). Protein concentrations were determined by BCA assay. One hundred micrograms of protein was loaded into separate wells of a 4 to 20% Tris-HCl polyacrylamide gel (Bio-Rad Laboratories, Hercules, CA), separated by electrophoresis, and transferred to a polyvinylidene fluoride (PVDF) membrane (Bio-Rad Laboratories). The membranes were blocked with 5% (wt/vol) skim milk in TBST buffer (10 mM Tris base, 150 mM NaCl, 0.1% Tween 20) for 1 h at room temperature. The blot was then incubated with an anti-LKT monoclonal antibody (MM601) for 1 h. Blots were washed and incubated for 1 h at room temperature with an anti-mouse IgG Fc fragment conjugated to horseradish peroxidase (HRP) (GE Health Sciences, Chalfont St. Giles, United Kingdom). Blots were washed, ECL chemiluminescence substrate (Pierce) was added to detect HRP, and bands were visualized by exposure to film (MidSci, St. Louis, MO).
Immunofluorescence and electron microscopy.
To perform immunofluorescence microscopy, bPMNs (3 × 106) were incubated on poly-l-lysine (Electron Microscopy Sciences, Hatfield, PA)-coated glass slides (Fisher Scientific, Hanover Park, IL) for 30 min. Slides were then incubated at 37°C with 0.5 U LKT, 5 × 107 fluorescein-labeled M. haemolytica cells, 0.5 U LKT, and 180 U DNase, 100 nM PMA, or 250 nM M. haemolytica LPS for 1 h. Slides were washed three times with PBS and fixed for 10 min with 4% paraformaldehyde (PFA). The slides were then washed, permeabilized with cold acetone for 5 min, washed again, and blocked with 1% bovine serum albumin (BSA; Pierce) in PBS for 20 min at room temperature. Slides were incubated for 1 h with 1 μM TOPRO. In some experiments, an antihistone monoclonal antibody (AbCam, Cambridge, MA) was added and slides were washed and then incubated for 1 h at room temperature with an Alexa Fluor 488-conjugated anti-mouse IgG antibody (Molecular Probes, Eugene, OR) in 1% BSA (in PBS). Slides were washed and examined by confocal microscopy (Nikon Eclipse TE2000-U; Nikon Corporation, Tokyo, Japan).
To perform transmission electron microscopy (TEM), bPMNs (3 × 106) were incubated on poly-l-lysine-coated glass slides for 30 min, washed twice with PBS, and then incubated with 0.5 U LKT for 1 h. Slides were washed three times with PBS and fixed for 30 min with Karnovsky's fixative (5% glutaraldehyde, 4% paraformaldehyde in 0.08 M sodium phosphate buffer) (Electron Microscopy Sciences). TEM was performed as described previously (39). Images were taken at the UW Medical School Electron Microscope Facility using a Philips CM120 STEM microscope.
To perform scanning electron microscopy, bPMNs (3 × 106) were incubated on poly-l-lysine-coated glass slides for 30 min, washed twice with PBS, and then incubated with 0.5 U LKT or 5 × 107 M. haemolytica cells for 1 h. Slides were washed and fixed using 4% paraformaldehyde in PBS, postfixed with 2.5% glutaraldehyde, and prepared as previously described (11). Images were taken at the Biological and Biophysical Preparation, Imaging and Characterization Laboratory at the University of Wisconsin—Madison with a Hitachi S900 microscope (Hitachi, Japan).
Bacterial trapping and killing.
M. haemolytica cells were grown to log phase as described earlier, washed three times in PBS, and resuspended for 15 min on ice in 0.5 mg/ml fluorescein isothiocyanate (FITC; Sigma-Aldrich) in 50 mM sodium carbonate buffer. The M. haemolytica cells were then washed three times with PBS and resuspended at a concentration of 5 × 109 bacteria/ml in serum-free RPMI 1640. Cell viability after FITC labeling was assessed by dilution plating both FITC-labeled and unlabeled M. haemolytica cells. Dilutions were plated on blood agar, and CFU were quantified.
bPMNs were treated four different ways prior to the addition of M. haemolytica cells. First, 0.25 U LKT was incubated with bPMNs (106) at 37°C while rotating at 8 rpm for 30 min prior to the addition of 10 μg/ml cytochalasin D (Cyto D), which was further incubated for 30 min. Second, bPMNs (106) were incubated for 30 min while rotating at 37°C with 0.25 U LKT. Third, bPMNs were incubated with 100 nM PMA for 30 min. Fourth, bPMNs (106) were incubated with 180 U DNase I for the entire duration of the assay. Treated (LKT plus Cyto D, LKT, PMA, or DNase) and untreated bPMNs were washed and then incubated with FITC-labeled or nonlabeled M. haemolytica cells (107) in RPMI 1640 for various times at 37°C.
To quantify the bacteria trapped within NETs, samples were washed three times with PBS and fluorescence was determined using an automated plate reader. To determine bacterial killing by NETs, bPMNs incubated with M. haemolytica suspensions were serially diluted in PBS and plated on TSA with 5% sheep RBCs. At each time point, serial dilutions of M. haemolytica incubated in RPMI medium without bPMNs were also made and plated onto blood agar to quantify total CFU. The percent bactericidal activity of NETs was determined as described previously (34).
Immunohistochemistry.
Paraffin-embedded tissue from an M. haemolytica-infected calf and a healthy control calf was obtained from the UW-Madison Veterinary Medicine Teaching Hospital (kindly provided by E. Behling-Kelly). Sections were deparaffinized and incubated for 1 h at room temperature with 2 μM Sytox Orange (Invitrogen), washed, and examined using a Nikon Eclipse E800 immunofluorescence microscope. For some experiments, 180 U of DNase was added to the tissue sections and incubated overnight at 4°C, washed, and stained with Sytox Orange.
Statistical analysis.
Group means were compared by analysis of variance (ANOVA), followed by the Tukey-Kramer pairwise comparison test, as performed by the Instat statistical package (GraphPad, San Diego, CA). The level of significance was set at a P value of <0.05.
RESULTS
LKT causes NET formation.
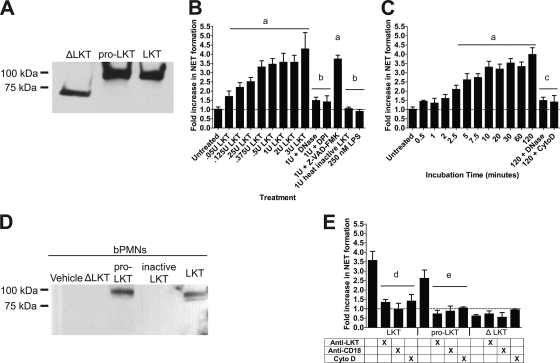
We first sought to determine if purified LKT causes NET formation by bovine neutrophils (bPMNs). We initially purified native LKT, pro-LKT (produced by an ΔlktC M. haemolytica strain), and a truncated ΔLKT (produced by an ΔlktA M. haemolytica strain) and confirmed LKT molecular weights using Western blot analysis (Fig. 1A). Purified LKTs were of the correct molecular weights as previously described (62). We observed NET formation in response to native LKT in both a dose- and time-dependent manner (Fig. 1B and C). NET formation was significantly reduced when bovine neutrophils were preincubated with DNase I or cytochalasin, which cleaves extracellular DNA and inhibits the intracellular transport of LKT, respectively (4). We also reduced NET formation when bPMNs were preincubated with the NADPH oxidase inhibitor DPI prior to addition of LKT. NET formation was not inhibited when bPMNs were incubated with the caspase family inhibitor Z-VAD-FMK. Purified M. haemolytica LPS did not induce NET formation (Fig. 1B), although it did stimulate PMN inducible nitric oxide synthase (iNOS) production (data not shown). Nor was NET formation observed in response to heat-inactivated LKT (100°C for 1 h). Taken together, these observations confirm that contaminating LPS does not cause NET formation (Fig. 1B).
FIG. 1.
