Abstract
Salmonella enterica is a versatile vaccine carrier for heterologous antigens. One strategy for vaccine antigen delivery is the use of live attenuated S. enterica strains that translocate heterologous antigens into antigen-presenting cells by means of type III secretion systems (T3SS). The feasibility of this approach has been demonstrated in various experimental vaccination studies. The efficacy of recombinant live vaccines is critically influenced by the optimal level of attenuation and many other factors. For the rational design of approaches involving translocation by T3SS, additional parameters are the level of expression of the heterologous antigens and the selection of carrier proteins for the delivery of antigens to desirable subcellular compartments of the target cell. We deployed the Salmonella pathogenicity island 2 (SPI2)-encoded T3SS for antigen delivery. The SPI2-T3SS and effector proteins are encoded by members of the large SsrAB regulon, including promoters with highly variable strength of expression. We investigated the effect of various in vivo-activated promoters of the SsrAB regulon on the efficacy of recombinant Salmonella vaccines. We observed that the use of promoters with higher strength results in greater synthesis of recombinant antigens and greater stimulation of T-cell responses in cell culture assays for the stimulation of T cells by the model antigen ovalbumin. In contrast, in vaccination experiments, promoters with a low level of expression resulted in the induction of higher amounts of T cells reactive to the model antigen listeriolysin. These results demonstrate that high-level expression of heterologous antigens does not necessarily result in optimal stimulation of immune responses.
Attenuated live bacteria provide an interesting platform for the generation of recombinant vaccines against various infectious diseases, as well as for therapeutic vaccination, for example, in cancer therapy. Various approaches have been devised to generate live carrier strains that can be used for in vivo expression and efficient display of heterologous antigens. Salmonella enterica is an attractive organism for the generation of live attenuated vaccines. Salmonella is a gastrointestinal Gram-negative pathogen with the ability to trigger an inflammatory response and diarrhea in the mild forms of disease, but it can also cross various barriers in the host in order to spread systemically, resulting in a life-threatening condition known as typhoid fever. Previous work indicated that Salmonella can also act as an efficient and versatile life carrier for mucosal vaccination (reviewed in reference 3). The various regulatory systems in Salmonella are well characterized and allow the construction of strains for efficient in vivo expression of heterologous antigens. S. enterica is an invasive and facultative intracellular pathogen that deploys two type III secretion systems (T3SS) for the manipulation of basic host cell functions (9). T3SS are complex molecular machines that mediate the translocation of effector proteins from the bacterial cytoplasm into the cytoplasm of host cells, thus acting as molecular syringes (4, 5). The T3SS encoded by Salmonella pathogenicity island 1 (SPI1) is required for the invasion of nonphagocytic cells by Salmonella, and the function of the SPI1-T3SS and its effectors is also linked to the inflammatory response of the intestinal mucosa. In contrast to the activity of the SPI1-T3SS in extracellular Salmonella, the SPI2-T3SS is specifically induced by intracellular Salmonella and mediates the translocation of a large number of effector proteins across the phagosomal membrane. Mutant strains deficient in the SPI2-T3SS or specific effector proteins such as SifA are highly attenuated for virulence (1) and have been considered as live attenuated vaccines against typhoid fever (21).
In addition to their function as important virulence factors for invasion and the intracellular lifestyle, the T3SS are also interesting tools for the delivery of heterologous antigens. This approach of inverted pathogenicity has first been proposed by Rüssmann and coworkers and subsequently extended to a larger number of T3SS in other bacteria and various vaccination approaches (19, 20, 22). We have previously demonstrated that effector proteins of the SPI2-T3SS can be used for the translocation of heterologous vaccine antigens using the SPI2-T3SS as a means of delivery (12). Effector proteins of the SPI2-T3SS are translocated into a variety of host cells, including antigen-presenting cells such as dendritic cells (DC) (7, 13). This specific feature renders the SPI2-T3SS delivery system very interesting for further exploitation in novel vaccination approaches (reviewed in reference 3).
Our previous studies used the SPI2-T3SS effector protein SseF as fusion partner for the translocation of fusion proteins (12, 24). For the generation of expression cassettes for the specific intracellular expression of fusion genes, the SPI2-derived promoter PsseA was used. In addition to SseF, a large number of further effector proteins are translocated by the SPI2-T3SS, and the expression of nearly all of these effectors is under the control of individual promoters. The majority of these promoters are under the control of the SsrAB two-component regulatory system, and genes in SPI2 and on various separate loci form a complex virulon that is coordinately regulated. We have recently performed a systematic comparative analysis of the expression levels of the genes in the SsrAB regulon and observed strong differences in the expression levels of the various promoters (25). Although PsseA, previously used for the generation of expression cassettes, belongs to a group of promoters with mid to high expression level, other promoters, such as PsifA or PsseJ, showed far higher expression levels under in vitro conditions inducing the SsrAB virulon, as well as in intracellular bacteria. Since the level of in vivo expression clearly is one of the parameters that affect the level of heterologous antigen in a recombinant strain, we speculated that the use of promoters with high expression levels could contribute to the optimization of the efficacy of immune response and protection after vaccination with recombinant Salmonella strains.
We hypothesized that the strength of intracellular activated promoters and the amount of heterologous antigen might be directly correlated with the immune response triggered by the vaccine. We used several promoters of the SsrAB regulon for the generation of expression cassettes for model antigens translocated by the SPI2-T3SS. The expression levels and immune responses mediated by cassettes with PsseJ, PsifA, PsifB, or PssaG were compared to that of the previously used PsseA. Interestingly, we observed that the highest immune response was induced by strains harboring expression cassettes with promoters yielding low- to mid-level expression in vitro.
MATERIALS AND METHODS
Bacterial strains and generation of plasmids.
Salmonella enterica serovar Typhimurium NCTC 12023 was used as a wild-type strain, and all strains used in the present study were isogenic derivatives of NCTC 12023. The ΔpurD ΔhtrA strain MvP728 (24) was used as an attenuated carrier strain. The characteristics of the strains are listed in Table 1. For the generation of recombinant plasmids (Table 2), Escherichia coli DH5α was used as a host.
TABLE 1.
S. enterica serovar Typhimurium strains used in this study
| Strain | Relevant characteristics | Source or reference |
|---|---|---|
| NCTC 12023 | Wild type | Lab collection |
| P2D6 | ssaV::mTn5 | Lab collection |
| MvP532 | ΔssrB::FRT | 16 |
| MvP678 | ΔhtrA | This study |
| MvP680 | ΔpurD | This study |
| MvP607 | PsifA::luc aph | 6 |
| MvP728 | ΔpurD ΔhtrA | 24 |
| MvP1102 | PsseA::luc aph | 25 |
| MvP1104 | PsifB::luc aph | 25 |
| MvP1105 | PsseG::luc aph | 25 |
| MvP1107 | PsseJ::luc aph | 25 |
TABLE 2.
