Abstract
Spirochetes belonging to the Borrelia burgdorferi sensu lato complex differ in resistance to complement-mediated killing by human serum. Here, we characterize complement sensitivity of a panel of B. lusitaniae isolates derived from ticks collected in Germany and Portugal as well as one patient-derived isolate, PoHL. All isolates are highly susceptible to complement-mediated lysis in human serum and activate complement predominantly by the alternative pathway, leading to an increased deposition of complement components C3, C6, and the terminal complement complex. Interestingly, serum-sensitive B. lusitaniae isolates were able to bind immune regulator factor H (CFH), and some strains also bound CFH-related protein 1 (CFHR1) and CFHR2. Moreover, CFH bound to the surface of B. lusitaniae was inefficient in mediating C3b conversion. Furthermore, the identification and characterization of a potential CFH-binding protein, OspE, revealed that this molecule possesses a significantly reduced binding capacity for CFH compared to that of CFH-binding OspE paralogs expressed by various serum-resistant Borrelia species. This finding suggests that a reduced binding capability of CFH is associated with an increased serum sensitivity of B. lusitaniae to human complement.
Lyme disease, the most prevalent vector-borne anthropozoonosis in Europe and North America, is caused by spirochetes of the Borrelia burgdorferi sensu lato complex (53). This complex of diverse spirochetes comprises at least 10 species: B. burgdorferi sensu stricto, B. afzelii, B. garinii, B. spielmanii, B. valaisiana, B. lusitaniae, B. japonica, B. turdi, B. sinica, and B. tanukii. Five additional groups, B. andersonii, B. bissettii, B. californiensis, B. carolinensis, and B. bavariensis, await species validation (38). In central Europe, B. burgdorferi sensu stricto, B. afzelii, B. garinii, B. spielmanii, and B. bavariensis are the causative agents of Lyme disease, while the pathogenic potential for B. bissettii, B. valaisiana, and B. lusitaniae remains unclear (9, 12, 49, 55). The isolation of B. lusitaniae from two Portuguese patients with clinical manifestations similar to the pathogenesis of Lyme disease suggests that this spirochete is pathogenic to humans (11-13). Compared to that of other genospecies in central or eastern Europe, the geographic distribution of B. lusitaniae is restricted to areas where lizards are widespread throughout Portugal, Spain, Morocco, and Tunisia or where they are distributed focally in Germany, Poland, France, and Switzerland (3, 37, 45).
Complement as a first line of defense is an essential arm of innate immunity and plays a central part in the recognition and elimination of invading microorganisms (60). This innate defense system is activated via three major pathways, the alternative, classical, and lectin pathways. In particular, the alternative pathway uses diverse recognition molecules that distinguish between foreign and “self” surfaces. Activation of each of these pathways leads to the cleavage of the central component C3 and the generation of its active splice fragments C3a and C3b. Deposition of the covalently bound opsonin C3b to acceptor cells is necessary for clearance of intruding microorganisms by phagocytosis, formation of the C3 convertase, and assembly of both the C5 convertase and the terminal complement complex (TCC). To protect “self” cell surfaces from excessive activation and harmful attack by complement, this system is well-balanced and finely tuned by various fluid-phase and membrane-anchored regulatory molecules (22, 35). The key fluid-phase inhibitors of the alternative pathway are factor H (CFH) and factor H-like protein 1 (FHL1). Both regulators act as cofactors for factor I-mediated inactivation of C3b to iC3b, inhibiting the formation of and accelerating the decay of the C3bBb convertase, and finally compete with factor B for binding to C3b (30, 42, 61, 62). CFH is composed of 20 individually folding protein domains, termed short consensus repeats (SCRs), of which the first four located at the N terminus exhibit the main complement regulatory activity (62). In solution, CFH forms dimers and oligomers and has a folded-back conformation (41). FHL1, a product of an alternatively spliced transcript of the CFH gene, consists of the seven N-terminal SCR domains of CFH and has a unique C-terminal extension of 4 hydrophobic amino acid residues (62). CFH-related protein 1 (CFHR1), which represents an additional member of the CFH protein family, inhibits complement activation by blocking C5 convertase and terminal complex formation (17).
Lyme disease spirochetes differ in their patterns of host specialization in a manner that is consistent with resistance or sensitivity to complement, which in turn allows B. burgdorferi sensu lato to selectively survive and persist in diverse animal hosts (32, 34). B. burgdorferi sensu stricto, B. afzelii, and B. spielmanii are resistant in vitro to the alternative pathway of complement activation in humans and sera derived from laboratory mice, whereas B. garinii is highly sensitive to such complement-mediated lysis (2, 5, 6, 19, 27, 34, 57). The inactivation of complement is associated with the ability of borreliae to bind host-derived fluid-phase complement regulators CFH and FHL1 (2, 29, 39, 54). Serum-resistant B. burgdorferi sensu stricto, B. afzelii, and B. spielmanii isolates express two major groups of molecules, collectively termed complement regulator-acquiring surface proteins (CRASPs), that serve as ligands for CFH and FHL1 or CFH and CFHR1 (16, 19, 28, 29, 59). The CFH/FHL1-binding proteins consist of CspA of B. burgdorferi sensu stricto, B. afzelii, and B. spielmanii and CspZ (15, 20, 24, 59). The CFH/CFHR1-binding proteins include ErpP, ErpC, and ErpA, i.e., members of the OspEF (Erp) protein family, OspE, and the p21 protein (1, 16, 18, 21, 25, 40, 51, 54). Expression of CspA or CspZ correlates with serum resistance in vitro, and heterologous expression of either CspA or CspZ converts a serum-susceptible into a serum-resistant phenotype, thus demonstrating an important role for each of the two molecules in evasion of complement-mediated killing (7, 15, 23, 52).
The unique association of B. lusitaniae with lizards and its somewhat weak pathogenic potential for humans may suggest particular properties of this genospecies toward serum complement. To analyze the serum sensitivity of B. lusitaniae, we examined isolates derived from lizard-feeding or questing ticks in Germany and Portugal and one isolate obtained from a Portuguese patient by measuring their binding capabilities for human complement regulators CFH and CFHR1.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Borrelial strains listed in Table 1 were cultured until mid-exponential phase (5 × 107 cells per ml) at 33°C in Barbour-Stoenner-Kelly (BSKII) medium as described previously (28). Cultures of Escherichia coli JM109 and TOP10 were propagated routinely in 2× YT medium (Becton Dickinson GmbH, Heidelberg, Germany) supplemented with ampicillin (100 μg/ml).
TABLE 1.
Borrelial strains used in this study
| Genospecies | Strain | Origin |
Complement resistancea | NHS/hiNHS ratio at day 10 | |
|---|---|---|---|---|---|
| Biological | Geographical | ||||
| B. lusitaniae | PoHL | Human, skin | Portugal | Sensitive | 3.22 |
| SDA1-N1 | Lizard-feeding tick, Ixodes ricinus | Portugal | Sensitive | 3.67 | |
| MT-M3 | Questing tick, I. ricinus | Portugal | Sensitive | 3.83 | |
| MT-M5 | Questing tick, I. ricinus | Portugal | Sensitive | 3.59 | |
| MT-M7 | Questing tick, I. ricinus | Portugal | Sensitive | 3.34 | |
| MT-M8 | Questing tick, I. ricinus | Portugal | Sensitive | 3.57 | |
| MT-W4 | Questing tick, I. ricinus | Portugal | Sensitive | 3.83 | |
| MT-W16 | Questing tick, I. ricinus | Portugal | Sensitive | 2.90 | |
| MT-W17 | Questing tick, I. ricinus | Portugal | Sensitive | 3.92 | |
| RBU Pm2-N6 | Lizard-feeding tick, I. ricinus | Germany | Sensitive | 3.86 | |
| RBU La5-L3 | Lizard-feeding tick, I. ricinus | Germany | Sensitive | 3.61 | |
| HHS La1-L3 | Lizard-feeding tick, I. ricinus | Germany | Sensitive | 3.21 | |
| BBWS2-W2 | Questing tick, I. ricinus | Germany | Sensitive | 3.81 | |
| ZWU3-N4 | Questing tick, I. ricinus | Germany | Sensitive | 2.74 | |
| ZWS-W1 | Questing tick, I. ricinus | Germany | Sensitive | 3.18 | |
| IP1-N1 | Questing tick, I. ricinus | Germany | Sensitive | 3.34 | |
| B. burgdorferi | B31-e2 | Tick, I. scapularis | United States | Resistant | 0.79 |
| LW2 | Human, skin | Germany | Resistant | 0.88 | |
| B. afzelii | FEM1-D15 | Human, skin | Germany | Resistant | 1.10 |
| B. spielmanii | A14S | Human, skin | Netherlands | Resistant | 1.37 |
| B. garinii | G1 | Human, CSFb | Germany | Sensitive | 2.80 |
Human sera, monoclonal and polyclonal antibodies, and human serum proteins.