LKT and pro-LKT cause NET formation by bovine neutrophils. (A) Purified native LKT, pro-LKT, and ΔLKT were separated by SDS-PAGE, blotted to PVDF, and probed using an anti-LKT antibody to estimate the molecular masses of the toxins. (B and C) bPMNs (106) were incubated with various amounts of LKT for 120 min (B) or were incubated with 1 U of LKT for various times (C). As a control, 106 bPMNs were preincubated with 180 U of DNase I, 10 μg/ml cytochalasin D (Cyto D), 10 μM DPI, or 200 μM Z-VAD-FMK (pan-caspase inhibitor) before LKT was added. (D) bPMNs (106) were incubated with 1 U LKT, heat-inactivated LKT, pro-LKT, or ΔLKT for 120 min. Cells were then washed, and lysates were prepared. Proteins were separated by SDS-PAGE, blotted to PVDF, and probed using an anti-LKT antibody. Untreated control cells were preincubated with the vehicle RPMI 1640. (E) bPMNs (106) were preincubated for 2 h with 1 U LKT, pro-LKT, or ΔLKT. Some bPMNs were incubated with an anti-CD18 antibody or 10 μg/ml cytochalasin D, or toxins were incubated with an anti-LKT antibody for 30 min prior to addition to bPMNs. Extracellular DNA was quantified by the addition of a 1:200 dilution of PicoGreen, and fluorescence was quantified using an automated plate reader. Data represent the means ± standard errors of the means of 5 independent experiments. a, P < 0.05 compared to untreated bPMNs; b, P < 0.05 compared to bPMNs treated with 1 U LKT; c, P < 0.05 compared to bPMNs treated with LKT for 120 min; d, P < 0.05 compared to bPMNs incubated with LKT; e, P < 0.05 compared to bPMNs incubated with pro-LKT.
To further examine LKT-induced NET formation, we purified pro-LKT from an ΔlktC M. haemolytica strain (kind gift from S. K. Highlander, Houston, TX) and a truncated ΔLKT from an ΔlktA M. haemolytica strain (kind gift from R. E. Briggs, Ames, IA). The ΔlktC M. haemolytica strain does not produce an active LKTC protein; therefore, acylation of the LKTA protein does not occur and the resulting pro-LKT is noncytolytic. The ΔlktA strain produces a truncated ΔLKT lacking amino acids 34 to 378, rendering it noncytotoxic (62). We incubated bPMNs with purified native LKT, heat-inactivated (inactive LKT) LKT, pro-LKT, or ΔLKT and analyzed binding to bPMN proteins by Western blotting. As previously demonstrated (62), native LKT and pro-LKT bind bPMNs, whereas the ΔLKT and heat-inactivated LKT did not (Fig. 1D). We next determined if unacylated pro-LKT was capable of causing NET formation. To our surprise, the pro-LKT caused NET formation (Fig. 1E), although it was slightly reduced compared to that caused by wild-type LKT. As expected, NET formation was not observed in response to the truncated ΔLKT (Fig. 1E). Likewise, preincubation of bPMNs with either anti-CD18 receptor antibody or cytochalasin D reduced NET formation in response to wild-type or pro-LKT. Furthermore, preincubation of LKT or pro-LKT with a neutralizing anti-LKT antibody (MM601) significantly diminished NET formation. These data confirm that LKT and pro-LKT, and not a contaminant in the LKT preparations, cause NET formation. These data also indicate that LKT causes NET formation via its interaction with the CD18 chain of LFA-1.
Incubation of bovine neutrophils with M. haemolytica cells causes NET formation.
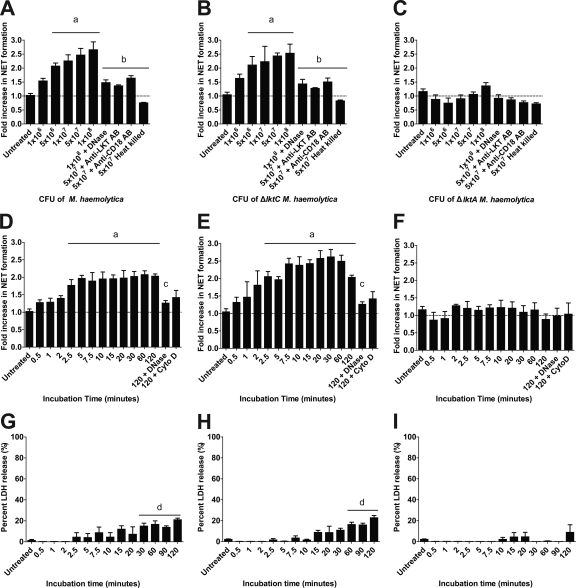
We next assessed if incubation of bPMNs with M. haemolytica cells would result in NET formation. We found that cells of M. haemolytica A1 (isolated from a pneumonic bovine lung) and an isogenic M. haemolytica ΔlktC strain, producing only pro-LKT, caused NET formation in a dose- and time-dependent manner. As expected, NET formation was not observed when bPMNs were incubated with cells of an M. haemolytica ΔlktA strain (Fig. 2). NET formation in response to wild-type or ΔlktC M. haemolytica was diminished by preincubation with DNase I, cytochalasin D, anti-LKT antibodies, or anti-CD18 antibodies (Fig. 2) but not with control antibodies (data not shown). NET formation did not occur in response to heat-treated (100°C for 1 h) wild-type, ΔlktC, or ΔlktA M. haemolytica cells (Fig. 2A to C). LDH release in response to M. haemolytica or ΔlktC M. haemolytica cells followed rather than preceded NET formation (Fig. 2G to I). LDH release did not occur in response to ΔlktA M. haemolytica cells.
FIG. 2.
Wild-type M. haemolytica and ΔlktC M. haemolytica cells cause NET formation by bovine neutrophils. (A to C) bPMNs (106) were incubated for 120 min with various numbers of M. haemolytica (A), ΔlktC M. haemolytica (B), or ΔlktA M. haemolytica (C) cells. In some experiments, 180 U of DNase I or an anti-CD18 antibody was preincubated with the bPMNs, or bacteria were preincubated with an anti-LKT antibody, for 30 min prior to addition to bPMNs. (D to F) bPMNs (106) were incubated for various times with 107 cells of M. haemolytica (D), ΔlktC M. haemolytica (E), or ΔlktA M. haemolytica (F). In some experiments, 180 U of DNase I or 10 μg/ml cytochalasin D (Cyto D) was preincubated with the bPMNs for 30 min. Extracellular DNA was quantified as described previously. (G to I) bPMNs (106) were incubated with 107 CFU of M. haemolytica (G), ΔlktC M. haemolytica (H), or ΔlktA M. haemolytica (I), and LDH release was quantified using the CytoTox 96 nonradioactive cytotoxicity assay as described by the manufacturer. Data represent the means ± standard errors of the means of 5 independent experiments. a, P < 0.05 compared to untreated bPMNs; b, P < 0.05 compared to bPMNs incubated with 5 × 107 bacteria; c, P < 0.05 compared to bPMNs incubated for 120 min with bacterial cells alone; d, P < 0.05 compared to LDH release by untreated bPMNs.
Repeated exposures to M. haemolytica cause a further increase in NET formation.
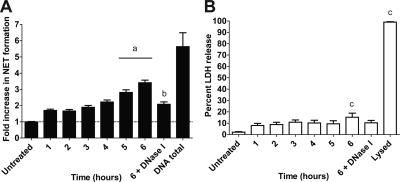
Previous research suggested that 10 to 33% of human neutrophils undergo NET formation in response to a single stimulus (13, 31). Whether NET formation increased further in response to repeated or sustained stimulation was not reported. Here, we show that repeated exposures (5 exposures) of bPMNs to M. haemolytica cells increased the percentage of bPMNs that form NETs, without a corresponding increase in LDH release (Fig. 3). Quantification of DNA in bPMN lysates (DNA total) confirmed that repeated exposure to M. haemolytica cells resulted in increased NET formation by bPMNs.
FIG. 3.
