Abstract
Anteiso-branched-chain fatty acids (BCFA) represent the dominant group of membrane fatty acids and have been established as crucial determinants in resistance against environmental stresses in Listeria monocytogenes, a facultative intracellular pathogen. Here, we investigate the role of anteiso-BCFA in L. monocytogenes virulence by using mutants deficient in branched-chain alpha-keto acid dehydrogenase (BKD), an enzyme complex involved in the synthesis of BCFA. In tissue culture models of infection, anteiso-BCFA contributed to intracellular growth and survival in macrophages and significantly enhanced plaque formation upon prolonged infection in L2 fibroblasts. The intracellular defects observed could be attributed partially to insufficient listeriolysin O (LLO) production, indicating a requirement for anteiso-BCFA in regulating virulence factor production. In a murine model of infection, the BKD-deficient mutant was highly attenuated, further emphasizing the importance of BKD-mediated metabolism in L. monocytogenes virulence. This study demonstrates an underappreciated role for BCFA in bacterial pathogenesis, which may provide insight into the development and application of antimicrobial agents.
Listeria monocytogenes is a food-borne bacterial pathogen that causes listeriosis, a disease with a high mortality rate and a wide range of clinical manifestations, including gastroenteritis, meningitis, and spontaneous miscarriage. To survive and propagate both inside and outside the host, L. monocytogenes exhibits resistance to many chemical and physical stresses. For example, L. monocytogenes has the ability to grow at refrigeration temperature (8), resulting in the prevalence of food contamination. One mechanism of stress resistance relies on maintaining optimal membrane fluidity by modulating membrane fatty acid composition. In L. monocytogenes, branched-chain fatty acids (BCFA), in particular, anteiso-C-15 and anteiso-C-17, represent the majority of membrane fatty acids and contribute to overall membrane fluidity and resistance against environmental stresses (1, 11, 27). However, it is unclear if BCFA-dependent resistance is necessary for L. monocytogenes to survive after encountering host defense mechanisms.
The branched-chain alpha-keto acid dehydrogenase (BKD) complex catalyzes decarboxylation of alpha-keto acids derived from branched-chain amino acids (BCAA), forming substrates for the biosynthesis of BCFA (Fig. 1 A). L. monocytogenes mutants with transposon insertions in genes encoding two different subunits of BKD exhibited marked BCFA deficiency, as well as severe growth defects at low temperature (1, 27) and at pH 5.0 and pH 9.0 (11). Supplementation with 2-methylbutyric acid (2MB) in growth medium enabled synthesis of anteiso-BCFA, the dominant species of membrane fatty acids in L. monocytogenes (Fig. 1A), and successfully restored growth of mutants deficient in BKD at low temperature (1, 27) and extreme pH conditions (11), demonstrating the necessary contribution of BKD and anteiso-BCFA to resistance against environmental stresses.
FIG. 1.
Disruption of the BKD operon leads to an altered protein lipoylation pattern. (A) Biochemical pathway for the synthesis of anteiso-branched-chain fatty acids (BCFA) in L. monocytogenes. Supplementation with 2-methylbutyric acid (2MB) bypasses the reaction catalyzed by BKD to form anteiso-BCFA, the predominant BCFA in the membrane of L. monocytogenes. CoA, coenzyme A. (B) Immunoblotting of lysates from bacterial cultures grown in BHI broth. Blots were probed with antibodies against lipoic acid (α-LA) or the E2 and E3 subunits of the pyruvate dehydrogenase complex (α-PDH). Prior to lysis, samples were normalized by optical density (600 nm). The arrow indicates a lipoylated protein present in WT bacteria but absent in the cld-2 mutant and the two independent transductants (BKD-1 and BKD-2). Molecular mass markers (in kilodaltons) are given at left.
Although the importance of anteiso-BCFA in resistance to environmental stresses has been recognized, there is limited available information on the role of anteiso-BCFA inside host environments. A Staphylococcus aureus mutant deficient in BCFA exhibited multiple in vitro and in vivo defects, including reduced adhesion to epithelial cells and attenuation in mice, although the BCFA-dependent molecular determinants for these phenotypes have not yet been identified (24). In addition, heterologous expression of a Synechocystis fatty acid desaturase in Salmonella enterica serovar Typhimurium significantly altered the membrane dynamics, resulting in a decrease in intracellular persistence (22). Moreover, disruptions in the membrane fatty acid profile of Pseudomonas aeruginosa caused dysregulation of the quorum-sensing system (4), potentially altering pathogenesis. Therefore, perturbations of membrane fatty acid composition may have a profound influence on an organism's ability to modulate stress resistance and facilitate virulence. A better understanding will provide alternative avenues to develop interventions for infection.
Membranes not only provide a barrier to the external environment but also provide a physical framework for membrane-associated functions, such as cell division, motility, transport, secretion, and signal transduction. The intracellular life cycle of L. monocytogenes depends on several key virulence factors that are anchored on the bacterial surface or secreted across the bacterial membrane and released into the extracellular milieu. The bacterium first enters the host cell through phagocytosis or through bacterial surface internalin-mediated uptake (5). Within host phagosomes or vacuoles, L. monocytogenes secretes across the bacterial membrane listeriolysin O (LLO), a cholesterol-dependent cytolysin that forms pores in the phagosomal membrane to facilitate escape into the host cytosol (23). Other secreted virulence factors include phospholipases, which mediate vacuolar escape, cell-to-cell spread, and possibly nutrient acquisition (10, 14). L. monocytogenes replicates in the host cytosol and acquires actin-based motility mediated by the bacterial surface protein ActA. Propelled by the force of actin polymerization, the bacterium can invade neighboring cells without exiting the protected intracellular niche (19).