Plasmids used in this study
| Plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| pBluescript SK (pSK) | High-copy-number vector; Ampr | Lab stock |
| pWSK29 | Low-copy-number vector; Ampr | Lab stock |
| p2077 | PsseAsscBsseFsseG::M45 in pSK | 8 |
| p2506 | PsseA OVA::M45 in p2064 | 11 |
| p2629 | pWSK29 PsseAsscBsseF::OVA::M45 | This study |
| p3528 | pWSK29 PsifBsscBsseF::OVA::M45 | This study |
| p3529 | pWSK29 PssaGsscBsseF::OVA::M45 | This study |
| p3530 | pWSK29 PsifAsscBsseF::OVA::M45 | This study |
| p3531 | pWSK29 PsseJsscBsseF::OVA::M45 | This study |
| p2810 | pWSK29 PsseAsscBsseF::lisA51-363::HA | 12 |
| p3497 | pWSK29 PsifBsscBsseF::lisA51-363::HA | This study |
| p3498 | pWSK29 PssaGsscBsseF::lisA51-363::HA | This study |
| p3504 | pWSK29 PsifAsscBsseF::lisA51-363::HA | This study |
| p3499 | pWSK29 PsseJsscBsseF::lisA51-363::HA | This study |
Ampr, ampicillin resistance.
An expression cassette consisting of sscB and a gene fusion of sseF, ovalbumin (OVA), and the M45 tag under the control of SPI2 promoter PsseA was generated by “splicing by overlap extension PCR” (SOE-PCR). In the first round of PCR, reaction A was performed with primers SseF-C-For-EcoRI and SseF-Ova-SOE-P2 using the plasmid p2077 as a template. Reaction B was performed with SseF-Ova-SOE-P3 and Sequencing-Rev using the plasmid p2506 as a template. Both reactions were performed with the High Fidelity reagent system (Roche) and 25 amplification cycles to minimize PCR-generated sequence errors. The resulting products were purified by gel electrophoresis. In a second round of PCR, equimolar amounts of the two products of the first round of PCR were mixed and used as a template. The reaction was performed using the primers SseF-C-For-EcoRI and Sequencing-Rev. The resulting product of 1.6 kb was digested with PstI and XbaI, and a fragment of 1.2 kb was isolated. Plasmid p2096 harboring PsseA sscB sseF::M45 was digested with PstI and XbaI; the larger fragment was purified and ligated with the product of the SOE-PCR. Sequencing of the resulting plasmid p2629 confirmed that sseF::M45 was replaced by sseF::OVA::M45. For further plasmids for the expression of sseF::OVA::M45, DNA fragments containing the promoters of sifB, ssaG, sifA, or sseJ were amplified from genomic DNA of wild-type (WT) S. enterica serovar Typhimurium using target gene-specific primer pairs as listed in Table 3. The PCR product was digested by KpnI and EcoRI and subcloned in plasmid p2629 digested by KpnI and EcoRI to obtain plasmid p3528, p3529, p3530, or p3531 containing PsifB, PssaG, PsifA, or PsseJ, respectively.
TABLE 3.
Oligonucleotides used in this study
| Oligonucleotide | Sequence (5′-3′) |
|---|---|
| SseF-C-For-EcoRI | ATAGAATTCATATTCCGTCAGCGG |
| SseF-Ova-SOE-P2 | GAAAGGCATTGCTTGTGTGTCTGGTTCTCCCCGAGATGTATG |
| SseF-Ova-SOE-P3 | GACACACACGCAATGCCTTTC |
| Sequencing-Rev | AGCGGATAACAATTTCACACAGGA |
| SseJ-Pro-For-KpnI | TACGGTACCTCACATAAAACACTAGCAC |
| Pro-SseJ-Rep-EcoRI | ACGGAATTCCTCCTTACTTTATTAAACACGCG |
| SsaG-Pro-For-KpnI | ATGGGTACCTCCCTGGGCGGTAGATTA |
| SsaG-Pro-Rev-EcoRI | TCCGAATTCCTTAAAATAAATACATCGTAA |
| SifB-Pro-For-KpnI | ATGGGTACCTGCCCTACCGCTAAACATC |
| SifB-Pro-Rev-EcoRI | TCCGAATTCCACAAGTGATTATATGATAC |
| SifA-Pro-For-KpnI | TACGGTACCTCATAAGCGATTAATTGCGCAAC |
| SifA-Pro-Rev-EcoRI | ACGGAATTCTTAATCTCACTTATACTGGA |
| LisA-51-For-EcoRV | CTAGATATCACGCCAATCGAAAAGAAAC |
| HA-Rev-XbaI | CCATCTAGATTAAGCGTAGTCTGGG |
For the construction of plasmids for the expression of sseF::lisA::HA gene fusion, a 1,007-bp fragment for lisA was amplified from p2810 and digested by EcoRV and XbaI and inserted into p3528, p3529, p3530, or p3531 digested by EcoRV and XbaI. The resulting plasmids p3497, p3498, p3504, or p3499 expressed sseF::lisA::HA under the control of PsifB, PssaG, PsifA, or PsseJ, respectively.
DC infection and reporter analyses.
The preparation and culture of bone marrow cells from C57BL/6 mice for generation of bone marrow-derived DC (BM-DC) has been previously described (17). After 6 days of culture in RPMI 1640 medium (PAA; Colbe) containing 10% heat-inactivated fetal calf serum (Gibco-BRL, Grand Island, NY) and granulocyte-macrophage colony-stimulating factor at 37°C in an atmosphere of 5% CO2, the BM-DC were suspended in small flasks for experiments at a density of 8 × 106 cells per flask and allowed to adhere for at least 12 h.
Bacterial strains were grown overnight in LB, and the optical density at 600 nm of the cultures was adjusted to 0.2 in 1 ml of phosphate-buffered saline (PBS). Aliquots of this suspension were added to flasks in order to yield an multiplicity of infection (MOI) of ∼100. The flask were centrifuged at 500 × g for 5 min to synchronize the infection and subsequently incubated at 37°C in an atmosphere of 5% CO2 for 30 min. Thereafter, the flasks were washed thrice with RPMI 1640 medium and incubated with RPMI 1640 medium containing 100 μg of gentamicin ml−1 for 1 h, followed by RPMI 1640 medium containing 25 μg of gentamicin ml−1 for the rest of the experiment.