Repeated exposure to M. haemolytica results in increased NET formation by bovine neutrophils. M. haemolytica cells (107) were added to 106 bPMNs every hour for 1 to 6 h and NET formation (A) and LDH release (B) were measured at 6 h. DNase I (180 U) was also added to one group of cells that was treated hourly for 6 h. Total DNA was determined by lysing 106 bPMNs, and extracellular DNA was quantified as described previously. Data represent the means ± standard errors of the means of 5 independent experiments. a, P < 0.05 compared to bPMNs treated for 1 h; b, P < 0.05 compared to bPMNs treated for 6 h; c, P < 0.05 compared to untreated bPMNs.
bPMN NETs trap and kill M. haemolytica cells.
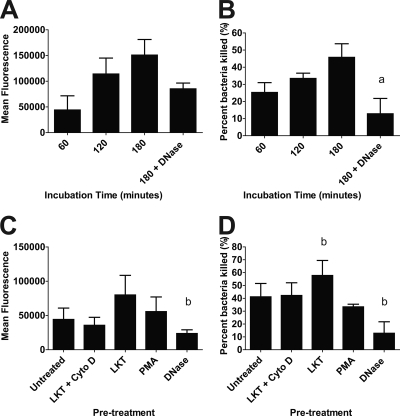
Other investigators have shown that NETs are capable of trapping and killing extracellular pathogens (12, 27, 28, 31, 34, 52, 64). Using fluorescein-labeled M. haemolytica cells, we found that bacterial cells were trapped, in a time-dependent manner, by NETs (Fig. 4A). In similar experiments, M. haemolytica cells were incubated with bPMNs for various times and viability of the bacteria was assessed. Up to 50% of the M. haemolytica cells were killed during a 180-min incubation period (Fig. 4B). To confirm that bacteria were trapped in NETs, we added DNase I to cleave extracellular DNA and thereby free trapped bacterial cells. DNase I treatment resulted in a significant reduction in the number of M. haemolytica cells trapped and killed by bPMNs (Fig. 4A and B). We infer that the remaining proportion of bacterial cells that were killed by DNase I-treated bPMNs represent bacterial cells that were phagocytosed and killed intracellularly.
FIG. 4.
Preincubating bovine neutrophils with LKT increases the trapping and killing of M. haemolytica within NETs. (A and B) bPMNs (106) were incubated with 107 fluorescein-labeled M. haemolytica (A) or unlabeled M. haemolytica (B) cells for 60 to 180 min. (C and D) Similarly, treated bPMNs (LKT plus Cyto D, LKT, PMA, or DNase) or untreated bPMNs were incubated with 107 fluorescein-labeled M. haemolytica cells (C) or unlabeled M. haemolytica cells (D) for 180 min. In panels C and D, 106 bPMNs were incubated with (i) 0.25 U LKT for 30 min followed by 10 μg/ml cytochalasin D (LKT + Cyto D), (ii) 0.25 U LKT, (iii) 100 nM PMA for 30 min at 37°C, or (iv) 180 U DNase I for the duration of the experiment. Fluorescence (A and C) was measured using an automated plate reader. Bacterial survival (B and D) was estimated by plating serial dilutions of lysates on TSA plus 5% sheep RBC plates. Data represent the means ± standard errors of the means of 5 independent experiments. a, P < 0.05 compared to bPMNs treated for 180 min; b, P < 0.05 compared to untreated bPMNs.
During pulmonary infection with M. haemolytica, bovine leukocytes may be exposed to LKT before they encounter bacterial cells. We, therefore, next examined whether LKT-induced NETs can trap and kill M. haemolytica. To do so, we incubated bPMNs with a small amount of LKT (0.25 U; <5% LDH release) for 30 min. In some experiments, we then added cytochalasin D to inhibit phagocytosis after LKT treatment. bPMNs were then incubated with M. haemolytica cells (10 bacterial cells per bPMN), and bacterial trapping and killing were quantified. Prior exposure to LKT resulted in NET formation that increased the numbers of M. haemolytica cells that were trapped and killed (Fig. 4C and D). In some experiments, bPMNs were incubated with DNase along with M. haemolytica cells during the experiment. Addition of DNase I reduced the numbers of bacteria that were trapped and killed (Fig. 4C and D), implicating DNA-containing NETs as the source of bacterial killing. We also used a Syto 9 and propidium iodide staining kit (Live/Dead BacLight bacterial viability kit; Invitrogen) to confirm that M. haemolytica cells were killed within NETs (data not shown) and to exclude the possibility that reduced numbers of CFU simply reflect clumped bacteria.
bPMN NETs contain nuclear DNA.
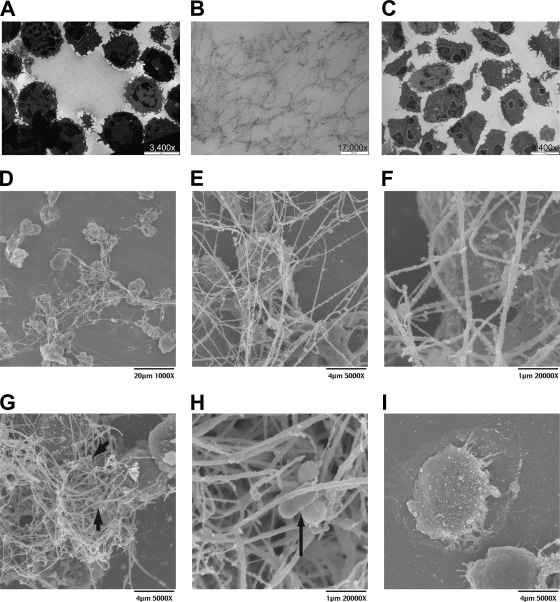
When we examined NETs using transmission electron microscopy (TEM) and scanning electron microscopy (SEM), we observed weblike structures extruded from the LKT-treated and M. haemolytica-treated bPMNs (Fig. 5). These structures were not seen with control untreated bPMNs. Some of the DNA strands were longer than 60 μm as illustrated in Fig. 5D. M. haemolytica cells were observed to be trapped within the web of DNA fibrils (Fig. 5G and H).
FIG. 5.
Transmission and scanning electron photomicrographs of NETs formed by bovine neutrophils in response to M. haemolytica LKT. LKT (0.5 U) or 5 × 107 M. haemolytica cells were incubated with 3 × 106 bPMNs for 60 min at 37°C. Cells were washed, fixed, and processed for TEM (A to C) or SEM (D to I) as described in Materials and Methods. Transmission electron photomicrographs were taken at a magnification of ×3,400 (bar, 5 μm) (A and C) or ×17,000 (bar, 200 nm) (B). (A) Large weblike structures were released from LKT-treated bPMNs. (B) Enlargement of panel A, to illustrate individual fibrils. (C) Control cells incubated in RPMI medium do not exhibit fibrils. (D to I) Scanning electron photomicrographs. (D) Micrograph demonstrates a large network of extracellular DNA strands released in response to LKT. (E) Higher magnification of panel D, illustrating bPMNs trapped within the mesh. (F) Higher magnification of panel E, illustrating in greater detail the ultrastructure of individual NET fibrils. (G) Complex network of DNA strands in which bPMNs and M. haemolytica cells are visible. (H) Higher magnification of panel G, illustrating more closely M. haemolytica cells trapped within a NET. Arrows indicate trapped M. haemolytica cells in panels G and H. (I) Control bPMNs incubated in RPMI medium do not exhibit fibrils. Photomicrographs are of representative cells from 3 independent experiments.
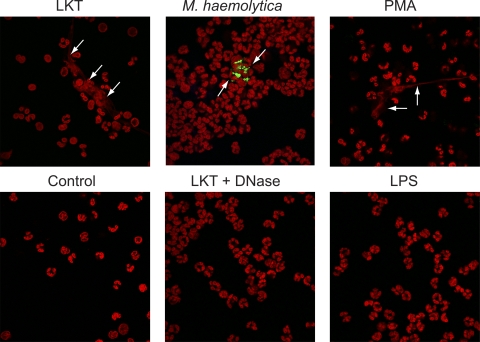
We next examined NETs by confocal microscopy. We incubated bPMNs with LKT, fluorescein-labeled M. haemolytica cells, or PMA and fluorescently stained nucleic acids with TOPRO. Confocal microscopy analysis confirmed the presence of large, extracellular strands and clumps of DNA released from bPMNs in response to LKT, M. haemolytica cells, or 100 nM PMA (Fig. 6). Release of extracellular DNA was not observed for untreated bPMNs, bPMNs incubated with M. haemolytica LPS, or bPMNs concomitantly incubated with LKT and DNase I (Fig. 6).