Despite the relevance in resistance against environmental stresses, the contribution of anteiso-BCFA to L. monocytogenes virulence remains unclear. Synthesis of anteiso-BCFA occurs primarily through the activity of BKD, which is a lipoylated holoenzyme complex. We previously showed that L. monocytogenes requires lipoate-dependent metabolism for intracellular growth by utilizing host-derived lipoyl peptides (16, 17, 21), a phenotype suggestive of a role for BKD in virulence. However, metabolic disruption does not always guarantee attenuation because of the potential for host metabolites to bypass the disruption (3, 6, 20). Therefore, even though anteiso-BCFA confer resistance in vitro, we cannot assume that deficiency in anteiso-BCFA necessarily leads to virulence defects.
In this study, we aim to elucidate the contribution of anteiso-BCFA during L. monocytogenes intracellular infection by utilizing the previously characterized cld-2 transposon insertion mutant (1, 11, 15, 26, 27) and two independently transduced BKD-deficient mutants in which the cld-2 transposon insertion was transduced into a clean wild-type (WT) background. We show that anteiso-BCFA facilitate intracellular infection of L. monocytogenes by enhancing survival, growth, and phagosomal escape. In the absence of anteiso-BCFA, L. monocytogenes also exhibited striking defects in long-term intracellular replication assays and in vivo fitness. Our findings demonstrate the importance of anteiso-BCFA in L. monocytogenes virulence and establish BKD as a potential target for antimicrobial agents.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
L. monocytogenes strains used in this study include wild-type (WT) strain 10403S; DP-L3903; the Δhly mutant, with a clean deletion of the gene encoding listeriolysin O; the cld-2 mutant, which harbors a Tn917 insertion in the gene encoding the E3 subunit of the BKD complex (25); and two transductants, BKD-1 and BKD-2, which are WT strains independently transduced with the cld-2 transposon allele. Transduction was accomplished by infecting WT bacteria with U153 bacteriophage stock packaged with genomic DNA from the cld-2 mutant and selecting for erythromycin resistance, a marker present on Tn917. Overnight cultures grown in filter-sterilized brain heart infusion (BHI) medium (Difco) with or without supplementation of 5 mM 2-methylbutyric acid (2MB) (Sigma) were used for infection and listeriolysin O (LLO) secretion assays. Mid-exponential-phase cultures grown in BHI medium with or without 2MB were frozen at −80°C as infection stocks and regrown as described previously (2) for mouse infections and escape assays.
Immunoblotting.
Protein samples were analyzed by SDS-PAGE and transferred onto nitrocellulose membranes (Trans-Blot SD Cell; Bio-Rad). The membranes were rinsed with distilled H2O (dH2O) and blocked in TBS-Tw (25 mM Tris-HCl, 150 mM NaCl, 0.05% Tween 20) with 3% nonfat dry milk at room temperature, followed by overnight incubation with anti-LLO antibody (ANT0006; Diatheva), anti-lipoic acid antibody (437695; Calbiochem), or anti-pyruvate dehydrogenase (PDH) subunit E2/E3 antibody (MSP06; MitoSciences) at 4°C. The membranes were then rinsed with dH2O and incubated with horseradish peroxidase-conjugated secondary antibodies, rinsed with dH2O, incubated in 1× TBS-Tw, and rinsed with dH2O again prior to chemiluminescent development (RPN2132; Amersham).
RAW 264.7 macrophage infections.
RAW 264.7 macrophages (5 × 106 cells per plate) were plated onto coverslips (12-mm diameter) in 24-well plates on the night prior to infections. Overnight cultures grown in BHI medium with or without 2MB were used to infect host cells at a multiplicity of infection (MOI) of 5 for 30 min in the presence or absence of 2MB. After infection, host cells were washed 3 times in phosphate-buffered saline (PBS) and incubated in medium containing gentamicin (10 μg/ml) with or without 2MB. The number of intracellular L. monocytogenes CFU was determined by lysing host cells in sterile water and plating the lysate on BHI agar.
Escape assay.
Host cells (6 × 106 cells per plate) were plated onto sterile coverslips (18 by 18 mm) in 6-well plates on the night prior to infections. Frozen infection stocks were thawed and back-diluted into respective fresh media for 90 min to reach mid-exponential phase to be used for infection at an MOI of 5. At 2 h postinfection (hpi), coverslips were fixed in paraformaldehyde (3.7% in PBS) overnight at 4°C. For immunofluorescence microscopy, each coverslip was first washed with TBS-T (25 mM Tris-HCl, 150 mM NaCl, 0.1% Triton X-100) and blocked with TBS-T with 1% bovine serum albumin (BSA). Subsequently, anti-Listeria serum (1:500 in TBS-T with 1% BSA; Difco) was added onto each coverslip for 1 h. Each coverslip was washed in 5 ml of TBS-T prior to incubation with secondary antibodies (rhodamine-phalloidin, 1:400; fluorescein isothiocyanate [FITC]-goat anti-rabbit antibody, 1:400 in TBS-T with 1% BSA). One hundred intracellular bacteria per experimental replicate were scored for the presence or absence of actin clouds.
BMDM isolation.