Nonimmune human serum (NHS) was tested for the presence of anti-Borrelia IgM and IgG antibodies by use of commercially available enzyme-linked immunosorbent assays (ELISAs) (Enzygnost borreliosis/IgM and Enzygnost Lyme link VlsE/IgG; Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany). Only sera proven to be negative for anti-Borrelia IgM or IgG antibodies were pooled and used as a source of CFH for ligand affinity blotting. Purified CFH, polyclonal goat anti-CFH antiserum, human factor I, and human complement C3b were purchased from Calbiochem, Bad Soden, Germany. The cloning, expression, and purification of CFH, FHL1, and CFHR1 have been described previously (16, 30, 31). Polyclonal rabbit anti-SCR1-4 antiserum and monoclonal antibody (MAb) JHD 7.10 were used for detection of FHL1 and CFHR1, respectively (17, 30). For the detection of CFH, MAb IXF9 was applied (43), and a polyclonal anti-glutathione S-transferase (GST) antibody from GE Healthcare, Freiburg, Germany, was used. Goat anti-human C3 (dilutions of 1/1,000 for immunofluorescence microscopy and 1/2,000 for Western blotting) and anti-human C6 (dilution of 1/50) antibodies were purchased from Calbiochem, and monoclonal anti-human C5b-9 antibody (dilution of 1/10) was from Quidel (San Diego, CA). If not stated otherwise, anti-SCR1-4 was used at a final dilution of 1/1,000; anti-GST was used at a final dilution of 1/2,000; and MAbs IXF9, JHD 7.10, and B22 were used undiluted.
Serum sensitivity testing.
Serum sensitivity of borrelial strains was assessed employing a growth inhibition assay as previously described (6, 27, 36). Briefly, highly motile spirochetes (1.25 × 107) diluted in a final volume of 100 μl in BSKII medium containing 240 μg ml−1 phenol red were incubated with 50% nonimmune human serum (NHS) or 50% heat-inactivated NHS (hiNHS) in microtiter plates for 10 days at 33°C (Costar, Cambridge, MA). Growth of spirochetes was monitored by daily measuring of the indicator color shift of the medium at 562/630 nm using an ELISA reader (PowerWave 200; Bio-Tek Instruments, Winooski, VT). For calculation of the growth curves, Mikrowin version 3.0 software (Mikrotek, Overath, Germany) was used.
Immunofluorescence assay for detection of deposited complement components.
For detection of activated complement components deposited on the borrelial surface, an immunofluorescence assay was performed as previously described (19). In brief, spirochetes (6 × 106) were incubated in 25% NHS or, as a control, in 25% hiNHS for 30 min at 37°C with gentle agitation. Ten microliters of cell suspension was spotted on glass slides, allowed to air dry overnight, and fixed in methanol. After 1 h of incubation at 37°C with polyclonal antibodies directed against the complement component C3 (Calbiochem) or C6 (Calbiochem) or a MAb directed against C5b-9 (Quidel), slides were washed and subsequently incubated with Alexa 488-conjugated antibodies directed against either goat or mouse antibodies (Molecular Probes). After being washed, the slides were mounted with ProLong Gold antifade reagent (Molecular Probes) containing DAPI (4′,6-diamidino-2-phenylindole).
Binding of complement proteins to spirochetes in EDTA-treated human serum.
Borreliae (1 × 109 cells) grown to mid-log phase were washed and resuspended in 750 μl NHS supplemented with 34 mM EDTA (pH 8.0) to avoid complement activation. After 1 h of incubation at room temperature and four washes with PBSA (0.15 M NaCl, 0.03 M phosphate, 0.02% sodium azide, pH 7.2) containing 0.05% Tween 20, proteins bound to the cell surface were eluted with 100 mM glycine-HCl (pH 2.0) for 15 min. Cells were removed by centrifugation at 14,000 × g for 10 min at 4°C, and both the supernatant and the last wash were separated by Laemmli SDS-PAGE under nonreducing conditions and analyzed by Western blotting as previously described (19).
Opsonization of borrelial cells and analysis of covalently bound C3 fragment.
Spirochetes from logarithmic-phase cultures were harvested, washed three times, and resuspended in veronal-buffered saline (VBS). Opsonization was carried out by incubation of borrelial cells (2 × 108) in 10% NHS, 10% NHS-EGTA, or 10% NHS-EDTA for 30 min at 37°C. To differentiate between the classical and alternative pathways of complement activation, NHS had been preincubated for 30 min at 37°C either with 10 mM EGTA, 4 mM MgCl2 in VBS to specifically inactivate the classical pathway or with 10 mM EDTA to abolish activation of both the classical and alternative pathways. Non-covalently bound C3 was removed by washing the spirochetes with phosphate-buffered saline (PBS) containing 500 mM NaCl. Activated and covalently bound C3 was subsequently eluted from the borrelial surface by incubation of the cells in 1 M hydroxylamine, 0.2 M Na2CO3 (pH 11) for 60 min at 37°C. After centrifugation, the supernatants were adjusted to pH 7.0 by addition of 2 M HCl, separated by Laemmli SDS-PAGE under reducing conditions, and analyzed by Western blotting as previously described (19).
Cofactor assays with whole borrelial cells.
Cofactor activity of CFH bound to borrelial cells was analyzed by measuring factor I-mediated conversion of C3b to iC3b, as previously described extensively (16, 19). In brief, 4 × 107 cells immobilized onto microtiter plates were incubated with purified CFH (50 ng) for 60 min at room temperature. After the cells were washed, human C3b (Calbiochem) and human factor I (Calbiochem) were added to the cells and the reaction mixtures were incubated for 60 min at 37°C. The cells were sedimented by centrifugation, and the supernatants were mixed with sample buffer, subjected to SDS-PAGE under reducing conditions, and transferred onto a nitrocellulose membrane. C3b degradation products were visualized by Western blotting using polyclonal goat anti-C3 IgG (Calbiochem) (dilution of 1/2,000) and 3,3′,5,5′-tetramethylbenzidine as the substrate.
Enzyme-linked immunosorbent assay.
Binding of CFH, CFHR1, and FHL1 to recombinant borrelial proteins was analyzed by ELISA as described previously (52). Briefly, purified GST fusion proteins were immobilized onto microtiter plates overnight at 4°C, and unspecific binding sites were blocked with 0.2% gelatin in PBS for 6 h at 4°C. CFH (Calbiochem), CFHR1, or FHL1 (5 μg/ml each) was added to the wells and incubated overnight at 4°C. After the wells were washed with PBS, protein complexes were identified using a polyclonal goat anti-CFH antibody followed by a secondary peroxidase-conjugated anti-goat IgG antibody. The reaction was developed with 1,2-phenylenediamine dihydrochloride (Sigma-Aldrich).
SDS-PAGE, ligand affinity blot analysis, and Western blot analysis.
Whole-cell lysates obtained from each borrelial isolate or from purified recombinant proteins (500 ng per lane) were subjected to 10% Tris-Tricine SDS-PAGE under reducing conditions and transferred to nitrocellulose as previously described (28).
PCR cloning and purification of recombinant proteins.
Various oligonucleotides listed in Table 2 were selected for amplification of the orthologous ospE genes of B. lusitaniae. Amplicons purified were cloned into the pCR2.1 TOPO vector (Invitrogen, Carlsbad, CA) and subsequently sequenced. The ospE-like gene of B. lusitaniae isolate MT-M8 was then subcloned into the pGEX 6P-1 expression vector by PCR using primers OspE 55(+) BamHI and OspE 3nc(−) XhoI, resulting in plasmid pGEX P38MT-M8. The recombinant protein contained an amino-terminal GST tag, with the OspE segment beginning with that protein's first amino acid following the cysteine lipidation site. Expression of the GST-OspE fusion protein in E. coli JM109 and affinity purification on a glutathione-Sepharose column were performed as recommended by the manufacturer (GE Healthcare, Freiburg, Germany). Generation of recombinantly expressed CspA, CspZ, and ErpP proteins has been described elsewhere (15, 24, 25).
TABLE 2.
Oligonucleotides used in this study
| Primer | Sequence (5′ to 3′)a |
|---|---|
| UHB(+) | GTTGGTTAAAATTACATTTGCG |
| E470(−) | CTAGTGATATTGCATATTCAG |
| OspE 34(+) | GCTGTTTTTGCACTCCCGGGTTCTTGTGGAAAGTTT |
| ErpP Hind(−) | CAGCACAAACAATCAAAGCTTTTTTATTCATAATTATTC |
| BsCRASP-3 145(+) BamHI | GCTGTTTTTGCACTGTTTGGATCCTGTGGAAATTTTAC |
| BsCRASP-3 nc(−) | ATTCATAATTATTCTCTTCTCGAGTTTGAATTTCTA |
| OspE 55(+) BamHI | GTTTGTCCTGATAAGTGGATCCAAAACTGATGAAAGC |
| OspE 3nc(−) XhoI | CTTTTTTATTCATAATTATTCTCGAGTATACTTTAAACTTCTA |
| FlaB lusF | CACCAGCATCACTTTCAGGATCTCAAGC |
| FlaB lusR | GAGCTCCTTCTTGTTGAACACC |
| OspE F2 | GGGGGATCATTTAAAACTGGTATG |
| OspE R2 | CTTCTTAAACTCTTCTAATGGTATTGC |
Nucleotides underlined indicate introduced substitutions of the respective residues to generate an appropriate restriction site.