FIG. 6.
Confocal photomicrographs reveal extracellular DNA released from bovine neutrophils in response to LKT, M. haemolytica cells, or PMA. bPMNs (3 × 106) were allowed to attach to glass slides and then were incubated with 0.5 U LKT, 5 × 107 fluorescein-labeled M. haemolytica cells, or 100 nM PMA. Controls include untreated bPMNs (control) and bPMNs incubated with 0.5 U LKT plus 180 U DNase I, or 250 nM LPS, for 60 min. Cells were fixed, permeabilized, stained for DNA using TOPRO, and examined by confocal microscopy. Arrows illustrate representative NETs. Photomicrographs are of representative cells from 3 independent experiments.
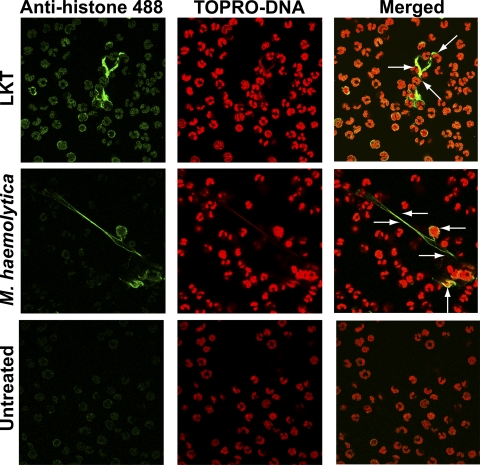
To determine the origin of the extracellular DNA in NETs, we incubated bPMNs with LKT or M. haemolytica cells and then fluorescently stained cells for DNA (red) and histones (green). Confocal microscopy revealed that DNA and histones colocalized within the nucleus in untreated control bPMNs (Fig. 7). Similarly, DNA and histones colocalized to both the extracellular DNA and nuclei of bPMNs treated with LKT or M. haemolytica cells. These observations suggest that the extracellular DNA is of nuclear origin (Fig. 7).
FIG. 7.
Colocalization of DNA and histones in NETs produced in response to LKT or M. haemolytica. bPMNs (3 × 106) were allowed to attach to glass slides and then incubated with 0.5 U LKT or 5 × 107 M. haemolytica cells for 60 min. Cells were fixed, permeabilized, stained for DNA using TOPRO (red), and probed for histones using an antihistone antibody followed by an anti-mouse antibody labeled with Alexa Fluor 488 (green). Cells were examined by confocal microscopy. Arrows indicate areas of colocalization of signal (yellow-orange) for extracellular DNA and histones. Photomicrographs are of representative cells from 3 independent experiments.
Extracellular DNA is observed in histological sections of lung tissue from an M. haemolytica-infected calf.
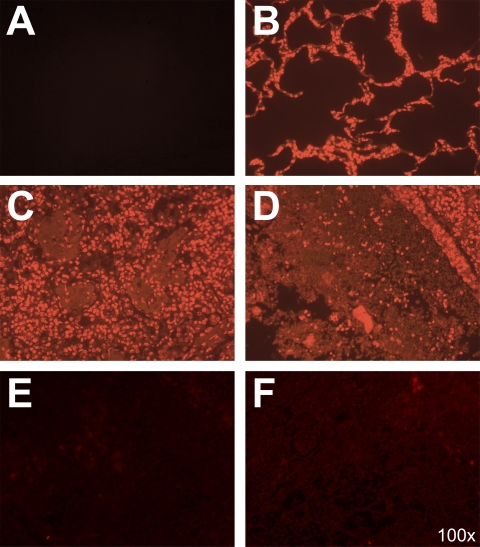
To provide evidence that neutrophils produce extracellular DNA in the lungs during M. haemolytica infection, we obtained lung samples from a calf that was euthanized because of severe respiratory disease. Subsequent microbiological testing confirmed that M. haemolytica was the cause of illness. Lung sections were prepared, deparaffinized, and stained with Sytox Orange (DNA stain). Microbiological examination revealed substantial amounts of extracellular DNA enmeshed around inflammatory cells (Fig. 8C and D). Extracellular DNA and infiltrating leukocytes were not observed in lung sections from a healthy control calf (Fig. 8B). As a control, some M. haemolytica-infected lung sections were not stained with Sytox Orange to demonstrate the absence of background fluorescence (Fig. 8A). To confirm that Sytox Orange staining was specific for DNA, we preincubated M. haemolytica-infected lung sections with DNase I, which reduced Sytox Orange staining compared to that in lung sections not treated with DNase (Fig. 8E and F). These data confirm the presence of extracellular DNA in the lung of an infected calf, which was not seen when lung sections were obtained from an uninfected calf.
FIG. 8.
Extracellular DNA is present in the lungs of an M. haemolytica-infected calf. Tissue sections (10 μm) were obtained from an M. haemolytica-infected calf and a healthy calf, deparaffinized, and incubated with Sytox Orange to stain DNA, or PBS as a control for autofluorescence at 570 nm. (A) Control lung tissue from an M. haemolytica-infected calf that was not stained with Sytox Orange to illustrate the absence of autofluorescence at 570 nm. (B) Lung section from a healthy bovine lung displays normal alveolar structure with no extracellular DNA staining. (C and D) Lung tissue obtained from an M. haemolytica-infected calf that was stained with Sytox Orange to detect DNA. (C) Cellular infiltrates in the alveoli with extracellular DNA present. (D) Consolidation of the lung and extensive extracellular DNA staining. (E and F) Lung tissues from an M. haemolytica-infected calf were incubated for 24 h with 180 U DNase at 4°C, washed, and incubated with Sytox Orange. Fluorescence was reduced compared to that in panels C and D, indicating that the Sytox staining was specific for DNA within the tissue.
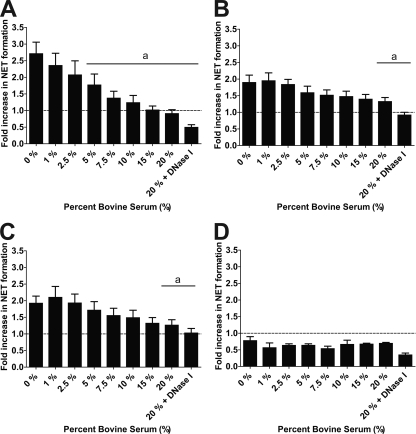
Preincubation of bPMNs with bovine serum reduces NET formation.
Serum proteins are present in the extravascular fluid that accumulates during pneumonia. Although serum contains DNase I, its functional role has not been clearly defined (71). Other investigators have reported that serum reduces human NET formation (12, 65). Here, we show that concomitant incubation of bPMNs with LKT or M. haemolytica cells, and increasing amounts of adult bovine serum, decreased NET formation (Fig. 9). From these results we cannot determine whether NETs do not form or are cleaved by DNase in bovine serum. However, we confirmed the ability of bovine serum to degrade lambda DNA as measured by PicoGreen binding (data not shown).
FIG. 9.
Coincubation with bovine serum causes a reduction in NET formation. bPMNs (106) were incubated with 1 U LKT (A), 107 M. haemolytica cells (B), 107 ΔlktC M. haemolytica cells (C), or 107 ΔlktA M. haemolytica cells (D) for 120 min with increasing amounts of normal adult bovine serum. In some experiments, 180 U of DNase I was also added to the 20% serum group. Extracellular DNA was quantified as described above. Data represent the means ± standard errors of the means of 5 independent experiments. a, P < 0.05 compared to bPMNs incubated without bovine serum.