Primary bone marrow-derived macrophages (BMDM) were isolated from femurs of female C57BL/6 mice. BMDM were differentiated by culturing bone marrow cells in Dulbecco's modified Eagle medium (DMEM) (11960; Gibco) containing 20% heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, 1 mM sodium pyruvate, 55 μM β-mercaptoethanol, and 30% L929 conditioned medium for 6 days; fresh media were added on day 3. Cells were harvested by being washed with cold PBS on day 6 and used for infections on day 7 or were frozen and stored in liquid nitrogen.
LLO secretion assay.
Supernatants of overnight bacterial cultures were normalized with medium based on optical density (600 nm). Proteins in the supernatant fraction were precipitated in 10% trichloroacetic acid (TCA), followed by two cold acetone washes.
Hemolytic assay.
The supernatant samples were reduced with 5 mM dithiothreitol for 1 h at room temperature and serially diluted in hemolysis assay buffer (125 mM NaCl, 35 mM Na2HPO4, pH 5.5) in 96-well plates. PBS-washed sheep red blood cells were added to diluted supernatant samples at approximately 6.2 × 108 cells per well. Plates were incubated at 37°C for 30 min and were centrifuged to pellet intact red blood cells. The supernatants were transferred to clean, optically clear 96-well plates and analyzed for absorbance at 541 nm. Triton X-100 was used as a positive control for complete lysis. As negative controls, uninoculated BHI medium and culture supernatant from the Δhly mutant did not produce any detectable hemolytic activity (data not shown). The absorbance reading was then normalized by culture optical density (600 nm).
L2 plaque assay.
L2 fibroblasts were cultured in 6-well plates to approximately 80 to 90% confluence. After 30 min of infection, 3 ml of agarose overlay (L2 tissue culture medium with 10 μg/ml gentamicin and 0.7% agarose with or without 5 mM 2MB) was added to each well. Plaques were allowed to develop for 4 to 5 days prior to visualization by neutral red staining. Plaque sizes (30 plaques) were determined in Adobe Photoshop and expressed as percentages of the mean diameter of plaques formed by infection with WT bacteria.
Mouse infections.
Frozen infection stocks were thawed and back-diluted into fresh BHI medium to grow at 37°C with shaking for 90 min to reach mid-exponential phase for inoculation. Female C57BL/6 mice (Jackson Laboratory) were injected intraperitoneally with a total of 5 × 105 L. monocytogenes cells per animal. The bacterial load was assessed by determining the number of L. monocytogenes CFU recovered from livers and spleens. Organs were harvested from infected mice 24 and 72 hpi and homogenized in PBS containing 0.1% NP-40.
Statistical analysis.
All statistical analyses were carried out using GraphPad Prism 5 software. Unpaired t tests were used to determine the statistical significance in the differences between samples for the intracellular growth assay and the actin colocalization assay. The Mann-Whitney test was used to determine the statistical significance in the differences between samples for the L2 fibroblast plaque assay and the murine infection experiments.
RESULTS
Disruption in the BKD operon leads to an altered protein lipoylation pattern.
The previously characterized cld-2 mutant harbors a transposon insertion in the lpd gene, which encodes the E3 subunit of the BKD holoenzyme (25, 26). To ensure that the transposon insertion would be the sole cause of any mutant phenotypes observed, two independent transduction experiments were performed to transduce the transposon into a clean WT background. One transductant from each transduction experiment, named BKD-1 and BKD-2, along with the cld-2 mutant was used in this study.
The BKD complex contains a lipoylated E2 subunit, encoded by a separate open reading frame downstream of lpd. Therefore, it is likely that the transposon insertion disrupted expression of the E2 subunit, resulting in loss of a functional BKD complex. In the absence of a specific antibody for the BKD complex in L. monocytogenes, we used an anti-lipoic acid antibody to test for the presence of a lipoylated BKD complex in the mutants. The BKD complex is one of three lipoylated complexes predicted from the sequenced L. monocytogenes genome (12). In addition to BKD, pyruvate dehydrogenase (PDH) and the glycine cleavage system (GCV) each contain a lipoylated subunit, which is required for the activity of these complexes. Therefore, we hypothesized that disruption in the BKD operon by transposon insertion would result in an altered protein lipoylation pattern. Bacterial lysates were prepared and analyzed by SDS-PAGE followed by immunoblotting. The predicted molecular masses for the lipoylated subunits in PDH, BKD, and GCV, based on the primary amino acid sequences, are 58 kDa, 45 kDa, and 14 kDa, respectively. When probed with an anti-lipoic acid antibody (Fig. 1B, top), all strains tested contained a prominent band (∼75 kDa) in the lysate. This band likely represents the lipoylated E2 subunit from the PDH complex, because a band of the same size reacted with an anti-PDH antibody (Fig. 1B, bottom). The E2 subunit of PDH migrates higher than its predicted size of 58 kDa as a result of lipoylation. In addition, a minor band slightly above the 50-kDa mark was detected by the anti-lipoic acid antibody only in the WT lysate but not in the three mutant strains. This band was not detected by the anti-PDH antibody and likely corresponded to the E2 subunit of the BKD complex. Therefore, all three mutant strains, the cld-2 mutant, BKD-1, and BKD-2, exhibited an altered protein lipoylation pattern consistent with the transposon disruption in the BKD operon. Because a lipoylated subunit is required for BKD complex activity, these data suggest that all three mutants have lost BKD-dependent metabolism as a result of the transposon insertion.
Anteiso-BCFA are required for optimal intracellular growth in macrophages.