Analysis of B. lusitaniae mRNA levels.
Total RNA was extracted from cultured spirochetes (1 × 109 cells) grown to mid-log phase by using TRIzol reagent and Max bacterial enhancement reagent (Invitrogen, Carlsbad, CA) as described previously (8). Briefly, isolated RNA was resuspended in water and treated with DNase I (Ambion, Austin, TX). Following inactivation of DNase I using DNase inactivation reagent (Ambion), a 1-μg aliquot of each DNA-free RNA preparation was reverse transcribed using a first-strand cDNA synthesis kit (Roche Diagnostic GmbH, Mannheim, Germany) with random hexamers and avian myeloblastosis virus (AMV) reverse transcriptase. As controls, reaction mixtures containing all components except AMV reverse transcriptase were prepared and treated similarly. Templates and primers were annealed for 10 min at room temperature, followed by cDNA synthesis at 42°C for 1 h. Reverse transcriptase was inactivated by incubating the reaction mixtures at 95°C for 5 min, followed by 10 min at 4°C. All cDNAs and appropriate controls were diluted 10-fold before being used as templates for reverse transcriptase PCR (RT-PCR) or quantitative real-time PCR.
RT-PCR was performed using Taq polymerase (Invitrogen) and oligonucleotides FlaB lusF and FlaB lusR or OspE F2 and OspE R2 for amplification of the flaB or ospE genes of B. lusitaniae, respectively (Table 2). All amplicons were 198 bp in size. RT-PCR conditions consisted of a 1-min initial 94°C denaturation, followed by 45 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 30 s. To verify amplicon sizes and purity, all RT-PCR products were separated by agarose gel electrophoresis, and the amplified DNA fragments were visualized by ethidium bromide. In addition, amplicons were sequenced to confirm their identities.
Quantitative real-time PCR was performed using a LightCycler thermal cycler (Roche Applied Science) as recommended by the manufacturer. In brief, each reaction mixture contained cDNA (50 ng), LightCycler FastStart DNA Master SYBR green I (Roche Applied Science), and oligonucleotide primers FlaB lusF and FlaB lusR or OspE F2 and OspE R2. All cDNA samples were analyzed in triplicate. The conditions for quantitative real time PCR consisted of a 2-min initial 94°C denaturation, followed by 45 cycles of 94°C for 5 s, 50°C for 5 s, and 72°C for 30 s. To generate standard curves, serial dilutions of purified amplicons of the flaB and ospE genes, respectively, were included in every assay. To calculate the copy number of the respective transcript present in each cDNA sample and for melting-curve analysis, Light Cycler software version 3.5 (Roche Applied Science) was utilized.
Nucleotide sequence analysis.
The deduced amino acid sequence of the OspE protein of B. lusitaniae MT-M8 was aligned using the DNAstar Lasergene 99 software package. The secondary-structure prediction was obtained using GOR4 (J. Garnier et al., 1996), available at http://www.expasy.org.
Nucleotide sequence accession number.
The OspE-encoding ospE gene sequence reported in this paper has been deposited in the EMBL/GenBank databases under accession no. FN822242.
RESULTS
Serum sensitivity of B. lusitaniae isolates.
To assess the sensitivity of B. lusitaniae to complement-mediated killing, 16 isolates of different biological and geographical origins (Table 1) were grown in the presence of 50% nonimmune human serum (NHS) or in 50% heat-inactivated NHS (hiNHS) for up to 10 days. In the presence of NHS, growth of B. lusitaniae isolates, including the human isolate PoHL, was strongly inhibited, as evidenced by minor changes in absorbance values (NHS/hiNHS ratios of 2.8 to 4.0) (Table 1). When identical experimental conditions were employed, serum-resistant strains B. burgdorferi B31-e2, B. afzelii FEM1-D15, and B. spielmanii A14S showed growth in NHS, as indicated by a significantly lower NHS/hiNHS ratio of <1.4 at day 10. In the presence of hiNHS, the growth of borrelial isolates was unaffected. Taken together, these results indicate that all B. lusitaniae isolates were highly susceptible to human complement and were classified as serum-sensitive strains.
Detection of deposited complement components C3, C6, and TCC on the surface of B. lusitaniae.
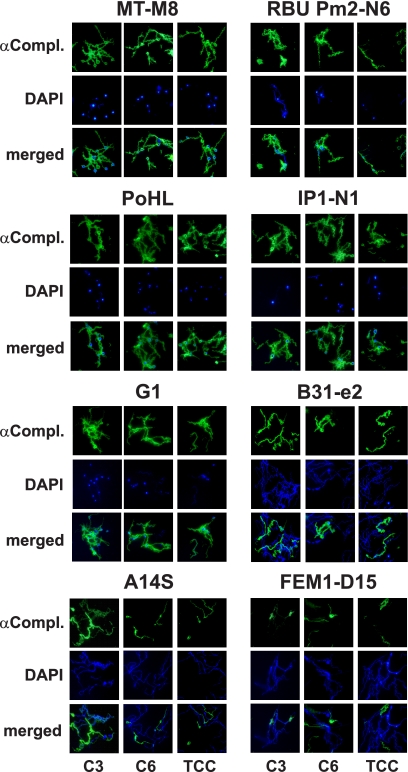
Next, we analyzed deposition of the complement components C3, C6, and TCC on the surface of B. lusitaniae isolates following incubation in NHS by immunofluorescence microscopy. Large amounts of C3, C6, and TCC were deposited on the surface of the serum-sensitive B. lusitaniae isolates, in particular, MT-M8, RBU Pm2-N6, PoHL, and IP1-N1, as well as the serum-sensitive control strain B. garinii G1 (Fig. 1). The remaining B. lusitaniae isolates showed the same prominent labeling (data not shown). In contrast, serum-resistant strains B. burgdorferi B31-e2, B. spielmanii A14S, and B. afzelii FEM1-D15 showed no or marginal fluorescent staining for C3, C6, and TCC. Counterstaining with DAPI was performed to identify all spirochetes in a given field. Cells depositing complement components showed extensive bleb formation and cell fragmentation. Although the cells themselves were DAPI negative, the blebs appeared to accumulate DNA, suggesting that the DAPI-negative cells observed represent “cell ghosts.” When spirochetes were incubated with hiNHS, cell morphology remained intact and fluorescent staining was undetectable (data not shown). Thus, incubation in NHS leads to complement-mediated lysis of B. lusitaniae isolates.
FIG. 1.
Deposition of complement components C3, C6, and TCC on the surface of B. lusitaniae. Complement components deposited on the surfaces of four representative B. lusitaniae isolates (MT-M8, RBU Pm2-N6, PoHL, and IP1-N1), B. garinii G1, B. burgdorferi B31-e2, B. spielmanii A14S, and B. afzelii FEM1-D15 were visualized by indirect immunofluorescence microscopy. Spirochetes were incubated with 25% NHS for 30 min at 37°C with gentle agitation, and bound C3, C6, and TCC were analyzed with specific antibodies against each component (αCompl.) and appropriate Alexa 488-conjugated secondary antibodies. For visualization of the spirochetes in a given microscopic field, the DNA-binding dye DAPI was used. The spirochetes were observed at a magnification of ×100. The data were recorded with a DS-5Mc charge-coupled-device (CCD) camera (Nikon) mounted on an Olympus CX40 fluorescence microscope. Each panel shown is representative of at least 20 microscope fields.
Complement activation and C3 deposition and degradation after opsonization.