At 12 h after infection, the supernatants were removed, and the cells were washed three times with PBS and lysed in 0.1% Triton X-100. An aliquot of the lysate was used to determine the number of viable intracellular bacteria by plating serial dilution onto agar plates and counting of CFU. The samples were centrifuged for 5 min to harvest the released bacteria. The pellets containing bacteria were resuspended in 250 μl of freshly prepared luciferase (Luc) assay lysis buffer and further processed for Luc assays as described previously (25). Briefly, samples were frozen at −20°C and allowed to thaw at room temperature. After thawing, the samples were incubated for 15 min at room temperature with repeated mixing on a Vortex mixer. Aliquots (25 μl) of the lysate were transferred in triplicates into wells of white mitrotiter plates (Nunc, Wiesbaden, Germany), and 50 μl of the Luc reagent mixture was added. Light emission was recorded by using an Ascent Fluroscan FL luminometer (Labsystems). Assays were performed in triplicates, and background photon counts were subtracted.
SPI2-T3SS-dependent translocation of fusion proteins by intracellular Salmonella.
BM-DC from C57BL/6 mice were infected with various Salmonella strains harboring plasmids for the expression of sseF::lisA::HA or sseF::OVA::M45 under the control of various in vivo-activated promoters. As carrier strains, Salmonella WT or a double-mutant strain MvP728 was used. At 16 h after infection, the cells were fixed and processed for immunostaining of Salmonella lipopolysaccharide (rabbit anti-Salmonella O1,4,5; Difco/BD), the hemagglutinin (HA) epitope tag (Roche), and the DC-specific marker CD11c (Armenian hamster anti-CD11c; BD). The cells were analyzed by microscopy using a Zeiss LSM700 laser scanning confocal microscope.
Quantification of T-cell stimulation.
The SL-H2-Kb-specific, murine CD8+ T-cell hybridoma B3Z expresses the lacZ reporter gene under the control of the NFAT enhancer (14). The cell line was kindly provided by Nilabh Shastri at the University of California at Berkeley. Briefly, 105 BM-DC from C57BL/6 mice per well of 96-well plates were infected with bacterial strains grown to stationary phase. Infection was performed for 1 h at an MOI of 25 or 100 for WT or MvP728 strains, respectively. The plate was centrifuged for 5 min at 500 × g to synchronize the infection. After the infection period, noninternalized bacteria were removed by two washes with PBS. To kill remaining extracellular bacteria, infected cells were incubated for 1 h in medium containing 100 μg of gentamicin ml−1. After a washing step, medium containing 25 μg of gentamicin ml−1 was added. B3Z T cells were added to the plate and cocultured with a DC/T-cell ratio ranging from 1:8 to 1:0.125 in a total volume of 200 μl per well for 24 h. The cells were centrifuged at maximal speed and lysed by the addition of 100 μl of substrate solution (0.15 mM chlorophenyl red β-galactopyranoside and 0.5% [vol/vol] Nonidet P-40 in PBS). After incubation for 6 to 8 h at 37°C, the absorbance was determined at 595 nm.
For stimulation assays, 105 BM-DC from C57BL/6 mice were seeded per well of a 96-well plate. The cell were infected with bacterial strains at an MOI of 25 or stimulated with 50 μg of ovalbumin ml−1 or 1 μg of concanavalin A ml−1 (both from Sigma, Deisenhofen, Germany). All experiments were performed in parallel for 1 h. The cells were washed twice with PBS and incubated with RPMI 1640 medium containing 100 μg of gentamicin ml−1 for 1 h. After infection, plates were gamma-irradiated (3,600 rads) prior to coculture with either 2 × 105 spleen cells from OT-I (10) or OT-II mice in a final ratio of 2:1. OT-I and OT-II mice express a transgenic T-cell receptor (TCR) (Vα2/Vβ5) specific for the OVA-derived peptides presented in the context of either major histocompatibility complex (MHC) class I or MHC class II, respectively. The transgene status was confirmed by fluorescence-activated cell sorting (FACS) with anti-mouse CD4 (clone RM4-5), anti-mouse CD8α (clone 5H10), and anti-mouse Vα2 (clone B20.1) from BD Biosciences. After 48 h of coincubation, the proliferation was measured by determining the 3H uptake for an additional 24 h. All experiments were performed in triplicates and repeated at least three times.
Vaccination experiments.
BALB/c female mice, 4 to 6 weeks old, were purchased from Jackson Laboratory and maintained under standard conditions in the CHLA Animal Facility and were treated according to an IACUC-approved protocol. For immunization, cohorts of 7 mice were immunized by orogastric application of 1 × 109 CFU per mouse of Salmonella MvP728 ΔpurD ΔhtrA strain or MvP728 harboring plasmid p2810 (PsseA), p3497 (PsifB), p3498 (PssaG), p3504 (PsifA), p3499 (PsseJ), or vector pWSK29 as a control. The inocula were applied in 200 μl of 5% sodium bicarbonate by using 20× gastric gavage needles (Popper & Sons, Inc.). Booster immunizations were applied at days 14 and 28 after primary vaccination using the same conditions. The inoculum used was always verified by serial dilution and plating on LB agar plates in the presence of the appropriate antibiotic. To analyze the shedding of Salmonella strains by vaccinated mice, approximately 100 mg of fresh fecal pellets were homogenized in 1 ml of PBS, and bacteria were enumerated on LB agar plates containing selective antibiotics. To analyze the persistence of Salmonella strains in the organs of vaccinated mice, the animals were sacrificed, and various organs were removed and homogenized in sterile PBS. The numbers of viable bacteria present in the organs were determined by plating serial dilutions onto Leifson agar plates or onto LB agar plates containing appropriate antibiotics.
MHC pentamer staining and FACS analysis.
Blood samples were collected 10, 21, and 35 days after immunization from vaccinated mice or control groups. Blood cells were harvested, erythrocytes were lysed by the addition of ammonium chloride, and the cells were subsequently resuspended in RPMI complete medium. Subsequently, cells were stained with R-PE-labeled H-2Kd/GYKDGNEYI Pro5 pentamer (ProImmune; Listeria-specific H-2Kd-Llo pentamers) and rat anti-mouse CD8-fluorescein isothiocyanate (ProImmune). After washing, murine CD8+ T cells stained with Pro5 pentamers can be analyzed by flow cytometry (FACSCalibur; BD Biosciences), and the frequency of antigen-specific T cells was determined. A similar approach was used to quantify listeriolysin (Llo)-specific CD8+ T cells in spleen cell suspension obtained from vaccinated mice.
Statistical analyses.
Analyses for unpaired groups and the control group were performed by the one-way analysis of variance with a Bonferroni t test using SigmaPlot 11. Statistical significance is denoted as defined in the figures.
RESULTS
Activity of SPI2 promoters of intracellular Salmonella in DC.