DISCUSSION
We believe this is the first report of NET formation in response to a bacterial exotoxin. Here, we show that the M. haemolytica leukotoxin causes NET formation in a dose- and time-dependent manner (Fig. 1B and C). DNase I was used to cleave extracellular DNA produced in response to LKT and demonstrate that the fluorescence signal was due to PicoGreen binding to extracellular DNA. We used several methods to assess whether LKT, and not a contaminant, causes NET formation. These included bPMNs incubated with (i) cytochalasin D, an inhibitor of LKT-mediated internalization; (ii) antibodies to LKT or its CD18 receptor; (iii) heat-inactivated LKT or bacterial cells (100°C for 1 h); (iv) a truncated ΔLKT; and (v) purified M. haemolytica LPS (Fig. 1). These data indicate that LKT causes NET formation via binding to its CD18 receptor and that M. haemolytica LPS alone (25 nM to 2.5 μM) does not cause NET formation by bPMNs. Similar results were seen by Clark et al. (20), who reported that LPS did not stimulate NET production by human neutrophils, unless platelets were also present.
Other investigators have found that NET formation requires NADPH oxidase activity (31). We confirmed these findings by preincubating bPMNs with DPI, a selective NADPH oxidase inhibitor, and found that DPI inhibited NET formation as expected (Fig. 1B). To test whether extracellular DNA was released as a result of apoptosis, we incubated bPMNs with the pan-caspase inhibitor Z-VAD-FMK. Addition of Z-VAD-FMK did not reduce NET formation, indicating that NET formation is independent of apoptosis (Fig. 1B). We also confirmed that the release of extracellular DNA preceded LDH release, suggesting that NET formation was not simply due to necrotic cell death (Fig. 2). Overall, these are consistent with NET formation by bPMNs being NADPH oxidase dependent and independent of necrosis and apoptosis.
One of our most striking observations was that unacylated pro-LKT triggers NET formation, despite having negligible activity in standard cytotoxicity assays (Fig. 1E). We confirmed that the pro-LKT bound bPMNs to a similar extent as did wild-type LKT, as reported by others (62), whereas a truncated ΔLKT did not (Fig. 1D). The latter finding differs somewhat from that of Thumbikat et al. (62), who reported that the ΔLKT was capable of binding CD18 from bovine lymphoblastoid (BL-3) cells (a pre-B-cell line). Our data indicate that binding of LKT or pro-LKT to the CD18 receptor on bPMNs causes NET formation, despite the lack of cytotoxicity for pro-LKT in standard cytotoxicity assays. We believe that this is the first report of a biological function for the unacylated pro-LKT. It is unknown how much unacylated pro-LKT, if any, is produced by wild-type M. haemolytica cells during an infection. However, the data in the present study confirm that pro-LKT does have biological activity, despite its lack of substantial cytotoxicity for bPMNs.
We observed NET production in response to cells of wild-type M. haemolytica and cells of an M. haemolytica ΔlktC strain (Fig. 2). It is important to note that cells of the M. haemolytica ΔlktA strain did not stimulate NET formation by bPMNs. These observations provide additional evidence that LKT is of primary importance for NET formation in response to M. haemolytica cells. This conclusion was further strengthened by using antibodies to either LKT or its CD18 receptor, which significantly reduced NET formation (Fig. 2). As expected, NET formation did not occur when human neutrophils were treated with M. haemolytica or its LKT (data not shown). Because there are previous reports of NET production in response to a variety of Gram-positive and Gram-negative bacterial cells (12), it was surprising that eliminating or neutralizing LKT by itself significantly reduced NET formation. Although LKT is a major virulence factor for M. haemolytica, one might expect that other bacterial molecules would contribute to NET formation. For example, one study showed that pilus-deficient group A streptococci caused less NET formation by human neutrophils than did wild-type streptococci, although strong direct evidence that pili were the principal cause of NET formation was lacking (2). Other reports of NET formation in response to bacteria, fungi, and protozoans did not identify a specific microbial component responsible for these responses. It would be interesting to investigate toxins or virulence factors produced by other NET-causing pathogens, to determine whether any of these are principally responsible for NET formation by those microbes.
The signaling mechanisms that stimulate and regulate NET formation have not yet been established. Because LKT binding to CD18 was required for NET formation (Fig. 1 and 2), we infer that signaling downstream of CD18 is important for bovine neutrophil DNA release. Previous reports have demonstrated that neutrophils from several vertebrate species, human eosinophils and mast cells, and chicken heterophils all form extracellular traps (12, 13, 19, 34, 38, 43, 48, 49, 68, 73). All these cells express CD18, which is involved in transendothelial migration of leukocytes from the bloodstream into tissue at sites of infection (45, 47, 54, 60, 61, 75). Because CD18 is expressed by cell types capable of forming extracellular traps, perhaps binding of pathogen-associated molecular patterns (PAMPs) to CD18 leads to downstream signaling that culminates in extracellular trap release. Further examination of the role that CD18 plays in extracellular trap formation by different species of leukocytes could lead to a better understanding of the mechanisms that regulate NET formation.
Previous research demonstrated that only 10 to 33% of human neutrophils formed NETs in response to a single exposure to a stimulus in vitro (13, 31). Here we asked whether repeated exposure of bPMNs to M. haemolytica cells results in a greater number of bPMNs forming NETs. We found that this is the case, with repeated addition of M. haemolytica cells (hourly for up to 5 h) resulting in a significant increase in NET formation, with only a modest increase in LDH release (Fig. 3). Overall, we interpret these results as indicating that only a proportion of neutrophils undergo NET formation in response to a single stimulus but that these increase in number as the insult continues. Because neutrophils are terminally differentiated cells with a relatively short life span in the circulation (57), it would be of interest to determine whether age, or relative senescence of individual PMNs, influences NET formation.
This is not the first report that bovine PMNs can form NETs in vitro. One group demonstrated that bovine PMNs form NETs that trap mastitis-inducing bacterial cells even in the presence of milk, which is inhibitory for phagocytosis (43). A second group of investigators demonstrated that beta-hydroxybutyrate, produced during ketosis or hyperketonemia, reduced phagocytosis and NET-mediated killing of E. coli P4 by bPMNs (34). Here, we report that bovine NETs are capable of trapping and killing the pneumonic pathogen M. haemolytica in vitro (Fig. 4A and B). We also provide evidence that preformed NETs (formed in response to LKT) trap and kill more M. haemolytica cells than do NETs produced in response to the bacterial cells themselves (Fig. 4C and D). As reported in previous studies (12, 34, 64, 66), we inhibited phagocytosis using cytochalasin D to confirm that bacterial death was due to NET-mediated extracellular killing and not phagocytosis and intracellular killing. Curiously, NETs formed in response to PMA were not increased in their ability to trap and kill M. haemolytica cells (Fig. 4C and D).
Older histopathological studies of M. haemolytica-infected ruminants reported streaming leukocytes, defined as elongated inflammatory cells with streaming nuclei, within the alveoli of pneumonic cattle, sheep, and goats (14, 16, 72). Further examination of the origin of streaming leukocytes indicated that they were mostly of neutrophil origin (1). One investigator observed bacterial cells lining streaming leukocytes in the alveoli of M. haemolytica-infected goats (72). The mechanism responsible for streaming leukocyte formation has not been established, nor is it known what role streaming leukocytes might play in the clearance of M. haemolytica. We interpret these older reports of streaming leukocytes as being similar to the NET formation that we observed in vitro. Using TEM and SEM, we observed long strands of extracellular DNA, which were gathered into aggregates that spanned over 60 μm in length (Fig. 5), being released from bPMNs incubated with M. haemolytica cells or its LKT. DNA strands appeared to enmesh intact PMNs as well, forming large aggregates in which M. haemolytica cells were observed (Fig. 5G and H). Confocal microscopy and TOPRO staining confirmed that these strands consisted of DNA and associated histones emanating from bPMNs. DNase I treatment eliminated these strands, confirming that they were dependent on DNA release. When we added fluorescein-labeled M. haemolytica cells, we could observe bacterial cells trapped within aggregates of extracellular DNA and bPMNs. We confirmed that the fluorescein-labeled M. haemolytica cells used in these experiments were not clumped prior to incubation with bPMNs (data not shown). These data are reminiscent of previous histological reports by Yener and colleagues (72), who observed bacterial microcolonies among the streaming leukocytes in the alveoli of M. haemolytica-infected goats.