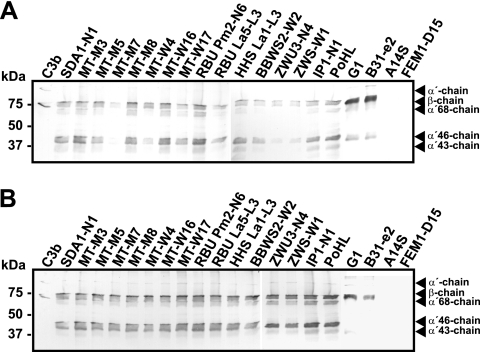
Borreliae can activate complement by either the classical or the alternative pathway (4, 6, 57). To compare the contributions of the alternative and classical pathways to complement activation, each B. lusitaniae isolate was incubated in 10% NHS, 10% EGTA-chelated NHS (for specific inhibition of the classical pathway), or 10% EDTA-chelated NHS (for inhibition of both complement pathways). After incubation, covalently bound C3 was released from the bacterial surface by using hydroxylamine, and C3 degradation products were analyzed by Western blotting. B. lusitaniae isolates showed no significant differences in complement activation and C3b deposition (Fig. 2). All isolates bound the 75-kDa β-chain common to C3b and iC3b, the 68-kDa α′-chain of iC3b, and the 43-kDa α′-chain of C3c after incubation with NHS (Fig. 2A). The presence of the 105-kDa α′-chain indicates deposition of intact C3b on the cell surface. All B. lusitaniae isolates, B. garinii strain G1, and B. burgdorferi strain B31-e2 activated C3, whereas no covalently bound C3 was released from the surface of B. spielmanii A14S or B. afzelii FEM1-D15. In addition, similar C3 fragmentation patterns were obtained from B. lusitaniae isolates, B. garinii G1, and B. burgdorferi B31-e2 following incubation in NHS-EGTA, indicating that these particular Borrelia isolates activate complement predominately via the alternative pathway (Fig. 2B). Opsonization of C3 was undetectable when B. spielmanii A14S and B. afzelii FEM1-D15 were incubated with EGTA-chelated NHS. The restricted complement activation of these particular isolates could be explained either by efficient release of covalently bound C3 due to binding of complement regulators or by inhibition of C3 deposition through the production of a slime layer as described previously (27). As expected, when spirochetes were incubated with EDTA-chelated serum, C3 was not activated (data not shown). Thus, all B. lusitaniae isolates activated complement mainly by the alternative pathway.
FIG. 2.
Complement activation and C3 deposition. B. lusitaniae isolates and control strains B. garinii G1, B. burgdorferi B31-e2, B. spielmanii A14S, and B. afzelii FEM1-D15 were incubated in 10% NHS (A) or 10% EGTA-chelated NHS (B) for 30 min. After opsonization, covalently bound C3 fragments were released from the bacterial surface by using hydroxylamine, and the resulting degradation products were analyzed by Western blotting. The 105-kDa band represents the α′-chain of C3b, and the 75-kDa β-chain is common to all C3 fragments. The degradation fragment of 68 kDa indicates the α′-chain of iC3b (α′68-chain), and the 43-kDa band represents C3c. Purified C3b was used as a control and was identified by the uncleaved 115-kDa α-chain. The mobility of the marker proteins (Precision Plus protein standard) is indicated on the left.
Identification of serum protein binding to B. lusitaniae.
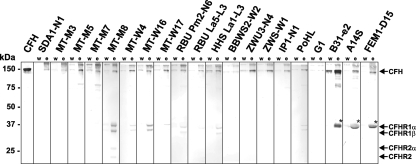
To further examine whether B. lusitaniae can bind to human serum proteins and in particular to members of the CFH family, borrelial cells were incubated in NHS-EDTA to avoid complement activation, followed by extensive washings and elution of bound proteins from the spirochetal surface using 0.1 M glycine-HCl (pH 2.0). The last wash and the eluted fraction were then separated by SDS-PAGE and subjected to Western blotting with a polyclonal anti-CFH antiserum. A faint band of 150 kDa corresponding to CFH was detected in the eluted fractions of all B. lusitaniae isolates (Fig. 3). Isolates SDA1-N1, MT-M8, MT-W4, MT-W16, RBU Pm2-N6, RBU La5-L3, HHS La1-L3, and PoHL in addition bound CFHR1α (43 kDa) and CFHR1β (37 kDa), the two glycosylated forms of CFHR1. Moreover, isolate MT-M8 acquired CFHR2 (24 kDa) and its glycosylated form CFHR2α (29 kDa) on the surface. As expected, serum-sensitive B. garinii isolate G1 did not bind CFH or any other members of the CFH protein family. In contrast, serum-resistant isolates B. burgdorferi B31-e2, B. spielmanii A14S, and B. afzelii FEM1-D15 bound CFH and FHL1 to their surfaces, as previously described (19).
FIG. 3.
Binding of serum proteins by B. lusitaniae isolates. B. lusitaniae isolates as well as control strains B. garinii G1, B. burgdorferi B31-e2, B. spielmanii A14S, and B. afzelii FEM1-D15 incubated in NHS-EDTA were extensively washed with PBSA containing 0.05% Tween 20, and bound proteins were eluted using 0.1 M glycine (pH 2.0). The last wash (w) and the eluate fraction (e) obtained from each isolate were separated using nonreducing 12.5% SDS-PAGE, transferred to nitrocellulose, and probed with a polyclonal anti-CFH antiserum (Calbiochem, Darmstadt, Germany). Purified CFH was used as a positive control. The mobility of the marker proteins (Precision Plus protein standard) is indicated on the left. The band corresponding to FHL1 is indicated by an asterisk.
Cofactor activity of CFH bound to B. lusitaniae.
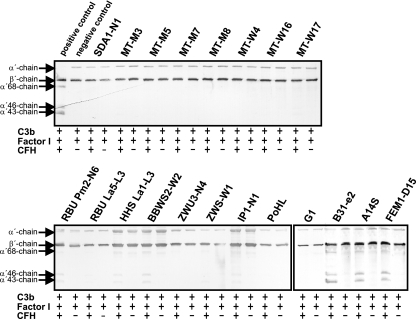
To investigate the conflicting observation that serum-sensitive B. lusitaniae isolates could acquire CFH on their surfaces, a cofactor assay was employed. Intact borrelial cells with CFH attached to the surface were analyzed for the capacity to inactive C3b. To this end, we incubated spirochetes with purified CFH, factor I, and C3b for 60 min and analyzed the C3b inactivation products by Western blotting. CFH, when bound to 13 of the 16 isolates, failed to produce the characteristic C3b cleavage pattern (68-, 46-, and 43-kDa α′-chain) (Fig. 4). When incubated with isolates RBU Pm2-N6, HHS La1-L3, BBWS2-W2, and IP1-N1, however, CFH retained some of its complement regulatory activity, based on the appearance of the 68-, 46-, and 43-kDa bands. When serum-sensitive B. garinii G1 was used, C3b degradation could not be observed. In contrast, CFH bound to serum-resistant B. burgdorferi B31-e2, B. spielmanii A14S, and B. afzelii FEM1-D15 retained its cofactor activity, as indicated by the presence of representative C3b inactivation products. Taken together, these experiments indicate that CFH bound to the majority of the tested B. lusitaniae isolates failed to inactivate C3b.
FIG. 4.
Analysis of the functional activities of CFH bound to B. lusitaniae. Spirochetes immobilized to microtiter plates were used to capture CFH. After sequential addition of C3b and factor I, bound CFH retained cofactor activity by enabling factor I-mediated cleavage of C3b to iC3b. Following incubation, the mixture was separated by SDS-PAGE under reducing conditions and transferred to nitrocellulose, and C3b and its degradation products were analyzed using a C3 antiserum (Calbiochem, Darmstadt, Germany). As a positive control, purified CFH (50 ng) was incubated with C3b and factor I, and as a negative control, complement proteins were incubated in the absence of CFH. The mobilities of the α′-chain and the β-chain of C3b and the cleavage products of the α′-chain (α′-68, α′-46, and α′-43) are indicated. +, incubation with all complement proteins; −, incubation in the absence of CFH.
Identification of CFH-binding proteins in B. lusitaniae.
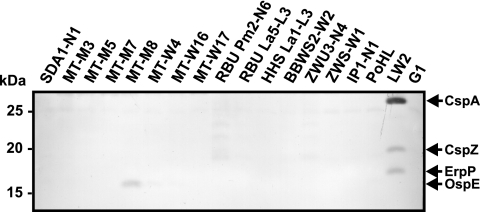
To extend our analysis on the identification of potential CFH-binding proteins produced by diverse borrelia species, ligand affinity blotting (28) was performed with cell lysates obtained from the 16 B. lusitaniae isolates. Following incubation with NHS and a polyclonal anti-CFH antiserum, a CFH-binding protein of approximately 16 kDa was identified solely for B. lusitaniae isolate MT-M8 (Fig. 5). Concerning isolates RBU Pm2-N6 and ZWU3-N4, very weak signals could also be detected. For comparison, cell lysate obtained from serum-resistant B. burgdorferi sensu stricto isolate LW2 showed three CFH-binding CRASP proteins (CspA, ErpP, and ErpA). In agreement with our previous results, CFH-binding proteins were undetectable in the serum-sensitive B. garinii isolate G1 (19, 28, 52). Taken together, these results indicate that only 1 of the 16 B. lusitaniae isolates tested expresses a 16-kDa protein that possesses CFH-binding capability.
FIG. 5.