To determine whether altering membrane anteiso-BCFA content affects L. monocytogenes virulence, we compared intracellular growth of the WT and the BKD-deficient mutants in RAW 264.7 peritoneal macrophages in the presence or absence of 2MB in the tissue culture medium (Fig. 2). In the absence of 2MB, both BKD-deficient mutants exhibited a marked defect in intracellular survival and growth compared to the levels for WT bacteria (Fig. 2A), suggesting that RAW 264.7 macrophages do not harbor metabolites that allow bypass of the defective BKD complex. Moreover, there were equivalent levels of intracellular WT and BKD-deficient mutant bacteria at 1 hpi, an observation suggesting no uptake or invasion defects for L. monocytogenes deficient in anteiso-BCFA in RAW 264.7 macrophages. Notably, supplementation with 2MB completely restored intracellular survival and growth of the BKD-deficient mutants (Fig. 2B), indicating a crucial role for anteiso-BCFA during intracellular infection. The initial loss of CFU suggests that anteiso-BCFA enhance L. monocytogenes resistance against intracellular host defense. A similar decline in intracellular CFU was also observed during infection with the cld-2 mutant in the absence of 2MB (data not shown). These results indicate that anteiso-BCFA are required for L. monocytogenes intracellular survival and growth.
FIG. 2.
Anteiso-BCFA enhance intracellular growth of L. monocytogenes in RAW 264.7 macrophages. Stationary-phase bacterial cultures were used to infect RAW 264.7 macrophages at an MOI of 5. At the times indicated, host cells were lysed to enumerate intracellular CFU. (A) No 2MB was used for the growth of bacterial inoculum or during infection. (B) Supplementation of 2MB was used for the growth of bacterial inoculum and during infection for the synthesis of anteiso-BCFA. Averages of triplicates from a single experiment are plotted, with error bars representing standard deviations. The data shown here are representative of at least 3 independent experiments. Statistically significant differences between the WT and BKD-1 are represented by asterisks (**, P < 0.01; ***, P < 0.001). P values were determined using the unpaired t test in GraphPad Prism 5 software.
BCFA deficit decreases colocalization of L. monocytogenes with cytosolic actin.
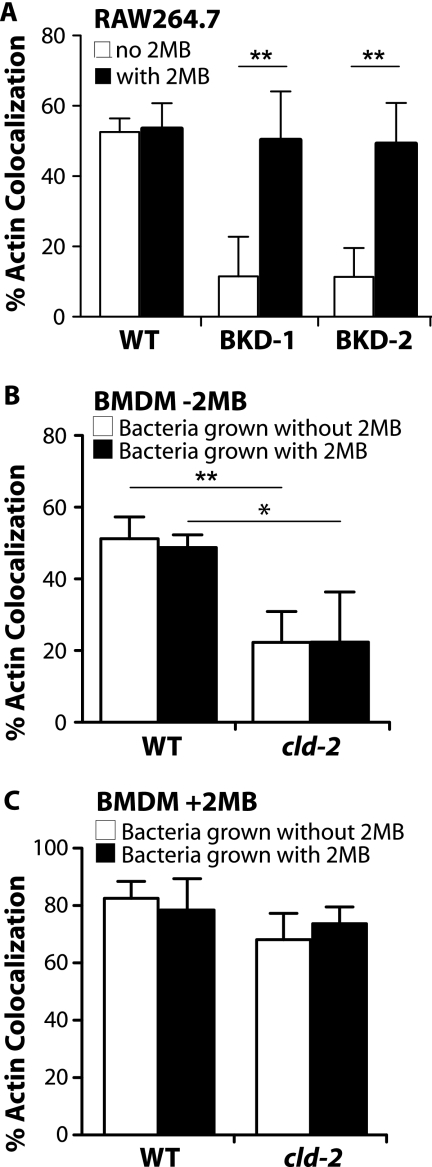
The marked defect in intracellular growth, including the initial loss of CFU, observed with the BKD-deficient mutants led us to hypothesize that anteiso-BCFA may be crucial immediately after L. monocytogenes enters a host cell. Prior to growth in the host cytosol, L. monocytogenes must escape the phagosome, a degradative environment that potentially contributes to early killing of the BKD-deficient mutants. Therefore, we tested whether anteiso-BCFA contribute to L. monocytogenes phagosomal escape by enumerating the proportion of bacteria associated with cytosolic F-actin as an indicator for cytosolic entry in the presence or absence of 2MB using fluorescence microscopy. Mid-exponential-phase cultures of WT or BKD-deficient mutant bacteria were used to infect RAW 264.7 macrophages for 30 min with or without 2MB. In RAW 264.7 macrophages (Fig. 3 A) at 2 hpi, WT bacteria exhibited comparable levels of actin colocalization regardless of 2MB supplementation. However, supplementation with 2MB notably enhanced the proportion of BKD-deficient mutants positive for actin nucleation (Fig. 3A). Similar results were obtained with the cld-2 mutant (data not shown) and with infections in bone marrow-derived macrophages (BMDM) (data not shown), suggesting that anteiso-BCFA are important for actin colocalization.
FIG. 3.