These in vitro observations led us to seek evidence for DNA release in response to M. haemolytica in vivo. Immunohistochemistry revealed extensive leukocyte infiltration and extracellular DNA deposition in the lungs of an M. haemolytica-infected calf but not a healthy calf (Fig. 8). We observed a significant reduction in Sytox Orange staining (DNA stain) when lung sections were preincubated with DNase I. These data demonstrate the presence of extracellular DNA in the lungs during M. haemolytica infection in vivo. Although at this time we cannot confirm the cells from which the DNA originates, we presume that it is derived from the numerous leukocytes within the alveoli. We speculate that DNA may be released from streaming leukocytes within the lungs of M. haemolytica-infected cattle.
Once NETs form, there must mechanisms for their remodeling and removal. It has been known since the 1950s that human serum contains DNase I (71). Here, we present evidence that bovine serum has DNase activity that is capable of cleaving bovine NETs in vitro (Fig. 9). These data suggest that leakage of plasma proteins into the inflamed tissue could provide DNase I that functions in part to remove NETs formed in response to microbes like M. haemolytica. Although other investigators have used purified DNase I to degrade NETs (12, 15, 20, 31, 35, 43, 52, 64), we are unaware of published evidence for NET degradation by serum DNase in vivo.
The results of this study provide evidence for a novel response of bPMNs to infection with the respiratory pathogen M. haemolytica. At this point, it is difficult to assess whether NET production in response to LKT is beneficial to host defense. We provide evidence that NET production in response to LKT leads to decreased M. haemolytica survival. However, we do not know whether bacteria that survive within NETs are “hidden” from the innate immune system. If the bacterial cells that are trapped in the NETs continue to secrete LKT at that location, it could exacerbate inflammation in the lung. Future examination of the importance of NET formation to the host, and how bacterial cells respond to trap formation, will lead to a better understanding of the pathogenesis of M. haemolytica pneumonia in cattle.
Acknowledgments
This work was supported by the USDA Natural Research Initiative (2004-14841 and 2006-17522) and the Walter and Marth Renk Endowed Laboratory for Food Safety.
We thank Anantharaman Muthuswamy and Erica Behling-Kelly for the paraffin-embedded lung sections (Madison, WI). We also thank Joseph Heintz, Ralph Albrecht, and Randy Massey for their assistance with the scanning and transmission electron microscopy, respectively.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 7 September 2010.
REFERENCES
- 1.Ackermann, M. R., B. M. DeBey, T. J. Stabel, J. H. Gold, K. B. Register, and J. T. Meehan. 1994. Distribution of anti-CD68 (EBM11) immunoreactivity in formalin-fixed, paraffin-embedded bovine tissues. Vet. Pathol. 31:340-348. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, L. E., H. C. Maisey, A. M. Timmer, S. H. Rooijakkers, R. L. Gallo, M. von Kockritz-Blickwede, and V. Nizet. 2010. M1T1 group A streptococcal pili promote epithelial colonization but diminish systemic virulence through neutrophil extracellular entrapment. J. Mol. Med. 88:371-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambagala, T. C., A. P. Ambagala, and S. Srikumaran. 1999. The leukotoxin of Pasteurella haemolytica binds to beta(2) integrins on bovine leukocytes. FEMS Microbol. Lett. 179:161-167. [DOI] [PubMed] [Google Scholar]
- 4.Atapattu, D. N., R. M. Albrecht, D. J. McClenahan, and C. J. Czuprynski. 2008. Dynamin-2-dependent targeting of Mannheimia haemolytica leukotoxin to mitochondrial cyclophilin D in bovine lymphoblastoid cells. Infect. Immun. 76:5357-5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atapattu, D. N., and C. J. Czuprynski. 2007. Mannheimia haemolytica leukotoxin binds to lipid rafts in bovine lymphoblastoid cells and is internalized in a dynamin-2- and clathrin-dependent manner. Infect. Immun. 75:4719-4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atapattu, D. N., and C. J. Czuprynski. 2005. Mannheimia haemolytica leukotoxin induces apoptosis of bovine lymphoblastoid cells (BL-3) via a caspase-9-dependent mitochondrial pathway. Infect. Immun. 73:5504-5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behrendt, J. H., A. Ruiz, H. Zahner, A. Taubert, and C. Hermosilla. 2010. Neutrophil extracellular trap formation as innate immune reactions against the apicomplexan parasite Eimeria bovis. Vet. Immunol. Immunopathol. 133:1-8. [DOI] [PubMed] [Google Scholar]
- 8.Beiter, K., F. Wartha, B. Albiger, S. Normark, A. Zychlinsky, and B. Henriques-Normark. 2006. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr. Biol. 16:401-407. [DOI] [PubMed] [Google Scholar]
- 9.Bianchi, M., A. Hakkim, V. Brinkmann, U. Siler, R. A. Seger, A. Zychlinsky, and J. Reichenbach. 2009. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood 114:2619-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breider, M. A., R. D. Walker, F. M. Hopkins, T. W. Schultz, and T. L. Bowersock. 1988. Pulmonary lesions induced by Pasteurella haemolytica in neutrophil sufficient and neutrophil deficient calves. Can. J. Vet. Res. 52:205-209. [PMC free article] [PubMed] [Google Scholar]
- 11.Brinkmann, V., B. Laube, U. Abu Abed, C. Goosman, and A. Zychlinsky. 2010. Neutrophil extracellular trap: how to generate and visualize them. J. Vis. Exp. 36:pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brinkmann, V., U. Reichard, C. Goosmann, B. Fauler, Y. Uhlemann, D. S. Weiss, Y. Weinrauch, and A. Zychlinsky. 2004. Neutrophil extracellular traps kill bacteria. Science 303:1532-1535. [DOI] [PubMed] [Google Scholar]
- 13.Brinkmann, V., and A. Zychlinsky. 2007. Beneficial suicide: why neutrophils die to make NETs. Nat. Rev. 5:577-582. [DOI] [PubMed] [Google Scholar]
- 14.Brogden, K. A., B. DeBey, F. Audibert, H. Lehmkuhl, and L. Chedid. 1995. Protection of ruminants by Pasteurella haemolytica A1 capsular polysaccharide vaccines containing muramyl dipeptide analogs. Vaccine 13:1677-1684. [DOI] [PubMed] [Google Scholar]
- 15.Buchanan, J. T., A. J. Simpson, R. K. Aziz, G. Y. Liu, S. A. Kristian, M. Kotb, J. Feramisco, and V. Nizet. 2006. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr. Biol. 16:396-400. [DOI] [PubMed] [Google Scholar]
- 16.Caswell, J. L., D. M. Middleton, S. D. Sorden, and J. R. Gordon. 1998. Expression of the neutrophil chemoattractant interleukin-8 in the lesions of bovine pneumonic pasteurellosis. Vet. Pathol. 35:124-131. [DOI] [PubMed] [Google Scholar]
- 17.Caverly, J. M., Z. A. Radi, C. B. Andreasen, R. A. Dixon, K. A. Brogden, and M. R. Ackermann. 2001. Comparison of bronchoalveolar lavage fluid obtained from Mannheimia haemolytica-inoculated calves with and without prior treatment with the selectin inhibitor TBC1269. Am. J. Vet. Res. 62:665-672. [DOI] [PubMed] [Google Scholar]