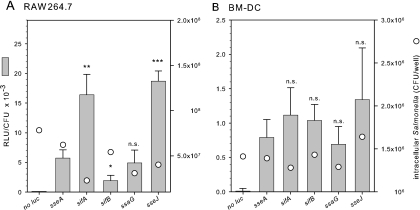
We previously performed a comprehensive and comparative analysis of the expression levels of promoters of genes in the SsrAB virulon (25). This analysis defined promoters that showed very high SsrAB-dependent expression levels under in vitro conditions, as well as in Salmonella inside cell line macrophages. Namely, reporter fusions to sseJ and sifA showed the highest intracellular expression. Other genes of the SsrAB virulon showed moderate or low expression, e.g., sseA or sifB, respectively. We speculated that the use of promoters of highly expressed genes of the SsrAB regulon might be useful to generate expression cassettes for high-level expression of heterologous antigens by Salmonella live carrier strains. DC are considered the most relevant population of antigen-presenting cells, and Salmonella can translocate heterologous antigens into DC in a SPI2-T3SS. We analyzed whether the intracellular expression of the SsrAB virulon showed differences similar to that observed in cell line macrophages. We selected genes of the SsrAB regulon with moderate and high expression levels in vitro and in macrophages for further analyses. The reporter activities of strains harboring luciferase fusions to selected genes were analyzed in RAW264.7 cells and murine BM-DC (Fig. 1). We observed that distinct differences in the expression levels were obvious, with sseJ::luc and sifA::luc showing the highest level of reporter activities in RAW264.7 cells. These observations were similar to the expression levels observed for the various reporter fusions in our previous study (25). In contrast to the reporter activities determined for intracellular Salmonella in RAW264.7 macrophages, the differences in the expression levels between sseA, ssaG, sifB, sifA, and sseJ were less pronounced for Salmonella in BM-DC. The difference between minimal and maximal expression was ∼2-fold. The relative expression levels in BM-DC were much lower in BM-DC compared to RAW264.7 cells at ca. 0.7 to 1.4 and ca. 2 to 19, respectively, relative luciferase units per intracellular Salmonella cell. We noticed rather large variations between the assays. These observations indicate that promoters of SsrAB-controlled genes are active in BM-DC but show much lower range of differences in promoter activity.
FIG. 1.
Promoter activities of intracellular Salmonella in macrophages and murine DC. Various S. enterica serovar Typhimurium strains harboring chromosomal promoter fusions to genes of the SsrAB virulon were used to infect the macrophagelike murine cell line RAW264.7 (A) or murine BM-DC (B) at an MOI of 10 or 25, respectively. Phagocytosis of the bacteria was allowed for 1 h and subsequently noninternalized bacteria were killed by addition of gentamicin. At 12 h after infection, infected cells were lysed in order to release intracellular bacteria. A fraction of the lysate was used to quantify the amount of viable intracellular bacteria by plating of serial dilutions onto agar plates and determination of CFU (circles). Equal amounts of the lysates were used to determine the luciferase activities of the recovered intracellular bacteria and reporter activities were expressed as relative luciferase units (RLU) per CFU (bars). Means and standard deviations of triplicate assays are shown. Statistical analyses were performed comparing the various promoter fusions to the sseB::luc reporter and are indicated as follows: n.s., not significant; *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
Generation and evaluation of expression cassettes.
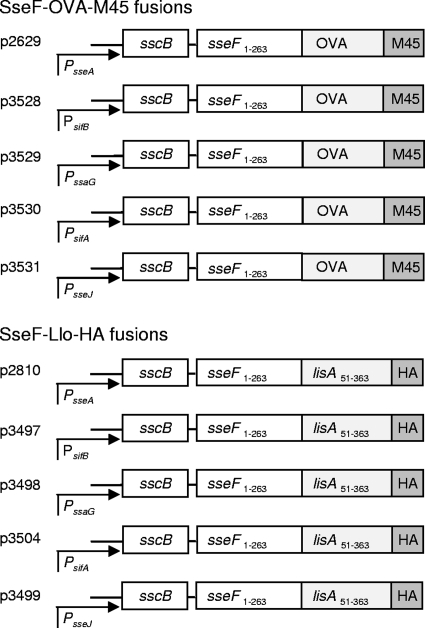
The promoters of several genes of the SsrAB regulon were used for further studies. In order to test of efficacy of various promoters of the SsrAB regulon in vaccination approaches with live attenuated carrier strains, expression cassettes containing promoters of sseA, ssaG, sifB, sifA, or sseJ and a gene encoding a hybrid protein consisting of SPI2-T3SS translocated effector proteins SseF and model antigens OVA or Llo were generated. For either model antigen, a large panel of tools is available for the characterization of immune responses. The expression cassettes were located on low-copy-number plasmids that have previously been shown to be compatible with SsrAB-regulated in vivo expression. The modular design of the various expression cassettes is presented in Fig. 2.
FIG. 2.
Generation of expression cassettes for the expression of heterologous vaccine antigens by promoters of the SsrAB virulon. Expression cassettes consist of hybrid genes for the expression of fusion proteins consisting of SPI2 effector protein SseF, model antigens Llo or OVA, and a C-terminal epitope tag (M45 or HA) for the standardized detection of the amounts of fusion protein. Cassettes also contain sscB encoding a specific chaperone for SseF. The expression is controlled by various in vivo-activated promoters of the SsrAB regulon that control the expression of genes within SPI2 (PsseA, PssaG) or genes encoding SPI2 effector proteins outside of SPI2 (PsifA, PsifB, and PsseJ). All plasmids were generated on the basis of the low-copy-number vector pWSK29, and plasmid designations are indicated by p2629, etc.
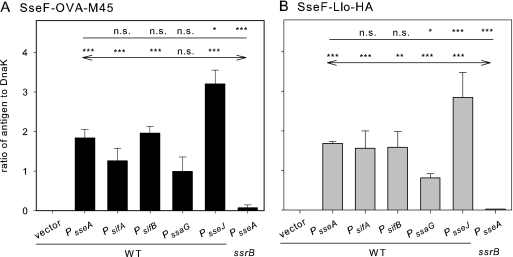
Next, strains harboring the expression cassettes were tested for the levels of recombinant fusion proteins. We first used in vitro culture conditions known to induce the expression of genes of the SsrAB regulon and the synthesis of SPI2 effector proteins. The synthesis of SseF-OVA-M45 and SseF-Llo-HA fusion proteins was observed for all constructs analyzed here (Fig. 3). In order to quantify the amounts of recombinant protein produced by the various strains, Western blots were analyzed by using the Odyssey detection system, and signals for model antigens were normalized by using the levels of constitutively synthesized control protein DnaK. For both model antigens, highest amounts of recombinant proteins were detected if PsseJ was used. The amounts were ∼2-fold higher than those obtained for constructs with PsseA used in our previous studies. Constructs with PsifA were at a comparable level protein, while expression under the control of PssaG was the lowest. In the absence of SsrB, the transcriptional activator of the SsrAB regulon, only very low levels of synthesis of fusion proteins were observed.
FIG. 3.