Anteiso-BCFA increase L. monocytogenes colocalization with actin. Exponential-phase bacterial cultures were used to infect RAW 264.7 macrophages (A) and bone marrow-derived macrophages (B and C) for 30 min. To estimate the proportion of cytosolic bacteria, actin colocalization was determined 2 hpi by counting the number of bacteria stained positive with phalloidin out of a total of 100 bacteria stained positive with an anti-Listeria antibody, using immunofluorescence microscopy. (A) “No 2MB” and “with 2MB” refer to supplementation of 5 mM 2MB in BHI medium during growth of bacterial cultures prior to infection and in tissue culture medium during infection. To further determine where 2MB utilization was required, supplementation of 2MB was either omitted (B) or included (C) in the tissue culture medium during infection. Averages from 3 independent experiments are plotted, with error bars representing standard deviations. Statistically significant differences between samples were determined using the unpaired t test in GraphPad Prism 5 software and are represented by asterisks (*, P < 0.05; **, P < 0.01).
To further demonstrate the role of anteiso-BCFA during intracellular infection, we infected BMDM with WT or cld-2 mutant bacteria grown with or without 2MB but did not supplement with 2MB during infection. The cld-2 mutant can synthesize WT levels of anteiso-BCFA only in the presence of 2MB. By supplementing with 2MB only during growth of the bacterial inoculum but not in tissue culture medium, we therefore introduced the cld-2 mutant to BMDM with either wild-type or deficient levels of anteiso-BCFA and eliminated synthesis of anteiso-BCFA from inside BMDM. At 2 hpi, WT bacteria grown with or without 2MB did not exhibit a difference in actin colocalization (Fig. 3B). However, the cld-2 mutant exhibited less actin colocalization than did the WT, regardless of the level of anteiso-BCFA present prior to entry (Fig. 3B). Notably, when 2MB was added to the tissue culture medium during infection, the cld-2 mutant exhibited levels of actin colocalization similar to that of the WT regardless of the presence or absence of 2MB in the bacterial growth medium (Fig. 3C). Together, these results demonstrate that the content of anteiso-BCFA in L. monocytogenes prior to entry is not a key virulence determinant for BMDM infection and reveal an acute requirement for anteiso-BCFA after entry, suggesting active fatty acid synthesis (FAS) early in infection.
Anteiso-BCFA modulate production of listeriolysin O.
Listeriolysin O is a cholesterol-dependent cytolysin necessary to perforate phagosomal membranes for L. monocytogenes to escape into the cytosol. The reduced actin nucleation observed in the absence of anteiso-BCFA could be caused by either defects in LLO production, resulting in a delay in phagosomal escape and subsequent actin nucleation, or defects in ActA production, which would reduce actin nucleation even if mutant bacteria had escaped into the cytosol. Because mutants deficient in ActA do not exhibit a delay in intracellular growth during primary infection (13), we hypothesized that the reduced actin nucleation was indicative of delayed escape caused by insufficient LLO production.
To determine if anteiso-BCFA contribute to LLO production or secretion, we analyzed the abundance and activity of secreted LLO in the supernatant of bacterial cultures grown with or without 2MB. The abundance of LLO in the supernatant was analyzed by SDS-PAGE and immunoblotting using an anti-LLO antibody (Fig. 4 A). In the absence of 2MB, the amount of LLO in the supernatant from both BKD-deficient mutants was greatly reduced compared to that in the supernatant from WT bacteria. Only with prolonged film exposure were we able to detect LLO in supernatant from the BKD-deficient mutants on the blot (data not shown). However, in the presence of 2MB supplementation to restore anteiso-BCFA content, the amount of LLO in the supernatant was notably enhanced in both BKD-deficient mutants. This result demonstrates a requirement for anteiso-BCFA in LLO production.
FIG. 4.
Bacterial LLO production requires anteiso-BCFA. Abundance (A) and activity (B and C) of LLO in the supernatant of overnight bacterial cultures grown in BHI medium with or without 2MB. (A) Bacterial culture supernatant fractions were first normalized by culture optical density. Proteins were extracted from the supernatant by TCA precipitation and analyzed by SDS-PAGE and immunoblotting with an anti-LLO antibody. Recombinant LLO (rLLO) was used as a positive control. Supernatant of the Δhly mutant (Δhly) was used as a negative control. (B and C) Hemolytic assays were performed by mixing sheep red blood cells with serially diluted supernatant samples. Hemolytic activity measured by absorbance at 541 nm was also normalized by culture optical density (OD). Averages of triplicates from a single experiment are plotted, with error bars representing standard deviations. The data shown here are representative of at least 3 independent experiments.
This observation was further validated by testing the activity of LLO secreted using a hemolytic assay with sheep red blood cells. There was little to no activity in the supernatant of the BKD-deficient mutants in the absence of 2MB (Fig. 4B). However, lytic activities in the culture supernatant of the BKD-deficient mutants were restored to the WT level when 2MB was included in the growth medium (Fig. 4C). Similar results were obtained with the cld-2 mutant (data not shown), further supporting a role for anteiso-BCFA in modulating LLO production.
Anteiso-BCFA are necessary for sustained intracellular replication.
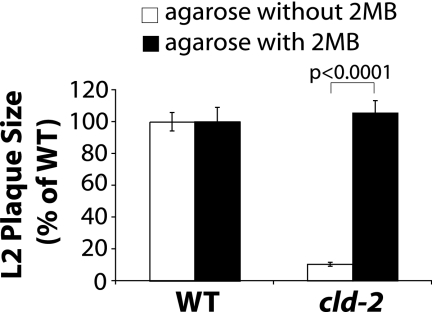
It remained unclear whether BKD-deficient mutant bacteria would be able to sustain multiple rounds of infection if initial defects in survival and growth were to accumulate with each infection event. To address this question, we performed infections in L2 fibroblast monolayers with or without 2MB and measured plaque size as an indicator of prolonged replication and spread to neighboring cells (Fig. 5). Supplementation with 2MB did not affect plaque formation by WT bacteria. However, the cld-2 mutant and both BKD-deficient mutants (data not shown) exhibited a severe defect in plaque formation after several days of infection. This defect was completely rescued by 2MB supplementation during infection. These results indicate that anteiso-BCFA are critical to sustain prolonged intracellular replication in a cell culture model of infection.