- 18.Chang, Y. F., R. Young, D. Post, and D. K. Struck. 1987. Identification and characterization of the Pasteurella haemolytica leukotoxin. Infect. Immun. 55:2348-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuammitri, P., J. Ostojic, C. B. Andreasen, S. B. Redmond, S. J. Lamont, and D. Palic. 2009. Chicken heterophil extracellular traps (HETs): novel defense mechanism of chicken heterophils. Vet. Immunol. Immunopathol. 129:126-131. [DOI] [PubMed] [Google Scholar]
- 20.Clark, S. R., A. C. Ma, S. A. Tavener, B. McDonald, Z. Goodarzi, M. M. Kelly, K. D. Patel, S. Chakrabarti, E. McAvoy, G. D. Sinclair, E. M. Keys, E. Allen-Vercoe, R. Devinney, C. J. Doig, F. H. Green, and P. Kubes. 2007. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 13:463-469. [DOI] [PubMed] [Google Scholar]
- 21.Dassanayake, R. P., W. Liu, W. C. Davis, W. J. Foreyt, and S. Srikumaran. 2008. Bighorn sheep beta2-integrin LFA-1 serves as a receptor for Mannheimia haemolytica leukotoxin. J. Wildl. Dis. 44:743-747. [DOI] [PubMed] [Google Scholar]
- 22.Dassanayake, R. P., S. K. Maheswaran, and S. Srikumaran. 2007. Monomeric expression of bovine beta2-integrin subunits reveals their role in Mannheimia haemolytica leukotoxin-induced biological effects. Infect. Immun. 75:5004-5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dassanayake, R. P., S. Shanthalingam, W. C. Davis, and S. Srikumaran. 2007. Mannheimia haemolytica leukotoxin-induced cytolysis of ovine (Ovis aries) leukocytes is mediated by CD18, the beta subunit of beta2-integrins. Microb. Pathog. 42:167-173. [DOI] [PubMed] [Google Scholar]
- 24.Deshpande, M. S., T. C. Ambagala, A. P. Ambagala, M. E. Kehrli, Jr., and S. Srikumaran. 2002. Bovine CD18 is necessary and sufficient to mediate Mannheimia (Pasteurella) haemolytica leukotoxin-induced cytolysis. Infect. Immun. 70:5058-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dileepan, T., M. S. Kannan, B. Walcheck, P. Thumbikat, and S. K. Maheswaran. 2005. Mapping of the binding site for Mannheimia haemolytica leukotoxin within bovine CD18. Infect. Immun. 73:5233-5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dileepan, T., P. Thumbikat, B. Walcheck, M. S. Kannan, and S. K. Maheswaran. 2005. Recombinant expression of bovine LFA-1 and characterization of its role as a receptor for Mannheimia haemolytica leukotoxin. Microb. Pathog. 38:249-257. [DOI] [PubMed] [Google Scholar]
- 27.Dolgushin, I., and S. Andreeva. 2009. Neutrophil extracellular traps: method of detection and assessment of bacterial trapping efficacy. Zh. Mikrobiol. Epidemiol. Immunobiol. 2:65-67. [PubMed] [Google Scholar]
- 28.Ermert, D., A. Zychlinsky, and C. Urban. 2009. Fungal and bacterial killing by neutrophils. Methods Mol. Biol. 470:293-312. [DOI] [PubMed] [Google Scholar]
- 29.Fedorova, N. D., and S. K. Highlander. 1997. Generation of targeted nonpolar gene insertions and operon fusions in Pasteurella haemolytica and creation of a strain that produces and secretes inactive leukotoxin. Infect. Immun. 65:2593-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frey, J., and P. Kuhnert. 2002. RTX toxins in Pasteurellaceae. Int. J. Med. Microbiol. 292:149-158. [DOI] [PubMed] [Google Scholar]
- 31.Fuchs, T. A., U. Abed, C. Goosmann, R. Hurwitz, I. Schulze, V. Wahn, Y. Weinrauch, V. Brinkmann, and A. Zychlinsky. 2007. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176:231-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gentry, M. J., and S. Srikumaran. 1991. Neutralizing monoclonal antibodies to Pasteurella haemolytica leukotoxin affinity-purify the toxin from crude culture supernatants. Microb. Pathog. 10:411-417. [DOI] [PubMed] [Google Scholar]
- 33.Gopinath, R. S., T. C. Ambagala, M. S. Deshpande, R. O. Donis, and S. Srikumaran. 2005. Mannheimia (Pasteurella) haemolytica leukotoxin binding domain lies within amino acids 1 to 291 of bovine CD18. Infect. Immun. 73:6179-6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grinberg, N., S. Elazar, I. Rosenshine, and N. Y. Shpigel. 2008. Beta-hydroxybutyrate abrogates formation of bovine neutrophil extracellular traps and bactericidal activity against mammary pathogenic Escherichia coli. Infect. Immun. 76:2802-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guimaraes-Costa, A. B., M. T. Nascimento, G. S. Froment, R. P. Soares, F. N. Morgado, F. Conceicao-Silva, and E. M. Saraiva. 2009. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc. Natl. Acad. Sci. U. S. A. 106:6748-6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Highlander, S. K., M. Chidambaram, M. J. Engler, and G. M. Weinstock. 1989. DNA sequence of the Pasteurella haemolytica leukotoxin gene cluster. DNA 8:15-28. [DOI] [PubMed] [Google Scholar]
- 37.Highlander, S. K., M. J. Engler, and G. M. Weinstock. 1990. Secretion and expression of the Pasteurella haemolytica leukotoxin. J. Bacteriol. 172:2343-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katzenback, B. A., and M. Belosevic. 2009. Isolation and functional characterization of neutrophil-like cells, from goldfish (Carassius auratus L.) kidney. Dev. Comp. Immunol. 33:601-611. [DOI] [PubMed] [Google Scholar]
- 39.Krautgartner, W. D., and L. Vitkov. 2008. Visualization of neutrophil extracellular traps in TEM. Micron 39:367-372. [DOI] [PubMed] [Google Scholar]
- 40.Lawrence, P. K., R. P. Dassanayake, D. P. Knowles, and S. Srikumaran. 2007. Transfection of non-susceptible cells with Ovis aries recombinant lymphocyte function-associated antigen 1 renders susceptibility to Mannheimia haemolytica leukotoxin. Vet. Microbiol. 125:91-99. [DOI] [PubMed] [Google Scholar]
- 41.Lawrence, P. K., W. R. Nelson, W. Liu, D. P. Knowles, W. J. Foreyt, and S. Srikumaran. 2008. Beta(2) integrin Mac-1 is a receptor for Mannheimia haemolytica leukotoxin on bovine and ovine leukocytes. Vet. Immunol. Immunopathol. 122:285-294. [DOI] [PubMed] [Google Scholar]
- 42.Leite, F., C. Malazdrewich, H. S. Yoo, S. K. Maheswaran, and C. J. Czuprynski. 1999. Use of TUNEL staining to detect apoptotic cells in the lungs of cattle experimentally infected with Pasteurella haemolytica. Microb. Pathog. 27:179-185. [DOI] [PubMed] [Google Scholar]
- 43.Lippolis, J. D., T. A. Reinhardt, J. P. Goff, and R. L. Horst. 2006. Neutrophil extracellular trap formation by bovine neutrophils is not inhibited by milk. Vet. Immunol. Immunopathol. 113:248-255. [DOI] [PubMed] [Google Scholar]
- 44.Liu, W., K. A. Brayton, W. C. Davis, K. Mansfield, J. Lagerquist, W. Foreyt, and S. Srikumaran. 2007. Mannheimia (Pasteurella) haemolytica leukotoxin utilizes CD18 as its receptor on bighorn sheep leukocytes. J. Wildl. Dis. 43:75-81. [DOI] [PubMed] [Google Scholar]
- 45.Matsuyama, T., and T. Iida. 2002. Tilapia mast cell lysates enhance neutrophil adhesion to cultured vascular endothelial cells. Fish Shellfish Immunol. 13:243-250. [DOI] [PubMed] [Google Scholar]
- 46.McClenahan, D., K. Hellenbrand, D. Atapattu, N. Aulik, D. Carlton, A. Kapur, and C. Czuprynski. 2008. Effects of lipopolysaccharide and Mannheimia haemolytica leukotoxin on bovine lung microvascular endothelial cells and alveolar epithelial cells. Clin. Vaccine Immunol. 15:338-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moyes, R. B., M. H. Kogut, R. E. Droleskey, and J. R. DeLoach. 1998. Differential expression of adhesion molecules by chicken heterophils activated in vivo with Salmonella enteritidis-immune lymphokines. Vet. Immunol. Immunopathol. 62:83-95. [DOI] [PubMed] [Google Scholar]