Synthesis of fusion proteins with model antigens. The S. enterica serovar Typhimurium wild-type strain with the empty plasmid vector (vector) or harboring plasmids of the expression of sseF::OVA::M45 (A) or sseF::lisA::HA (B) under the control of various SsrAB-controlled promoters was grown in SPI2-inducing minimal media (PCN-P, pH 5.8). As negative controls, ssrB-deficient strain (MvP532, ΔssrB::FRT) harboring plasmids with PsseA-containing expression cassettes was used. Bacteria were harvested after 8 h of culture under inducing conditions, and equal amounts of bacteria (determined based on the optical density at 600 nm) were lysed and subjected to SDS-PAGE and Western blot analyses for the detection of the M45 (A) or HA (B) epitope tag. Blots were probed with fluorescently labeled secondary antibodies, and signal intensities were quantified by using the Odyssey system (Li-Cor). As loading controls, the cytosolic heat shock protein DnaK was detected on the same blot, and signals were quantified. The ratios of the M45 to DnaK or HA to DnaK signals were calculated, and means and standard deviations for three samples are shown. Statistical analyses were performed comparing signals for expression cassettes with various promoters to signals for expression cassettes with PsseA (indicated by plain lines) or by comparing various expression cassettes in a WT background to the PsseA expression cassette in an ssrB background (indicated by lines with arrows). Statistical analysis: n.s., not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
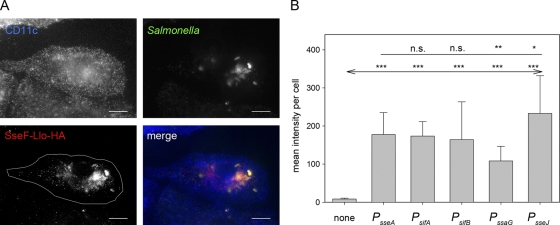
As a further control of the function of the expression cassettes, the translocation of the model antigens by intracellular Salmonella in BM-DC was analyzed. We investigated the translocation of SseF-OVA-M45 (data not shown) and SseF-Llo-HA (Fig. 4) fusion proteins by strains harboring expression cassettes with various SsrAB-regulated promoters. The use of the various expression cassettes resulted in the translocation of fusion proteins into BM-DC (see, for example, Fig. 4A), but the intensities of the immunofluorescence staining were variable, indicating different amounts of translocated protein. For quantification, infected CD11c+ cells harboring similar numbers of intracellular Salmonella were selected, and the signal intensities of the fluorescence channel for SseF-Llo-HA staining were quantified. Comparison of expression cassettes with various promoters indicated the highest and lowest amounts of translocated protein for the PsseJ- and PssaG-controlled expression cassettes, respectively, and similar levels for the expression cassettes containing PsifA, PsifB, or PsseA (Fig. 4B). Overall, the results for the translocation in BM-DC were in good correlation with the expression levels of the reporter fusions (Fig. 1B) and the amounts of protein synthesized in vitro (Fig. 3). These data indicate that the choice of the promoter had a limited effect on the amounts of heterologous protein produced and translocated by Salmonella residing in BM-DC.
FIG. 4.
Translocation of fusion proteins by intracellular Salmonella. WT S. enterica serovar Typhimurium harboring plasmids with cassettes for the expression of sseF::lisA::HA under the control of various promoters was used to infect BM-DC at an MOI of 25. The cells were fixed 16 h after infection and processed for immunostaining for DC marker CD11c (blue), intracellular Salmonella (green), and translocated fusion protein SseF-Llo-HA (red). CD11c+ and Salmonella-infected cells were identified by confocal laser scanning microscopy and imaged by using the ZEN software package (Zeiss). (A) Representative infected BM-DC with Salmonella WT harboring an expression cassette with PsseJ translocating SseF-Llo-HA. The white line in the SseF-Llo-HA micrograph defines the area used for quantification of the immunofluorescence signals. (B) After infection with Salmonella WT harboring plasmids with various expression cassettes, infected cells with similar amounts of intracellular Salmonella were selected for the various conditions, and the signal intensities of the Cy3 channel for the anti-HA strains were measured with identical exposure times. The mean signal intensities and standard deviations for at least 20 infected cells per strains are shown. Statistical analyses were performed comparing signals for expression cassettes with various promoters to signals for expression cassettes with PsseA (indicated by a plain line) or by comparison to mock-infected cells (indicated by a line with arrows). Statistical analysis: n.s., not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Quantification of T-cell responses to antigens presented by intracellular Salmonella.
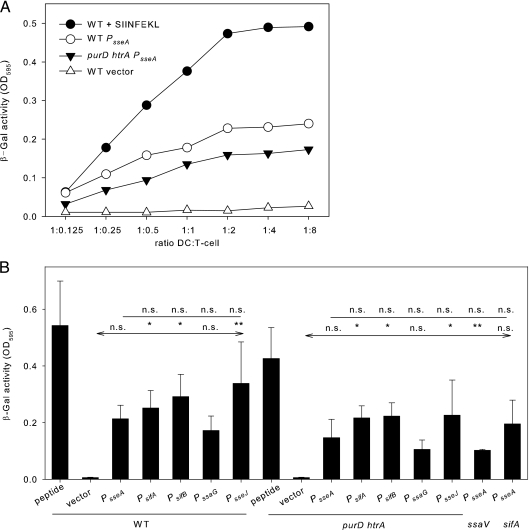
We next compared the antigen-dependent simulation of T cells after uptake of recombinant Salmonella strains to the expression of recombinant antigens under the control of various SsrAB-controlled promoters (Fig. 5). BM-DC were infected with S. enterica serovar Typhimurium WT or the attenuated carrier strain MvP728 lacking purD and htrA (24) harboring plasmids for the expression of sseF::OVA::M45 under the control of various promoters. Subsequently, infected BM-DC were incubated with the B3Z T-cell line. B3Z is a T-cell hybridoma that recognizes the OVA-derived SIINFEKL epitope in the context of H2Kb and expresses lacZ reporter gene under the control of the NFAT enhancer. The β-galactosidase activity is thus a measure of the antigen-dependent stimulation (23). Very low stimulation was observed with the vector controls without expression cassettes, whereas addition of the SIINFEKL peptide was used as positive control resulting in maximal stimulation. The use of the Salmonella WT or ΔpurD ΔhtrA strain with plasmids harboring various expression cassettes resulted in highly increased stimulation of the T-cell hybridoma. In either strain background, the greatest stimulation was obtained with expression cassettes with PsseJ, while the use of PssaG yielded the lowest efficiency (Fig. 5B). However, the differences between expression cassettes with various promoters were not significant. As further controls, the expression cassette with PsseA was introduced into Salmonella strain P2D6 that can expression heterologous antigens but is unable to translocate fusion proteins due to a defect in the SPI2-encoded T3SS. This strain induced higher T-cell stimulation than the vector control but lower stimulation compared to the WT strain with the PsseA expression-based expression cassette. This result indicates that a certain level of antigen presentation can occur without the translocation of the heterologous antigen. We also used the sifA-deficient strain MvP503 with the PsseA-based expression cassette. A sifA strain has been reported to escape into the cytoplasm of antigen-presenting cells (21). The T-cell stimulation by the sifA strain was comparable to that of the WT strain.