Identification of CFH-binding proteins of B. lusitaniae isolates. Cell lysates (30 μg each) obtained from diverse B. lusitaniae isolates and control strains B. burgdorferi sensu stricto LW2 and B. garinii G1 were separated by 10% Tris-Tricine SDS-PAGE and transferred to nitrocellulose. The membranes were incubated with NHS as a source for CFH, and binding of the proteins was detected by a polyclonal anti-CFH serum. The identified CRASP proteins, CspA, CspZ, and ErpP of B. burgdorferi LW2 and OspE of B. lusitaniae MT-M8, are indicated on the right, and the mobility of the marker proteins is indicated on the left.
Cloning and characterization of the CFH-binding protein of B. lusitaniae MT-M8.
According to the binding properties and the molecular mass, we speculated that the 16-kDa protein of isolate MT-M8 could represent a member of the polymorphic CFH/CFHR1-binding Erp family. Consequently, a set of oligonucleotide primers suitable to amplify various Erp-encoding genes was employed (25, 51) (Table 2). Sequence analysis of the achieved 766-bp amplicon revealed an open reading frame that exhibited 61.2% sequence identity to the erpA, erpC, and erpP genes of B. burgdorferi sensu stricto B31. This erp-like gene encodes a unique protein consisting of 192 amino acid residues, with a calculated molecular mass of 21.5 kDa. The N terminus of this protein is homologous to the consensus signal peptidase II cleavage sequence Leu(Ala, Ser)−4-Leu(Val, Phe, Ile)−3-Ile(Val, Gly)−2-Ala(Ser, Gly)−1-Cys+1 (14, 50), suggesting that the putative protein is lipidated and located at the outer surface of the spirochetes. To confirm the surface localization of the 16-kDa protein, we treated intact borrelial cells with proteolytic enzymes. Treatment with proteinase K at the lowest concentration (2.5 μg/ml) led to a complete elimination of the 16-kDa band, whereas trypsin treatment had no effect (data not shown). Because the CFH-binding protein of 16 kDa is readily accessible to proteinase K, it appears to be located on the outer surface of B. lusitaniae.
Binding properties of the recombinant CFH-binding protein of B. lusitaniae MT-M8.
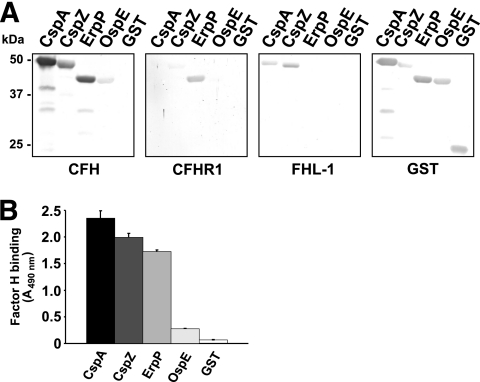
We analyzed the binding activity of the CFH-binding protein of B. lusitaniae MT-M8 to diverse members of the CFH protein family, in particular, CFH, FHL1, and CFHR1. A GST-tagged fusion protein using oligonucleotides provisionally termed OspE 55(+) BamHI and OspE 3nc(−) XhoI was expressed (Table 2). After cloning and overproduction in E. coli, ligand affinity blotting and ELISA were performed to investigate binding of CFH, CFHR1, and FHL1 (Fig. 6). The recombinant OspE derived from B. lusitaniae MT-M8 failed to bind FHL1 and CFHR1 and bound to CFH less efficiently than did CspA, CspZ, and ErpP of B. burgdorferi sensu stricto LW2 (20, 21, 29, 30) (Fig. 6A). Each of the three B. burgdorferi sensu stricto CRASP proteins used as controls bound to CFH and FHL1 (CspA and CspZ) or CFH and CFHR1 (ErpP). As expected, purified GST protein did not bind to any of the three complement regulators. Subsequently, we quantified the binding activity of each recombinant protein to CFH by ELISA (Fig. 6B). Consistent with our ligand affinity blot analyses, binding of CspA, CspZ, and ErpP to CFH was up to 6-fold more efficient than that of OspE of B. lusitaniae MT-M8.
FIG. 6.
Binding capability of OspE of B. lusitaniae MT-M8 to human serum proteins. (A) Binding capabilities of CFH, CFHR1, and FHL1 to purified GST fusion proteins were analyzed by ligand affinity blotting. Recombinant proteins (500 ng/lane) were subjected to 10% Tris-Tricine SDS-PAGE and blotted to nitrocellulose membranes. GST fusion proteins were detected by using anti-goat GST antibody. For detection of CFH and CFHR1 bound to CRASP proteins, membranes were incubated with NHS as a source of CFH or with purified CFHR1. Protein complexes were then visualized using MAb IXF9 or JHD 7.10, respectively. Binding of FHL1 was detected using MAb B22 or polyclonal anti-SCR1-4 antiserum specific for the N-terminal region of CFH and FHL1. The CFH/FHL1-binding CspA and CspZ proteins, the CFH/CFHR1-binding ErpP protein, and purified GST served as controls. (B) Binding of CFH to recombinant CRASP proteins was also quantified by ELISA. Proteins (500 ng each) were immobilized onto a microtiter plate and incubated with purified CFH. For detection of protein complexes, a polyclonal anti-CFH antiserum was used. Reaction mixtures were run in duplicate or triplicate, and all experiments were repeated at least twice, with very similar results; the figure displays a representative experiment. Error bars represent standard deviations (SDs).
OspE expression by B. lusitaniae isolates.
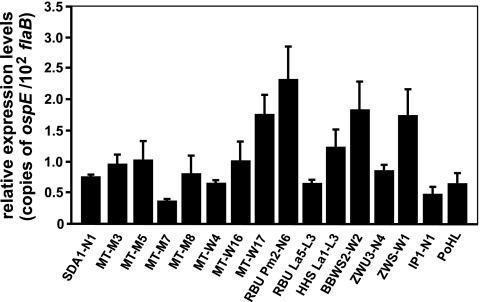
In order to examine expression levels of the ospE gene of B. lusitaniae in vitro, quantitative real-time PCR was performed. Total RNA isolated from each of the B. lusitaniae isolates grown to mid-logarithmic phase was reverse transcribed, and cDNAs were subjected to quantitative real-time PCR to measure the ospE transcript levels. As depicted in Fig. 7, ospE was expressed in all isolates, albeit at different levels. As expected, ospE expression levels were lower than flaB expression levels (Fig. 7). Overall, these results demonstrated that ospE transcripts were present in all B. lusitaniae isolates cultivated in vitro.
FIG. 7.
ospE expression levels among B. lusitaniae isolates. Illustrated are quantitative real-time PCR results from spirochetes grown at 33°C and harvested at mid-logarithmic growth phase. The relative expression levels of the ospE gene are presented as copies of ospE transcript per 102 copies of the constitutively expressed B. lusitaniae flaB gene. Each experiment was performed at least two times in triplicate, and error bars represent standard deviations (SDs).
Taken together, these experiments indicate that spirochetes of the genospecies B. lusitaniae are highly susceptible to human complement-mediated killing. All isolates activate complement mainly via the alternative pathway, resulting in deposition of complement components C3, C6, and TCC on the surface. Surprisingly, B. lusitaniae binds human complement regulators CFH and CFHR1. Based on its reduced activity, we hypothesize that CFH, when attached to the surface of B. lusitaniae, is sterically hindered and unable to retain full complement regulatory activity, thereby conferring a serum-sensitive phenotype.
DISCUSSION
B. burgdorferi sensu stricto, B. afzelii, and B. spielmanii, but not serum-sensitive B. garinii, recruit immune regulators of the alternative pathway, CFH and FHL1, which enable the bacteria to inhibit complement activation directly on their surface. In the present study, we demonstrate that 16 B. lusitaniae isolates originating from various geographical regions and biological sources were highly susceptible to lysis by human complement. Similarly to the serum-sensitive B. garinii isolate, they also deposited large amounts of activated complement components on their surface. Despite their serum sensitivity and in contrast to B. garinii, all B. lusitaniae isolates were able to bind CFH and, to some extent, CFHR1 and CFHR2 to their surface. The complement regulators attached to B. lusitaniae, however, failed to promote C3b degradation, thus permitting bacteriolysis. The CFH-binding OspE ortholog protein of isolate MT-M3 possessed a significantly reduced binding capacity for CFH compared to that of CRASP molecules that were previously characterized for serum-resistant Borrelia strains (15, 24, 25). Serum sensitivity of B. lusitaniae to human complement thus appears to be associated with a diminished binding capability for CFH.