- 48.Odaka, C., and T. Mizuochi. 2002. Macrophages are involved in DNA degradation of apoptotic cells in murine thymus after administration of hydrocortisone. Cell Death Differ. 9:104-112. [DOI] [PubMed] [Google Scholar]
- 49.Palic, D., C. B. Andreasen, J. Ostojic, R. M. Tell, and J. A. Roth. 2007. Zebrafish (Danio rerio) whole kidney assays to measure neutrophil extracellular trap release and degranulation of primary granules. J. Immunol. Methods 319:87-97. [DOI] [PubMed] [Google Scholar]
- 50.Palic, D., J. Ostojic, C. B. Andreasen, and J. A. Roth. 2007. Fish cast NETs: neutrophil extracellular traps are released from fish neutrophils. Dev. Comp. Immunol. 31:805-816. [DOI] [PubMed] [Google Scholar]
- 51.Papayannopoulos, V., and A. Zychlinsky. 2009. NETs: a new strategy for using old weapons. Trends Immunol. 30:513-521. [DOI] [PubMed] [Google Scholar]
- 52.Ramos-Kichik, V., R. Mondragon-Flores, M. Mondragon-Castelan, S. Gonzalez-Pozos, S. Muniz-Hernandez, O. Rojas-Espinosa, R. Chacon-Salinas, S. Estrada-Parra, and I. Estrada-Garcia. 2009. Neutrophil extracellular traps are induced by Mycobacterium tuberculosis. Tuberculosis (Edinb.) 89:29-37. [DOI] [PubMed] [Google Scholar]
- 53.Reid, S. D., W. Hong, K. E. Dew, D. R. Winn, B. Pang, J. Watt, D. T. Glover, S. K. Hollingshead, and W. E. Swords. 2009. Streptococcus pneumoniae forms surface-attached communities in the middle ear of experimentally infected chinchillas. J. Infect. Dis. 199:786-794. [DOI] [PubMed] [Google Scholar]
- 54.Rosenkranz, A. R., A. Coxon, M. Maurer, M. F. Gurish, K. F. Austen, D. S. Friend, S. J. Galli, and T. N. Mayadas. 1998. Impaired mast cell development and innate immunity in Mac-1 (CD11b/CD18, CR3)-deficient mice. J. Immunol. 161:6463-6467. [PubMed] [Google Scholar]
- 55.Shanthalingam, S., and S. Srikumaran. 2009. Intact signal peptide of CD18, the beta-subunit of beta2-integrins, renders ruminants susceptible to Mannheimia haemolytica leukotoxin. Proc. Natl. Acad. Sci. U. S. A. 106:15448-15453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slocombe, R. F., J. Malark, R. Ingersoll, F. J. Derksen, and N. E. Robinson. 1985. Importance of neutrophils in the pathogenesis of acute pneumonic pasteurellosis in calves. Am. J. Vet. Res. 46:2253-2258. [PubMed] [Google Scholar]
- 57.Squier, M. K., A. J. Sehnert, and J. J. Cohen. 1995. Apoptosis in leukocytes. J. Leukoc. Biol. 57:2-10. [DOI] [PubMed] [Google Scholar]
- 58.Strathdee, C. A., and R. Y. Lo. 1989. Cloning, nucleotide sequence, and characterization of genes encoding the secretion function of the Pasteurella haemolytica leukotoxin determinant. J. Bacteriol. 171:916-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sumby, P., K. D. Barbian, D. J. Gardner, A. R. Whitney, D. M. Welty, R. D. Long, J. R. Bailey, M. J. Parnell, N. P. Hoe, G. G. Adams, F. R. Deleo, and J. M. Musser. 2005. Extracellular deoxyribonuclease made by group A Streptococcus assists pathogenesis by enhancing evasion of the innate immune response. Proc. Natl. Acad. Sci. U. S. A. 102:1679-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teixeira, M. M., S. Reynia, M. Robinson, A. Shock, T. J. Williams, F. M. Williams, A. G. Rossi, and P. G. Hellewell. 1994. Role of CD18 in the accumulation of eosinophils and neutrophils and local oedema formation in inflammatory reactions in guinea-pig skin. Br. J. Pharmacol. 111:811-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teixeira, M. M., T. J. Williams, and P. G. Hellewell. 1995. Mechanisms and pharmacological manipulation of eosinophil accumulation in vivo. Trends Pharmacol. Sci. 16:418-423. [DOI] [PubMed] [Google Scholar]
- 62.Thumbikat, P., R. E. Briggs, M. S. Kannan, and S. K. Maheswaran. 2003. Biological effects of two genetically defined leukotoxin mutants of Mannheimia haemolytica. Microb. Pathog. 34:217-226. [DOI] [PubMed] [Google Scholar]
- 63.Thumbikat, P., T. Dileepan, M. S. Kannan, and S. K. Maheswaran. 2005. Characterization of Mannheimia (Pasteurella) haemolytica leukotoxin interaction with bovine alveolar macrophage beta2 integrins. Vet. Res. 36:771-786. [DOI] [PubMed] [Google Scholar]
- 64.Urban, C. F., U. Reichard, V. Brinkmann, and A. Zychlinsky. 2006. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell. Microbiol. 8:668-676. [DOI] [PubMed] [Google Scholar]
- 65.von Kockritz-Blickwede, M., O. A. Chow, and V. Nizet. 2009. Fetal calf serum contains heat-stable nucleases that degrade neutrophil extracellular traps. Blood 114:5245-5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walker, M. J., A. Hollands, M. L. Sanderson-Smith, J. N. Cole, J. K. Kirk, A. Henningham, J. D. McArthur, K. Dinkla, R. K. Aziz, R. G. Kansal, A. J. Simpson, J. T. Buchanan, G. S. Chhatwal, M. Kotb, and V. Nizet. 2007. DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat. Med. 13:981-985. [DOI] [PubMed] [Google Scholar]
- 67.Walker, R. D., F. M. Hopkins, T. W. Schultz, M. D. McCracken, and R. N. Moore. 1985. Changes in leukocyte populations in pulmonary lavage fluids of calves after inhalation of Pasteurella haemolytica. Am. J. Vet. Res. 46:2429-2433. [PubMed] [Google Scholar]
- 68.Wardini, A. B., A. B. Guimaraes-Costa, M. T. Nascimento, N. R. Nadaes, M. G. Danelli, C. Mazur, C. F. Benjamim, E. M. Saraiva, and L. H. Pinto-da-Silva. 2010. Characterization of neutrophil extracellular traps in cats naturally infected with feline leukemia virus. J. Gen. Virol. 91:259-264. [DOI] [PubMed] [Google Scholar]
- 69.Wartha, F., K. Beiter, S. Normark, and B. Henriques-Normark. 2007. Neutrophil extracellular traps: casting the NET over pathogenesis. Curr. Opin. Microbiol. 10:52-56. [DOI] [PubMed] [Google Scholar]
- 70.Welch, R. A. 2001. RTX toxin structure and function: a story of numerous anomalies and few analogies in toxin biology. Curr. Top. Microbiol. Immunol. 257:85-111. [DOI] [PubMed] [Google Scholar]
- 71.Wroblewski, F., and O. Bodansky. 1950. Presence of desoxyribonuclease activity in human serum. Proc. Soc. Exp. Biol. Med. 74:443-445. [DOI] [PubMed] [Google Scholar]
- 72.Yener, Z., F. Ilhan, Z. Ilhan, and Y. S. Saglam. 2009. Immunohistochemical detection of Mannheimia (Pasteurella) haemolytica antigens in goats with natural pneumonia. Vet. Res. Commun. 33:305-313. [DOI] [PubMed] [Google Scholar]
- 73.Yousefi, S., J. A. Gold, N. Andina, J. J. Lee, A. M. Kelly, E. Kozlowski, I. Schmid, A. Straumann, J. Reichenbach, G. J. Gleich, and H. U. Simon. 2008. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 14:949-953. [DOI] [PubMed] [Google Scholar]
- 74.Zecchinon, L., T. Fett, and D. Desmecht. 2005. How Mannheimia haemolytica defeats host defence through a kiss of death mechanism. Vet. Res. 36:133-156. [DOI] [PubMed] [Google Scholar]
- 75.Zen, K., and C. A. Parkos. 2003. Leukocyte-epithelial interactions. Curr. Opin. Cell Biol. 15:557-564. [DOI] [PubMed] [Google Scholar]