FIG. 5.
Stimulation of T-cell responses by recombinant Salmonella vaccines. Murine BM-DC were infected at an MOI of 25 with Salmonella WT, the ΔpurD ΔhtrA carrier strain, or ssaV or ssrB strains as indicated. Strains harbored the empty plasmid vector or plasmids with expression cassettes for sseF::OVA::M45 under the control of various SsrAB-controlled promoters as indicated. As a positive control, BM-DC were infected with WT Salmonella and stimulated with the SIINFEKL peptide. The infected BM-DC were incubated with B3Z reporter cell line, and after coculture for 24 h, the β-galactosidase substrate chlorophenyl red β-galactopyranoside was added. After additional incubation for 6 h, the reaction was stopped, and the β-galactosidase product was quantified photometrically by measurement of the absorbance at 595 nm. (A) T-cell stimulation was analyzed at various ratios of infected BM-DC to T cells. For further experiments, a DC/T-cell ratio of 1:4 was used. (B) Effect of various SsrAB-controlled promoters on the stimulation of T cell. The means and standard deviations of triplicate samples are shown, and the data sets are representative of six independent experiments. Statistical analyses were performed comparing stimulation by strains harboring expression cassettes with various promoters to strains with expression cassettes with PsseA (indicated by plain lines) or by comparing stimulation by strains with various expression cassettes to the vector control (indicated by lines with arrows). Statistical analysis: n.s., not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
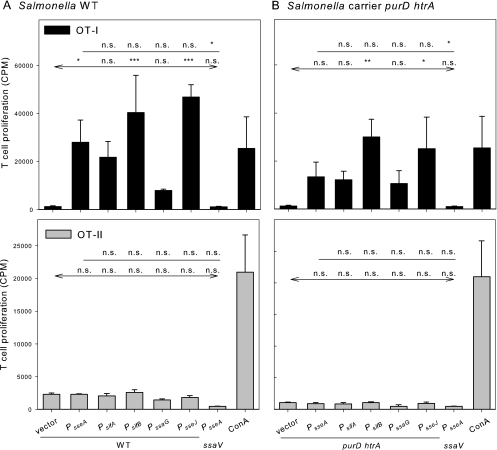
As a second approach, we used the OVA-specific T cells isolated from OT-I or OT-II transgenic mice. OT-I mice are transgenic for CD8+ T cells that recognize OVA epitope SIINFEKL (OVA257-276) in the context of MHC class I molecules, while OT-II mice are transgenic for CD4+ cells that respond to the OVA323-339 peptide ISQAVHAAHAEINEAGR in the context of the MHC class II I-A2 molecule. BM-DC were infected with various combinations of carrier strains and expression cassettes, and the proliferation of OT-I- and OT-II-derived T cells was determined as a measure of antigen-dependent stimulation (Fig. 6). Compared to the control stimulation of T cells by concanavalin A, high levels of stimulation of OT-I cells were obtained with Salmonella WT or the ΔpurD ΔhtrA carrier strain with the various expression cassettes for sseF::OVA::M45. In contrast, stimulation of OT-II cells was rather low and in the range of the vector control under the various conditions investigated in this assay. This indicates that peptides derived from translocated fusion proteins are predominantly presented in an MHC class I-restricted manner. The greatest stimulation was observed with strains harboring expression cassettes controlled by PsifB or PsseJ. The lowest rate of stimulation was recorded if strains with PssaG-controlled expression cassettes were used. A similar correlation between the specific promoter and the level of stimulation was observed for Salmonella WT and the attenuated carrier strain MvP728. However, lower antigen-dependent T-cell proliferation was observed in experiments with the attenuated carrier strain. These results indicated that expression cassettes with various SsrAB-controlled promoters are functional in a live attenuated carrier strain and that the level of antigen-dependent T-cell proliferation after antigen presentation by DC can be directed by the selection of promoters for control of expression cassettes.
FIG. 6.
Stimulation of CD4+ and CD8+ T-cell proliferation by recombinant Salmonella vaccines. BM-DC were infected with Salmonella WT (A) or the attenuated ΔpurD ΔhtrA carrier strain MvP728 (B), each harboring plasmids with various expression cassettes for expression of sseF::OVA:M45. As a control for the release of antigen independent of T3SS translocation, the T3SS-deficient ssaV strain expressing sseF::OVA::M45 under the control of PsseA was used. The infection of BM-DC was performed basically as described for Fig. 5. Infected BM-DC and intracellular bacteria were inactivated after 3 h of infection. Inactivated cells were added to T cells prepared from OT-I or OT-II mice as indicated, and T-cell stimulation was allowed for 24 h. For quantification, the incorporation of [3H]thymidine was determined and is expressed as counts per minute (CPM). The means and standard deviations of triplicate samples are shown for CPM of OT-1 cells (black bars) or OT-II cells (gray bars). The assays are representative of three assays with similar results. Statistical analyses were performed comparing stimulation by strains harboring expression cassettes with various promoters to strains with expression cassettes with PsseA (indicated by plain lines) or by comparing stimulation by strains with various expression cassettes to the vector control (indicated by lines with arrows). Statistical analysis: n.s., not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Quantification of T-cell responses to vaccination.
To translate the observations described above to a vaccination setting, the response to recombinant Salmonella strains with various expression cassettes for the sseF::lisA::HA was analyzed. As a carrier strain, the ΔpurD ΔhtrA strain MvP728 was used. This strain harbored expression cassettes for the expression of sseF::lisA::HA under the control of the various promoters evaluated above.