Spirochetes of the B. burgdorferi sensu lato complex oscillate in nature between diverse vertebrate hosts and ixodid tick vectors. The broad spectrum of reservoir hosts contributes differentially to the prevalence of particular genospecies associated with distinct patterns of host specialization (32, 33, 47). A growing body of experimental evidence suggests that the reservoir competence of a host for a particular borrelial species may be independent of extrinsic ecological factors but strongly correlates with the spirochetes’ serum sensitivity pattern to complement of a particular host group (32-34, 47). For example, rodent-associated B. afzelii is resistant to rodent complement but sensitive to avian complement, whereas bird-associated B. valaisiana and most B. garinii isolates react with the opposite serum sensitivity pattern (32-34). B. burgdorferi sensu stricto, on the other hand, is partially resistant to both rodent and avian complements, and this genospecies is adapted to both groups of hosts (44, 48). Concerning the sensitivity pattern to human complement, B. burgdorferi sensu stricto, B. afzelii, and B. spielmanii are resistant, whereas all B. garinii spirochetes, except those belonging to OspA serotype 4, are highly susceptible to complement-mediated killing (2, 4-6, 19, 26, 33, 34, 57). Due to the limited number of isolates that have been tested (4, 33), the resistance/sensitivity pattern of B. lusitaniae to human complement remains largely unknown. The association of B. lusitaniae with lizards as the most important reservoir hosts (13, 46, 58) implicates an adaptation of this genospecies to the hosts’ complement system. All B. lusitaniae isolates analyzed in the present study were readily killed by complement-active human serum, arguing for the inability of this genospecies to infect and survive in the human host. However, B. lusitaniae has been isolated from two Lyme disease patients, suggesting that it may have some pathogenic potential in humans (9-11). Of note, PoHL, a human-derived isolate included in this study, did not show any differences in serum susceptibility pattern or capability to bind complement regulatory proteins compared to those of the other B. lusitaniae isolates. Thus, it is tempting to speculate that this particular B. lusitaniae strain possesses other mechanisms to overcome human innate immune defenses, e.g., by the acquisition of proteins of the tick saliva sharing immunosuppressive or anticomplement activity during spirochete transmission, by the in vivo expression of complement binding proteins, or by an in vivo-formed slime layer. Otherwise, under certain circumstances, B. lusitaniae may establish infections in immune-sufficient or immunocompromised individuals. Studies on the prevalence of B. lusitaniae in patients with Lyme disease who reside in an area where infected lizards are abundant will help to elucidate the potential of this genospecies to cause clinical manifestations of Lyme disease.
Comparative studies on serum sensitivity of borreliae revealed distinct patterns of resistance/sensitivity to human complement for a particular genospecies, i.e., B. afzelii and B. burgdorferi sensu stricto isolates are either resistant or partially resistant, B. spielmanii exemplifies all phenotypes of serum sensitivity (resistant, intermediate, and sensitive), B. garinii OspA serotype 4 strains (more recently delineated as a distinct species and provisionally termed B. bavariensis) are intermediate or resistant, and B. garinii non-OspA serotype 4 strains display a sensitive phenotype (4, 6, 19, 27, 34, 57). As observed for B. garinii non-OspA serotype 4, B. lusitaniae strains also group with the serum-sensitive borreliae. Owing to the limited number of isolates hitherto analyzed, the serum sensitivity pattern of B. valaisiana, known to belong to the most common genospecies in Europe, is as yet inadequately resolved. Because its pathogenic potential for humans has not yet been ascertained, its sensitivity to human serum should be determined with a broad spectrum of isolates. The few B. valaisiana isolates analyzed so far appear to be sensitive to human serum and display a resistance/sensitivity pattern similar to that of B. lusitaniae for mammal sera, as these strains are closely related phylogenetically (34).
The borreliacidal effect of complement results in the three essential indicators of Borrelia mortality, namely, immobilization of viable spirochetes, extensive bleb formation, and finally bacteriolysis of the cells (57). When analyzing activation and deposition of complement by B. lusitaniae, we observed a virtually complete loss of cell vitality after incubation in 50% NHS, generation of blebs, and complete destruction of spirochetal morphology. The serum-resistant isolates B. burgdorferi B31-e2, B. spielmanii A14S, and B. afzelii FEM1-D15 did not succumb to human serum, with only small amounts of complement components, in particular, TCC, deposited on their surface. Consequently, very few spirochetes of each population showed signs of cell destruction. Our observation on the bacteriolysis of B. lusitaniae in human serum is in accord with previous findings indicating that serum-sensitive B. garinii non-OspA serotype 4 strains were strongly affected by complement (27, 57).
B. lusitaniae activates complement mainly via the alternative pathway, similarly to the serum-sensitive B. garinii strains, suggesting a major contribution of this pathway to opsonization of B. lusitaniae with activated C3b (6, 56, 57). However, the involvement of the classical or the lectin pathway in complement activation of this particular genospecies cannot be completely excluded. Activation of both the alternative and the classical pathway in the absence of antibodies has been described earlier for B. burgdorferi sensu stricto and B. afzelii strains (6, 56, 57). Seemingly, among Lyme disease spirochetes, B. afzelii and B. spielmanii resist complement-mediated lysis more efficiently than all other borrelial genospecies. Furthermore, the absence of covalently bound C3b on the cell surface of these particular genospecies and the reduced capacity to activate complement via the classical and alternative pathways might be explained via the production of a slime layer, as previously demonstrated for B. afzelii (27), or by binding of complement regulators (19, 29).
Serum-resistant, but not serum-sensitive, borreliae are able to control the activation of complement by recruiting host-derived fluid-phase complement regulators CFH and FHL1 (2, 29, 39, 54). Interestingly, all B. lusitaniae isolates were able to bind CFH, and some isolates also bound CFHR1 and CFHR2 to their surface, although they are serum sensitive. Evidently, CFH bound to the spirochetal surface failed to protect cells from complement-mediated killing. We therefore conclude that CFH bound inadequately to the surface of the pathogen or that the amount of surface-bound CFH was insufficient to inactivate C3b or to accelerate the decay of formed C3 convertase following activation of the complement cascade. In line with this assumption, C3b inactivation products were undetectable following incubation of B. lusitaniae with purified CFH. Either scenario may explain our observation that B. lusitaniae cells accumulated levels of lethal complement activation products (C3, C6, and TCC) on their surfaces.
Interaction of serum-resistant borreliae with CFH and FHL1 or CFHR1 is mediated by a group of distinct outer surface proteins called CRASPs (1, 16, 18, 19, 28, 39, 54). Hence, binding of CFH, CFHR1, and CFHR2 necessitates an interacting ligand on the spirochete surface. Applying ligand affinity blotting, we identified a potential binding protein of B. lusitaniae isolate MT-M8 that strongly resembles members of the polymorphic OspE protein family. Due to the extended identity between the C-terminal domains of CFH and those of CFHR1 and CFHR2, all CRASPs (i.e., ErpA, ErpC, and ErpP) interacting with this particular C-terminal region of CFH are also able to bind complement regulators CFHR1 and CFHR2 (16). The observation that the binding capacity of the OspE molecule of B. lusitaniae isolate MT-M8 to CFH is far lower than that of CspA, CspZ, and ErpP may explain why we detected no binding of CFHR1 and found no specific C3b cleavage products in the cofactor assay.
In conclusion, we have demonstrated that B. lusitaniae spirochetes are able to bind human complement regulators CFH and CFHR1, but not FHL1, on their surface without gaining resistance to complement-mediated lysis, as do serum-resistant B. burgdorferi sensu stricto, B. afzelii, and B. spielmanii spirochetes. The identification and characterization of the CFH-binding OspE protein of B. lusitaniae revealed that interaction with CFH is insufficient to affect the regulatory activity for factor I-mediated inactivation of C3b.
Acknowledgments
We thank Christa Hanssen-Hübner for skillful and excellent technical assistance and Corinna Siegel for helpful discussions and assisting with the graphic illustrations. We are indebted to Isabel Franca for generously providing B. lusitaniae strain PoHL.
This work was funded by Deutsche Forschungsgemeinschaft grant Kr3383/1-2 to P. Kraiczy.
This work forms part of the Ph.D. thesis of R.D.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 7 September 2010.