FIG. 5.
Anteiso-BCFA facilitate intracellular infection of L2 fibroblasts. Stationary-phase bacterial cultures were used to infect monolayers of L2 fibroblasts. After 30 min of infection, infected monolayers were overlaid with medium containing agarose and gentamicin, with or without 5 mM 2MB. Plaques, indicative of the spread of infection, were visualized by neutral red staining of the monolayer after 2 to 5 days of infection. Averages from 30 plaques are plotted as percentages of the average WT plaque size, with error bars representing standard deviations, for each infection. The data shown here are representative of at least 3 independent experiments. Statistically significant differences between cld-2 mutant plaque sizes from fibroblasts infected with or without 2MB supplementation were determined using the Mann-Whitney test in GraphPad Prism 5 software.
BKD enables full virulence in a murine model of listeriosis.
The requirement for anteiso-BCFA during infection in cultured cells predicts that the BKD-deficient mutant would have a significant in vivo defect. However, unlike in tissue culture systems, L. monocytogenes traverses through the animal host, encountering diverse environments, which might provide metabolites to bypass the requirement for BKD in vivo. We therefore analyzed the fitness of the BKD-deficient mutant in a murine infection model. Female C57BL/6 mice were infected intraperitoneally with 5 × 105 CFU WT or BKD-1 L. monocytogenes. After 24 or 72 hpi, mice infected with the BKD-1 mutant had a significantly lower bacterial burden in spleen (Fig. 6 A) and liver (Fig. 6B) than mice infected with WT bacteria. Similar results were obtained with the cld-2 mutant (data not shown). Together, these results suggest that the host environment does not provide suitable metabolites to bypass BKD deficiency in L. monocytogenes and demonstrate a clear requirement for BKD during in vivo infection.
FIG. 6.
BKD is required for virulence in vivo. Exponential-phase cultures of WT or BKD-1 mutant bacteria (5 × 105 CFU per mouse) were used to infect female C57BL/6 mice intraperitoneally. Spleens and livers were harvested and homogenized 24 and 72 hpi. Bars represent the geometric means of recovered bacterial CFU in each injection group. Dashed lines represent the limit of detection. Statistically significant differences between samples were determined using the Mann-Whitney test in GraphPad Prism 5 software and are represented by asterisks (*, P < 0.05; **, P < 0.01).
DISCUSSION
In this study, we tested the contribution of BKD-dependent metabolism to L. monocytogenes intracellular growth and virulence. Our data from metabolite supplementation experiments indicate that the role of BKD in pathogenesis is attributable primarily to synthesis of anteiso-BCFA. We found that at the cellular level, anteiso-BCFA enhanced L. monocytogenes intracellular survival, growth, and phagosomal escape potentially by promoting LLO production. Furthermore, our results in the murine model of listeriosis demonstrate a clear requirement of BKD for in vivo infection.
Anteiso-BCFA have been associated with resistance against environmental stresses in L. monocytogenes (1, 11, 27). Early in RAW 264.7 macrophage infections, we observed that deficiencies in anteiso-BCFA resulted in a marked loss of intracellular CFU (Fig. 2A), suggesting that the mechanism of anteiso-BCFA in resisting environmental stresses may also play a role in resisting host degradation. Furthermore, we found that pregrowing the cld-2 mutant inoculum with 2MB to restore anteiso-BCFA prior to entry into host cells was not sufficient to rescue the actin colocalization defect unless 2MB was available during infection (Fig. 3B), suggesting an acute requirement for anteiso-BCFA and active fatty acid synthesis during phagosomal escape. In the absence of replication, active fatty acid synthesis inside phagosomes is likely a necessary response to membrane perturbations caused by phagosomal antimicrobial factors. Based on these observations, we predict that L. monocytogenes deficient in anteiso-BCFA would be trapped and be exposed to degradation inside phagosomes while L. monocytogenes sufficient in anteiso-BCFA would be resistant to phagosomal degradation. The identity of the phagosomal antimicrobial factor or factors responsible for killing L. monocytogenes deficient in anteiso-BCFA remains to be determined. The molecular mechanism of anteiso-BCFA contribution to L. monocytogenes resistance against phagosomal degradation also requires more in-depth investigation. Nevertheless, maintenance of appropriate membrane fatty acid composition, a general feature in the bacterial response to environmental fluctuations, can potentially be exploited to significantly weaken bacterial pathogens during infection.
The requirement for anteiso-BCFA in LLO production leads to the question of how bacterial membrane composition may modulate virulence. PrfA, the master regulator of virulence factor transcription in L. monocytogenes, regulates transcription of LLO in response to growth phase, pH, and carbon source (9). Loss of membrane fluidity in the absence of anteiso-BCFA may alter bacterial physiology and consequently influence the activity of PrfA, resulting in decreased LLO production. The detailed molecular mechanism of how membrane fatty acid composition affects LLO production remains to be determined. However, if L. monocytogenes encounters membrane damage inside phagosomes, it is possible that LLO production can be regulated in response to membrane perturbation in a manner dependent on anteiso-BCFA. It is unclear how altering membrane fatty acid composition affects bacterial signal transduction or secretion systems, which all contain membrane-associated components. As production of virulence factors is a process shared by many pathogens, determining how physical properties of the bacterial membrane affect virulence factor regulation may illuminate new avenues for intervention in infectious disease.