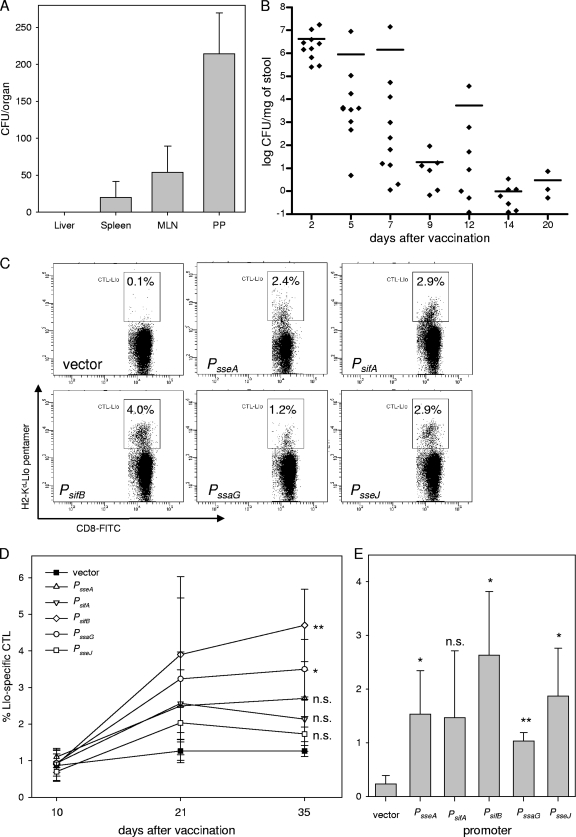
The performance of the carrier strain in a vaccinated organism was determined in mice that were orally vaccinated with MvP728 harboring p2810 for the expression of sseF::lisA::HA under the control of PsseA. The organ distribution was analyzed 15 days after immunization and indicated that a low number of the plasmid-harboring bacteria were present in gut-associated lymphatic tissue (Peyer's patches) but absent or highly reduced in systemic sites such as the liver, spleen, or mesenteric lymph nodes (Fig. 7A). Analyses of the fecal shedding indicated that the recombinant vaccine strain was present in the gastrointestinal tract for up to 20 days after vaccination. However, the amount of bacteria present in feces decreased continuously within the observation period (Fig. 7B). Similar observations were made for MvP728 harboring expression cassettes with other promoters (data not shown).
FIG. 7.
Immune response after vaccination with recombinant Salmonella carrier strains. Organ distribution (A) and fecal shedding (B) after oral vaccination of mice with the ΔpurD ΔhtrA carrier strain MvP728 harboring plasmid p2810. (A) Four mice were sacrificed 15 days after immunization, and serial dilutions of homogenates of liver, spleen, mesenteric lymph nodes (MLN), and Peyer's patches (PP) were plated onto agar plates for determination of the organ burden with Salmonella. For the spleen, PP, and MLN the CFU per organ is indicated, and for the liver the CFU per gram of liver is indicated. (B) The presence of the vaccine strain in fecal pellets was determined over a period of 20 days (days 2, 5, 7, 9, 12, 14, and 20 postvaccination). Approximately 100 mg of fresh fecal pellets was homogenized in 1 ml of PBS, and serial dilutions were platted on LB agar plates containing carbenicillin (50 μg ml−1) for bacterial enumeration of the fecal shedding of individual mice (limit of detection, 0.1 CFU mg of feces−1). The means are indicated by horizontal bars. (C to E) Groups of seven female mice were orally vaccinated with the ΔpurD ΔhtrA carrier strain MvP728 harboring plasmids for the expression of the sseF::lisA::HA gene fusion under the control of various promoters as indicated or the vector control. Booster immunizations were performed on days 14 and 28 after the primary vaccination. (D) Blood was collected at 10, 21, and 35 days after prime vaccination and pentamer staining for Llo-responsive CD8+ cells in peripheral blood was performed to determine immune responses at various time points after vaccination. The means and standard deviations are shown, and statistical analysis was performed for samples obtained 35 days after vaccination. (E) Mice were sacrificed 35 days after prime vaccination, the spleens were recovered, and suspensions of splenocytes were analyzed for Llo-responsive CD8+ cells as for panel D. Means and standard deviations of analyses of cohorts of immunized mice are given, and statistical analysis of vaccinated cohorts to the vector control was performed. Examples of FACS analyses of splenocytes are shown in panel C, and the percentages of gated cells are indicated. Statistical analysis: n.s., not significant; *, P < 0.05; **, P < 0.01.
To compare the performance of expression cassettes with various promoters of the SsrAB virulon, mice were immunized by oral application of the vector or vaccine strains, and two booster immunizations were performed on days 14 and 28. In order to quantify the immune response to vaccination, blood samples were analyzed 10, 21, and 35 days after the initial immunization. The amounts of Llo-specific CD8+ T cells were determined by using specific pentamers (Fig. 7C to E). The immunization with any of the strains harboring expression cassettes resulted in CD8+ T-cell responses that were higher than that of the vector control. At 35 days after prime immunization, the highest numbers of Llo-specific T cells in peripheral blood were detected in the group immunized with the vaccine strain containing the expression cassette controlled by PsifB (Fig. 7C). In contrast, the lowest amounts were observed for the vaccines with the PsseJ-containing expression cassette. In detail, the following order for the level of specific T-cell responses, as determined for the various expression cassettes: PsifB > PssaG > PsseA > PsifA > PsseJ > vector. Significant differences were obtained between PsifB and PsseJ (P < 0.01) and PsifB and PsifA (P < 0.05). The analysis of Llo-specific T cells in the spleen resulted in a similar pattern (Fig. 7D and E). The highest counts for specific CD8+ T cells were detected in the group vaccinated with the strain harboring the PsifB expression cassette, and the relative levels were PsifB > PsseJ > PsseA > PsifA > PssaG > vector. The difference between the various promoters, however, was not statistically significant (data not shown).
Interestingly, the results show that the use of promoters that resulted in high levels of expression of reporter gene fusions resulted in low to moderate efficacy in stimulation of immune responses if used for the generation of expression cassettes. In addition, organ-specific responses to the live vaccines have to be considered.
DISCUSSION
Live attenuated bacterial strains may be used as carriers displaying heterologous antigens for vaccination, and T3SS are useful means for injecting heterologous antigens directly into the cytoplasm of antigen-presenting cells (APC). The efficacy as vaccine of recombinant live attenuated bacteria depends on a variety of factors, including the level of virulence attenuation of the carrier, the selection of effector proteins as fusion partners or the strength of expression by intracellular activated promoters. For example, the intracellular activated SsrAB-virulon consists of more than 20 transcriptional units, and the strength of the various promoters varies within a large range (25). Here, we investigated the correlation between the promoter and the efficacy in experimental vaccination. We observed that the amount of protein and the stimulation of T-cell responses under in vitro conditions correlated well with the level of expression of the various promoters. We hypothesized that the use of strong promoters in expression cassettes would lead to higher amounts of recombinant antigen synthesized and translocated by the live carrier strains and, in turn, to higher stimulation of immune responses. Although we found that highly active promoters also result in increased levels of recombinant antigen synthesis and antigen-dependent T-cell stimulation in vitro, the correlation in a vaccination setting turned out to be inversed. In mice vaccinated with live attenuated Salmonella, the strongest stimulation of immune response was observed with expression cassettes controlled by the weaker promoters of the set investigated here. From these results, we conclude that the choice of an in vivo-activated promoter is in fact an important parameter for the construction of efficient recombinant vaccines. However, the analyses of the strength of the various promoters under in vitro conditions only allowed limited conclusions about the performance during vaccination of an organism. This observation may be explained by several factors. The additional, high-level expression of recombinant antigens by attenuated carrier strains clearly is an additional metabolic burden to the bacterial cell. This may result in a further attenuation of the carrier strains once confronted with the various lines of immune defense of the vaccinated organism. Carrier strains with a high level of expression of heterologous antigens would be more rapidly eliminated and thus reduced in the stimulation of immune responses. These adverse effects of expression of heterologous antigens are less pronounced in the in vitro assays used, since the carrier strains are not, or only in a limited form, exposed to immune defenses reactions.