REFERENCES
- 1.Alitalo, A., T. Meri, H. Lankinen, I. Seppala, P. Lahdenne, P. S. Hefty, D. Akins, and S. Meri. 2002. Complement inhibitor factor H binding to Lyme disease spirochetes is mediated by inducible expression of multiple plasmid-encoded outer surface protein E paralogs. J. Immunol. 169:3847-3853. [DOI] [PubMed] [Google Scholar]
- 2.Alitalo, A., T. Meri, L. Ramo, T. S. Jokiranta, T. Heikkila, I. J. T. Seppala, J. Oksi, M. Viljanen, and S. Meri. 2001. Complement evasion by Borrelia burgdorferi: serum-resistant strains promote C3b inactivation. Infect. Immun. 69:3685-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amore, G., L. Tomassone, E. Grego, C. Ragagli, L. Bertolotti, P. Nebbia, S. Rosati, and A. Mannelli. 2007. Borrelia lusitaniae in immature Ixodes ricinus (Acari: Ixodidae) feeding on common wall lizards in Tuscany, central Italy. J. Med. Entomol. 44:303-307. [DOI] [PubMed] [Google Scholar]
- 4.Bhide, M. R., M. Travnicek, M. Levkutova, J. Curlik, V. Revajova, and M. Levkut. 2005. Sensitivity of Borrelia genospecies to serum complement from different animals and human: a host-pathogen relationship. FEMS Immunol. Med. Microbiol. 43:165-172. [DOI] [PubMed] [Google Scholar]
- 5.Brade, V., I. Kleber, and G. Acker. 1992. Differences of two Borrelia burgdorferi strains in complement activation and serum resistance. Immunobiology 185:453-465. [DOI] [PubMed] [Google Scholar]
- 6.Breitner-Ruddock, S., R. Würzner, J. Schulze, and V. Brade. 1997. Heterogeneity in the complement-dependent bacteriolysis within the species of Borrelia burgdorferi. Med. Microbiol. Immunol. 185:253-260. [DOI] [PubMed] [Google Scholar]
- 7.Brooks, C. S., S. R. Vuppala, A. M. Jett, A. Alitalo, S. Meri, and D. R. Akins. 2005. Complement regulator-acquiring surface protein 1 imparts resistance to human serum in Borrelia burgdorferi. J. Immunol. 175:3299-3308. [DOI] [PubMed] [Google Scholar]
- 8.Bykowski, T., M. E. Woodman, A. E. Cooley, C. A. Brissette, V. Brade, R. Wallich, P. Kraiczy, and B. Stevenson. 2007. Coordinated expression of Borrelia burgdorferi complement regulator-acquiring surface proteins during the Lyme disease spirochete's mammal-tick infection cycle. Infect. Immun. 75:4227-4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collares-Pereira, M., S. Couceiro, I. Franca, K. Kurtenbach, S. M. Schafer, L. Vitorino, L. Goncalves, S. Baptista, M. L. Vieira, and C. Cunha. 2004. First isolation of Borrelia lusitaniae from a human patient. J. Clin. Microbiol. 42:1316-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.da Franca, I., L. Santos, T. Mesquita, M. Collares-Pereira, S. Baptista, L. Vieira, I. Viana, E. Vale, and C. Prates. 2005. Lyme borreliosis in Portugal caused by Borrelia lusitaniae? Clinical report on the first patient with a positive skin isolate. Wien. Klin. Wochenschr. 117:429-432. [DOI] [PubMed] [Google Scholar]
- 11.de Carvalho, I. L., J. E. Fonseca, J. G. Marques, A. Ullmann, A. Hojgaard, N. Zeidner, and M. S. Nuncio. 2008. Vasculitis-like syndrome associated with Borrelia lusitaniae infection. Clin. Rheumatol. 27:1587-1591. [DOI] [PubMed] [Google Scholar]
- 12.Diza, E., A. Papa, E. Vezyri, S. Tsounis, I. Milonas, and A. Antoniadis. 2004. Borrelia valaisiana in cerebrospinal fluid. Emerg. Infect. Dis. 10:1692-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dsouli, N., H. Younsi-Kabachii, D. Postic, S. Nouira, L. Gern, and A. Bouattour. 2006. Reservoir role of lizard Psammodromus algirus in transmission cycle of Borrelia burgdorferi sensu lato (Spirochaetaceae) in Tunisia. J. Med. Entomol. 43:737-742. [DOI] [PubMed] [Google Scholar]
- 14.Haake, D. A. 2000. Spirochaetal lipoproteins and pathogenesis. Microbiology 146:1491-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartmann, K., C. Corvey, C. Skerka, M. Kirschfink, M. Karas, V. Brade, J. C. Miller, B. Stevenson, R. Wallich, P. F. Zipfel, and P. Kraiczy. 2006. Functional characterization of BbCRASP-2, a distinct outer membrane protein of Borrelia burgdorferi that binds host complement regulators factor H and FHL-1. Mol. Microbiol. 61:1220-1236. [DOI] [PubMed] [Google Scholar]
- 16.Haupt, K., P. Kraiczy, R. Wallich, V. Brade, C. Skerka, and P. Zipfel. 2007. Binding of human factor H-related protein 1 to serum-resistant Borrelia burgdorferi is mediated by borrelial complement regulator-acquiring surface proteins. J. Infect. Dis. 196:124-133. [DOI] [PubMed] [Google Scholar]
- 17.Heinen, S., A. Hartmann, N. Lauer, U. Wiehl, H.-M. Dahse, S. Schirmer, K. Gropp, T. Enghardt, R. Wallich, S. Halbich, M. Mihlan, U. Schlotzer-Schrehardt, P. F. Zipfel, and C. Skerka. 2009. Factor H related protein 1 (CFHR-1) inhibits complement C5 convertase activity and terminal complex formation. Blood 114:2439-2447. [DOI] [PubMed] [Google Scholar]
- 18.Hellwage, J., T. Meri, T. Heikkila, A. Alitalo, J. Panelius, P. Lahdenne, I. J. T. Seppala, and S. Meri. 2001. The complement regulator factor H binds to the surface protein OspE of Borrelia burgdorferi. J. Biol. Chem. 276:8427-8435. [DOI] [PubMed] [Google Scholar]
- 19.Herzberger, P., C. Siegel, C. Skerka, V. Fingerle, U. Schulte-Spechtel, A. van Dam, B. Wilske, V. Brade, P. F. Zipfel, R. Wallich, and P. Kraiczy. 2007. Human pathogenic Borrelia spielmanii sp. nov. resists complement-mediated killing by direct binding of immune regulators factor H and factor H-like protein 1. Infect. Immun. 75:4817-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herzberger, P., C. Siegel, C. Skerka, V. Fingerle, U. Schulte-Spechtel, B. Wilske, V. Brade, P. F. Zipfel, R. Wallich, and P. Kraiczy. 2009. Identification and characterization of the factor H and FHL-1 binding complement regulator-acquiring surface protein 1 of the Lyme disease spirochete Borrelia spielmanii sp. nov. Int. J. Med. Microbiol. 299:141-154. [DOI] [PubMed] [Google Scholar]
- 21.Hovis, K. M., E. Tran, C. M. Sundy, E. Buckles, J. V. McDowell, and R. T. Marconi. 2006. Selective binding of Borrelia burgdorferi OspE paralogs to factor H and serum proteins from diverse animals: possible expansion of the role of OspE in Lyme disease pathogenesis. Infect. Immun. 74:1967-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Józsi, M., and P. F. Zipfel. 2008. Factor H family proteins and human diseases. Trends Immunol. 29:380-387. [DOI] [PubMed] [Google Scholar]
- 23.Kenedy, M. R., S. R. Vuppala, C. Siegel, P. Kraiczy, and D. R. Akins. 2009. CspA-mediated binding of human factor H inhibits complement deposition and confers serum resistance in Borrelia burgdorferi. Infect. Immun. 77:2773-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraiczy, P., J. Hellwage, C. Skerka, H. Becker, M. Kirschfink, M. M. Simon, V. Brade, P. F. Zipfel, and R. Wallich. 2004. Complement resistance of Borrelia burgdorferi correlates with the expression of BbCRASP-1, a novel linear plasmid-encoded surface protein that interacts with human factor H and FHL-1 and is unrelated to Erp proteins. J. Biol. Chem. 279:2421-2429. [DOI] [PubMed] [Google Scholar]
- 25.Kraiczy, P., J. Hellwage, C. Skerka, M. Kirschfink, V. Brade, P. F. Zipfel, and R. Wallich. 2003. Immune evasion of Borrelia burgdorferi: mapping of a complement-inhibitor factor H-binding site of BbCRASP-3, a novel member of the Erp protein family. Eur. J. Immunol. 33:697-707. [DOI] [PubMed] [Google Scholar]
- 26.Kraiczy, P., K.-P. Hunfeld, S. Peters, R. Wurzner, G. Acker, B. Wilske, and V. Brade. 2000. Borreliacidal activity of early Lyme disease sera against complement-resistant Borrelia afzelii FEM1 wild-type and an OspC-lacking FEM1 variant. J. Med. Microbiol. 49:917-928. [DOI] [PubMed] [Google Scholar]
- 27.Kraiczy, P., K. P. Hunfeld, S. Breitner-Ruddock, R. Wurzner, G. Acker, and V. Brade. 2000. Comparison of two laboratory methods for the determination of serum resistance in Borrelia burgdorferi isolates. Immunobiology 201:406-419. [DOI] [PubMed] [Google Scholar]
- 28.Kraiczy, P., C. Skerka, V. Brade, and P. F. Zipfel. 2001. Further characterization of complement regulator-acquiring surface proteins of Borrelia burgdorferi. Infect. Immun. 69:7800-7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraiczy, P., C. Skerka, M. Kirschfink, V. Brade, and P. F. Zipfel. 2001. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and factor H. Eur. J. Immunol. 31:1674-1684. [DOI] [PubMed] [Google Scholar]
- 30.Kühn, S., C. Skerka, and P. F. Zipfel. 1995. Mapping of the complement regulatory domains in the human factor H-like protein 1 and in factor H1. J. Immunol. 155:5663-5670. [PubMed] [Google Scholar]
- 31.Kühn, S., and P. F. Zipfel. 1995. The baculovirus expression vector pBSV-8His directs secretion of histidine-tagged proteins. Gene 162:225-229. [DOI] [PubMed] [Google Scholar]
- 32.Kurtenbach, K., S. De Michelis, S. Etti, S. M. Schafer, H. S. Sewell, V. Brade, and P. Kraiczy. 2002. Host association of Borrelia burgdorferi sensu lato—the key role of host complement. Trends Microbiol. 10:74-79. [DOI] [PubMed] [Google Scholar]
- 33.Kurtenbach, K., M. Peacey, S. G. Rijpkema, A. N. Hoodless, P. A. Nuttall, and S. E. Randolph. 2002. Borrelia burgdorferi s.l. in the vertebrate host, p. 117-148. In S. L. Gray, O. Kahl, R. S. Lane, and G. Stanek (ed.), Lyme borreliosis: biology of the infectious agents and epidemiology of disease. CABI Publishing, Wallingford, United Kingdom.