Inhibition of fatty acid synthesis (FAS) is an attractive antibacterial strategy because the bacterial FAS pathway is notably different from the mammalian FAS pathway. However, bacteria have adapted diverse mechanisms to decrease susceptibility to FAS inhibitors (13). Moreover, the efficacy of FAS inhibitors in vivo has been controversial because of the possibility for targeted pathogens to scavenge available fatty acids from serum to overcome FAS inhibition (6). For example, even though FAS inhibitors became inactive against Streptococcus agalactiae in serum, they remained potent against Staphylococcus aureus in the presence of human serum (3). These results indicate that the ability to utilize available fatty acids in the host environment varies by organism, resulting in differential susceptibility to FAS inhibitors in vivo. Therefore, it may be fruitful to consider targeting synthesis of specific fatty acids, such as anteiso-BCFA, thereby changing the overall fatty acid profile.
Disruption in membrane fatty acid content without complete inhibition of fatty acid synthesis is not lethal to target microorganisms, thus relaxing selective pressure and minimizing the appearance of resistant strains. For example, inhibition of unsaturated fatty acid production in the fungal pathogen Candida albicans genetically or chemically by targeting fatty acid desaturase was effective at blocking hyphal development (25), a key determinant of virulence (7). An effective inhibitor of the BKD complex in L. monocytogenes would increase sensitivity of the pathogen to environmental stresses and compromise in vivo fitness. Other pathogens, such as S. aureus (24) and Legionella pneumophila (18), also contain high percentages of BCFA in the membrane, broadening the possible application of a BKD inhibitor as an antimicrobial agent to increase the susceptibility of target pathogens to immune defenses and other antibiotic treatments.
Acknowledgments
We gratefully acknowledge B. J. Wilkinson for providing the cld-2 mutant strain and for generously sharing ideas and insights. We thank C. Alteri for critical review of the manuscript, and we thank members of the O'Riordan laboratory for helpful discussions.
This work was funded by a National Institutes of Health award to M. O'Riordan (R01AI064540).
Y. Sun is a trainee in the University of Michigan Molecular Mechanisms of Pathogenesis training program (T32AI007528).
Editor: S. M. Payne
Footnotes
Published ahead of print on 7 September 2010.
REFERENCES
- 1.Annous, B. A., L. A. Becker, D. O. Bayles, D. P. Labeda, and B. J. Wilkinson. 1997. Critical role of anteiso-C-15:0 fatty acid in the growth of Listeria monocytogenes at low temperatures. Appl. Environ. Microbiol. 63:3887-3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auerbuch, V., L. L. Lenz, and D. A. Portnoy. 2001. Development of a competitive index assay to evaluate the virulence of Listeria monocytogenes actA mutants during primary and secondary infection of mice. Infect. Immun. 69:5953-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balemans, W., N. Lounis, R. Gilissen, J. Guillemont, K. Simmen, K. Andries, and A. Koul. 2010. Essentiality of FASII pathway for Staphylococcus aureus. Nature 463:E3-E4. [DOI] [PubMed] [Google Scholar]
- 4.Baysse, C., M. Cullinane, V. Denervaud, E. Burrowes, J. M. Dow, J. P. Morrissey, L. Tam, J. T. Trevors, and F. O'Gara. 2005. Modulation of quorum sensing in Pseudomonas aeruginosa through alteration of membrane properties. Microbiology 151:2529-2542. [DOI] [PubMed] [Google Scholar]
- 5.Bonazzi, M., M. Lecuit, and P. Cossart. 2009. Listeria monocytogenes internalin and E-cadherin: from structure to pathogenesis. Cell. Microbiol. 11:693-702. [DOI] [PubMed] [Google Scholar]
- 6.Brinster, S., G. Lamberet, B. Staels, P. Trieu-Cuot, A. Gruss, and C. Poyart. 2009. Type II fatty acid synthesis is not a suitable antibiotic target for Gram-positive pathogens. Nature 458:83-86. [DOI] [PubMed] [Google Scholar]
- 7.Calderone, R. A., and W. A. Fonzi. 2001. Virulence factors of Candida albicans. Trends Microbiol. 9:327-335. [DOI] [PubMed] [Google Scholar]
- 8.Chan, Y. C., and M. Wiedmann. 2009. Physiology and genetics of Listeria monocytogenes survival and growth at cold temperatures. Crit. Rev. Food Sci. Nutr. 49:237-253. [DOI] [PubMed] [Google Scholar]
- 9.Freitag, N. E. 2006. From hot dogs to host cells: how the bacterial pathogen Listeria monocytogenes regulates virulence gene expression. Future Microbiol. 1:89-101. [DOI] [PubMed] [Google Scholar]
- 10.Freitag, N. E., G. C. Port, and M. D. Miner. 2009. Listeria monocytogenes—from saprophyte to intracellular pathogen. Nat. Rev. Microbiol. 7:623-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giotis, E. S., D. A. McDowell, I. S. Blair, and B. J. Wilkinson. 2007. Role of branched-chain fatty acids in pH stress tolerance in Listeria monocytogenes. Appl. Environ. Microbiol. 73:997-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glaser, P., L. Frangeul, C. Buchrieser, C. Rusniok, A. Amend, F. Baquero, P. Berche, H. Bloecker, P. Brandt, T. Chakraborty, A. Charbit, F. Chetouani, E. Couve, A. de Daruvar, P. Dehoux, E. Domann, G. Dominguez-Bernal, E. Duchaud, L. Durant, O. Dussurget, K. D. Entian, H. Fsihi, F. Garcia-Del Portillo, P. Garrido, L. Gautier, W. Goebel, N. Gomez-Lopez, T. Hain, J. Hauf, D. Jackson, L. M. Jones, U. Kaerst, J. Kreft, M. Kuhn, F. Kunst, G. Kurapkat, E. Madueno, A. Maitournam, J. M. Vicente, E. Ng, H. Nedjari, G. Nordsiek, S. Novella, B. de Pablos, J. C. Perez-Diaz, R. Purcell, B. Remmel, M. Rose, T. Schlueter, N. Simoes, A. Tierrez, J. A. Vazquez-Boland, H. Voss, J. Wehland, and P. Cossart. 2001. Comparative genomics of Listeria species. Science 294:849-852. [DOI] [PubMed] [Google Scholar]
- 13.Gouin, E., P. Dehoux, J. Mengaud, C. Kocks, and P. Cossart. 1995. iactA of Listeria ivanovii, although distantly related to Listeria monocytogenes actA, restores actin tail formation in an L. monocytogenes actA mutant. Infect. Immun. 63:2729-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph, B., and W. Goebel. 2007. Life of Listeria monocytogenes in the host cells' cytosol. Microbes Infect. 9:1188-1195. [DOI] [PubMed] [Google Scholar]
- 15.Julotok, M., A. K. Singh, C. Gatto, and B. J. Wilkinson. 2010. Influence of fatty acid precursors, including food preservatives, on the growth and fatty acid composition of Listeria monocytogenes at 37 and 10 degrees C. Appl. Environ. Microbiol. 76:1423-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keeney, K., L. Colosi, W. Weber, and M. O'Riordan. 2009. Generation of branched-chain fatty acids through lipoate-dependent metabolism facilitates intracellular growth of Listeria monocytogenes. J. Bacteriol. 191:2187-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keeney, K. M., J. A. Stuckey, and M. X. D. O'Riordan. 2007. LpIA1-dependent utilization of host lipoyl peptides enables Listeria cytosolic growth and virulence. Mol. Microbiol. 66:758-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambert, M. A., and C. W. Moss. 1984. Cellular fatty-acid compositions of Moraxella anatipestifer and Legionella pneumophila. Int. J. Syst. Bacteriol. 34:490-491. [Google Scholar]
- 19.Lambrechts, A., K. Gevaert, P. Cossart, J. Vandekerckhove, and M. Van Troys. 2008. Listeria comet tails: the actin-based motility machinery at work. Trends Cell Biol. 18:220-227. [DOI] [PubMed] [Google Scholar]
- 20.Marquis, H., H. G. A. Bouwer, D. J. Hinrichs, and D. A. Portnoy. 1993. Intracytoplasmic growth and virulence of Listeria monocytogenes autotrophic mutants. Infect. Immun. 61:3756-3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Riordan, M., M. A. Moors, and D. A. Portnoy. 2003. Listeria intracellular growth and virulence require host-derived lipoic acid. Science 302:462-464. [DOI] [PubMed] [Google Scholar]
- 22.Porta, A., Z. Torok, I. Horvath, S. Franceschelli, L. Vigh, and B. Maresca. 2010. Genetic modification of the Salmonella membrane physical state alters the pattern of heat shock response. J. Bacteriol. 192:1988-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schnupf, P., J. Zhou, A. Varshavsky, and D. A. Portnoy. 2007. Listeriolysin O secreted by Listeria monocytogenes into the host cell cytosol is degraded by the N-end rule pathway. Infect. Immun. 75:5135-5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh, V. K., D. S. Hattangady, E. S. Giotis, A. K. Singh, N. R. Chamberlain, M. K. Stuart, and B. J. Wilkinson. 2008. Insertional inactivation of branched-chain alpha-keto acid dehydrogenase in Staphylococcus aureus leads to decreased branched-chain membrane fatty acid content and increased susceptibility to certain stresses. Appl. Environ. Microbiol. 74:5882-5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu, D. M., S. Sillaots, J. Davison, W. Q. Hu, B. Jiang, S. Kauffman, N. Martel, P. Ocampo, C. Oh, S. Trosok, K. Veillette, H. Wang, M. H. Yang, L. Zhang, J. Becker, C. E. Martin, and T. Roemer. 2009. Chemical genetic profiling and characterization of small-molecule compounds that affect the biosynthesis of unsaturated fatty acids in Candida albicans. J. Biol. Chem. 284:19754-19764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu, K., D. O. Bayles, A. M. Xiong, R. K. Jayaswal, and B. J. Wilkinson. 2005. Precursor and temperature modulation of fatty acid composition and growth of Listeria monocytogenes cold-sensitive mutants with transposon-interrupted branched-chain alpha-keto acid dehydrogenase. Microbiology 151:615-623. [DOI] [PubMed] [Google Scholar]
- 27.Zhu, K., X. Ding, M. Julotok, and B. J. Wilkinson. 2005. Exogenous isoleucine and fatty acid shortening ensure the high content of anteiso-C-15:0 fatty acid required for low-temperature growth of Listeria monocytogenes. 4Appl. Environ. Microbiol. 71:8002-8007. [DOI] [PMC free article] [PubMed] [Google Scholar]