- 34.Kurtenbach, K., H.-S. Sewell, N. H. Ogden, S. E. Randolph, and P. A. Nuttall. 1998. Serum complement sensitivity as a key factor in Lyme disease ecology. Infect. Immun. 66:1248-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambris, J. D., D. Ricklin, and B. V. Geisbrecht. 2008. Complement evasion by human pathogens. Nat. Rev. Microbiol. 6:132-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma, J., and R. T. Coughlin. 1993. A simple, colorimetric microtiter assay for borreliacidal activity of antisera. J. Microbiol. Methods 17:145-153. [Google Scholar]
- 37.Majlathova, V., I. Majlath, M. Derdakova, B. Vichova, and B. Pet'ko. 2006. Borrelia lusitaniae and green lizards (Lacerta viridis), Karst region, Slovakia. Emerg. Infect. Dis. 12:1895-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Margos, G., S. A. Vollmer, M. Cornet, M. Garnier, V. Fingerle, B. Wilske, A. Bormane, L. Vitorino, M. Collares-Pereira, M. Drancourt, and K. Kurtenbach. 2009. A new Borrelia species defined by multilocus sequence analysis of housekeeping genes. Appl. Environ. Microbiol. 75:5410-5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDowell, J. V., J. Wolfgang, E. Tran, M. S. Metts, D. Hamilton, and R. T. Marconi. 2003. Comprehensive analysis of the factor H binding capabilities of Borrelia species associated with Lyme disease: delineation of two distinct classes of factor H binding proteins. Infect. Immun. 71:3597-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Metts, M. S., J. V. McDowell, M. Theisen, P. R. Hansen, and R. T. Marconi. 2003. Analysis of the OspE determinants involved in binding of factor H and OspE-targeting antibodies elicited during Borrelia burgdorferi infection in mice. Infect. Immun. 71:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okemefuna, A. I., R. Nan, J. Gor, and S. J. Perkins. 2009. Electrostatic interactions contribute to the folded-back conformation of wild type human factor H. J. Mol. Biol. 391:98-118. [DOI] [PubMed] [Google Scholar]
- 42.Pangburn, M. K., R. D. Schreiber, and H. J. Muller-Eberhard. 1977. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J. Exp. Med. 146:257-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prodinger, W. M., J. Hellwage, M. Spruth, M. P. Dierich, and P. F. Zipfel. 1998. The C-terminus of factor H: monoclonal antibodies inhibit heparin binding and identify epitopes common to factor H and factor H-related proteins. Biochem. J. 331:41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richter, D., B. Klug, A. Spielman, and F. R. Matuschka. 2004. Adaptation of diverse Lyme disease spirochetes in a natural rodent reservoir host. Infect. Immun. 72:2442-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richter, D., and F. R. Matuschka. 2006. Perpetuation of the Lyme disease spirochete Borrelia lusitaniae by lizards. Appl. Environ. Microbiol. 72:4627-4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richter, D., D. Postic, N. Sertour, I. Livey, F. R. Matuschka, and G. Baranton. 2006. Delineation of Borrelia burgdorferi sensu lato species by multilocus sequence analysis and confirmation of the delineation of Borrelia spielmanii sp. nov. Int. J. Syst. Evol. Microbiol. 56:873-881. [DOI] [PubMed] [Google Scholar]
- 47.Richter, D., D. B. Schlee, R. Allgower, and F.-R. Matuschka. 2004. Relationships of a novel Lyme disease spirochete, Borrelia spielmani sp. nov., with its hosts in central Europe. Appl. Environ. Microbiol. 70:6414-6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richter, D., A. Spielman, N. Komar, and F. R. Matuschka. 2000. Competence of American robins as reservoir hosts for Lyme disease spirochetes. Emerg. Infect. Dis. 6:133-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rijpkema, S. G., D. J. Tazelaar, M. J. Molkenboer, G. T. Noordhoek, G. Plantinga, L. M. Schouls, and J. F. Schellekens. 1997. Detection of Borrelia afzelii, Borrelia burgdorferi sensu stricto, Borrelia garinii and group VS116 by PCR in skin biopsies of patients with erythema migrans and acrodermatitis chronica atrophicans. Clin. Microbiol. Infect. 3:109-116. [DOI] [PubMed] [Google Scholar]
- 50.Schulze, R. J., and W. R. Zuckert. 2006. Borrelia burgdorferi lipoproteins are secreted to the outer surface by default. Mol. Microbiol. 59:1473-1484. [DOI] [PubMed] [Google Scholar]
- 51.Seling, A., C. Siegel, V. Fingerle, B. L. Jutras, C. A. Brissette, C. Skerka, R. Wallich, P. F. Zipfel, B. Stevenson, and P. Kraiczy. 2009. Functional characterization of Borrelia spielmanii outer surface proteins that interact with distinct members of the human factor H protein family and with plasminogen. Infect. Immun. 78:39-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siegel, C., J. Schreiber, K. Haupt, C. Skerka, V. Brade, M. M. Simon, B. Stevenson, R. Wallich, P. F. Zipfel, and P. Kraiczy. 2008. Deciphering the ligand-binding sites in the Borrelia burgdorferi complement regulator-acquiring surface protein 2 required for interactions with the human immune regulators factor H and factor H-like protein 1. J. Biol. Chem. 283:34855-34863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steere, A. C., J. Coburn, and L. Glickstein. 2004. The emergence of Lyme disease. J. Clin. Invest. 113:1093-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stevenson, B. 2002. Borrelia burgdorferi erp (ospE-related) gene sequences remain stable during mammalian infection. Infect. Immun. 70:5307-5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strle, F., R. N. Picken, Y. Cheng, J. Cimperman, V. Maraspin, S. Lotric-Furlan, E. Ruzic-Sabljic, and M. M. Picken. 1997. Clinical findings for patients with Lyme borreliosis caused by Borrelia burgdorferi sensu lato with genotypic and phenotypic similarities to strain 25015. Clin. Infect. Dis. 25:273-280. [DOI] [PubMed] [Google Scholar]
- 56.Suhonen, J., K. Hartiala, H. Tuominen-Gustafsson, and M. K. Viljanen. 2002. Sublethal concentrations of complement can effectively opsonize Borrelia burgdorferi. Scand. J. Immunol. 56:554-560. [DOI] [PubMed] [Google Scholar]
- 57.van Dam, A. P., A. Oei, R. Jaspars, C. Fijen, B. Wilske, L. Spanjaard, and J. Dankert. 1997. Complement-mediated serum sensitivity among spirochetes that cause Lyme disease. Infect. Immun. 65:1228-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vitorino, L. R., G. Margos, E. J. Feil, M. Collares-Pereira, L. Ze-Ze, and K. Kurtenbach. 2008. Fine-scale phylogeographic structure of Borrelia lusitaniae revealed by multilocus sequence typing. PLoS One 3:e4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wallich, R., J. Pattathu, V. Kitiratschky, C. Brenner, P. F. Zipfel, V. Brade, M. M. Simon, and P. Kraiczy. 2005. Identification and functional characterization of complement regulator-acquiring surface protein 1 of the Lyme disease spirochetes Borrelia afzelii and Borrelia garinii. Infect. Immun. 73:2351-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walport, M. J. 2001. Complement—second of two parts. N. Engl. J. Med. 344:1140-1144. [DOI] [PubMed] [Google Scholar]
- 61.Whaley, K., and S. Ruddy. 1976. Modulation of the alternative complement pathways by beta 1 H globulin. J. Exp. Med. 144:1147-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zipfel, P. F., and C. Skerka. 2009. Complement regulators and inhibitory proteins. Nat. Rev. Immunol. 9:729-740. [DOI] [PubMed] [Google Scholar]