Based on our results, we suggest PsifB and the previously used promoter PsseA as the optimal promoters for the generation of expression cassettes for recombinant antigens in Salmonella. However, the divergent nature of the antigens used in such approaches may require that new combinations of antigen and promoter are compared and further optimized. The modular design of the expression cassettes we described here should make the systematic evaluation and rational design of expression strategies rather easy. Since the expression level of heterologous antigens affects the viability of carrier strains and can lead to further attenuation, the optimal combination of expression cassettes and carrier strains has to be considered.
The presentation of antigen delivered into APC by the T3SS is likely to be distinct from that of naturally occurring cytosolic antigens. The later are mainly derived from defective ribosomal products of intracellular pathogens or self proteins. Such antigens are presented by MHC class I molecules. Phagocytosed extracellular antigens can also be presented to CD8+ lymphocytes through the process of cross presentation (2, 18). Professional APC such as DC are thus capable of activating CD8+ cells to provide immunity, for example, against viruses that do not infect APC, or against nonhematopoietic tumors. In the case of APC that phagocytosed Salmonella carrier strains, active translocation of heterologous proteins from the Salmonella-containing vacuole into the cytosol by the T3SS may be considered “pseudo-cross presentation.” The processing of these antigens is different, since intact fusion proteins are delivered into the cytoplasm of the target cell. A further parameter for rational design will be the selection of optimal T3SS proteins as vehicles for delivery of the heterologous antigen. In this and previous approaches (12, 24) we used SPI2-T3SS effector SseF as a fusion partner. SseF, as well as fusion proteins with SseF, are targeted to endosomal membranes and remain membrane associated for extended periods of time (15, 16). Other effector proteins of the SPI2-T3SS show a cytosolic localization and may be more prone to proteasomal degradation in the host cell. We consider the selection of the effector protein as fusion partner as a further important parameter for the rational design of recombinant vaccines. A systemic comparison of the efficacy of various SPI2-T3SS effectors in translocating heterologous vaccine antigens and in stimulating immune responses is currently in process in our lab (W. Halim and M. Hensel, unpublished data).
To our knowledge, the present study provides the first comparative analyses of the performance of the various promoters of a large virulon for the generation of expression cassettes for vaccination. In conclusion, our analysis showed that in vivo-induced promoters of expression cassettes for recombinant antigens are a critical parameter for the optimal design of a vaccination strategy. The performance and efficacy of such promoters in vaccination was inversely related to the performance estimated from the characterization in vitro. These results demonstrate the complex interplay between expression level, attenuation of live carrier strains, and properties of the recombinant antigen in a vaccinated host organism. For the rational design of recombinant vaccines, further studies are required for the comprehensive evaluation of these critical parameters.
Acknowledgments
This study was supported by grant HE 1964 to M.H. and by grants with collaborative research center SFB 796 from the Deutsche Forschungsgemeinschaft.
We thank Leonid Metelitsa for critical discussion of the results and Tanja Bergs for careful reading of the manuscript.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 23 August 2010.
REFERENCES
- 1.Beuzon, C. R., S. Meresse, K. E. Unsworth, J. Ruiz-Albert, S. Garvis, S. R. Waterman, T. A. Ryder, E. Boucrot, and D. W. Holden. 2000. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J. 19:3235-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanchard, N., and N. Shastri. 2010. Cross-presentation of peptides from intracellular pathogens by MHC class I molecules. Ann. N. Y. Acad. Sci. 1183:237-250. [DOI] [PubMed] [Google Scholar]
- 3.Cheminay, C., and M. Hensel. 2008. Rational design of Salmonella recombinant vaccines. Int. J. Med. Microbiol. 298:87-98. [DOI] [PubMed] [Google Scholar]
- 4.Cornelis, G. R. 2006. The type III secretion injectisome. Nat. Rev. Microbiol. 4:811-825. [DOI] [PubMed] [Google Scholar]
- 5.Galan, J. E., and H. Wolf-Watz. 2006. Protein delivery into eukaryotic cells by type III secretion machines. Nature 444:567-573. [DOI] [PubMed] [Google Scholar]
- 6.Gerlach, R. G., S. U. Hölzer, D. Jäckel, and M. Hensel. 2007. Rapid engineering of bacterial reporter gene fusions by using Red recombination. Appl. Environ. Microbiol. 73:4234-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halici, S., S. F. Zenk, J. Jantsch, and M. Hensel. 2008. Functional analysis of the Salmonella pathogenicity island 2-mediated inhibition of antigen presentation in dendritic cells. Infect. Immun. 76:4924-4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen-Wester, I., B. Stecher, and M. Hensel. 2002. Type III secretion of Salmonella enterica serovar Typhimurium translocated effectors and SseFG. Infect. Immun. 70:1403-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haraga, A., M. B. Ohlson, and S. I. Miller. 2008. Salmonellae interplay with host cells. Nat. Rev. Microbiol. 6:53-66. [DOI] [PubMed] [Google Scholar]
- 10.Hogquist, K. A., S. C. Jameson, W. R. Heath, J. L. Howard, M. J. Bevan, and F. R. Carbone. 1994. T-cell receptor antagonist peptides induce positive selection. Cell 76:17-27. [DOI] [PubMed] [Google Scholar]
- 11.Husseiny, M. I., and M. Hensel. 2005. Evaluation of an intracellular-activated promoter for the generation of live Salmonella recombinant vaccines. Vaccine 23:2580-2590. [DOI] [PubMed] [Google Scholar]
- 12.Husseiny, M. I., F. Wartha, and M. Hensel. 2007. Recombinant vaccines based on translocated effector proteins of Salmonella pathogenicity island 2. Vaccine 25:185-193. [DOI] [PubMed] [Google Scholar]
- 13.Jantsch, J., C. Cheminay, D. Chakravortty, T. Lindig, J. Hein, and M. Hensel. 2003. Intracellular activities of Salmonella enterica in murine dendritic cells. Cell. Microbiol. 5:933-945. [DOI] [PubMed] [Google Scholar]
- 14.Karttunen, J., S. Sanderson, and N. Shastri. 1992. Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proc. Natl. Acad. Sci. U. S. A. 89:6020-6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhle, V., and M. Hensel. 2002. SseF and SseG are translocated effectors of the type III secretion system of Salmonella pathogenicity island 2 that modulate aggregation of endosomal compartments. Cell. Microbiol. 4:813-824. [DOI] [PubMed] [Google Scholar]
- 16.Kuhle, V., D. Jäckel, and M. Hensel. 2004. Effector proteins encoded by Salmonella pathogenicity island 2 interfere with the microtubule cytoskeleton after translocation into host cells. Traffic 5:356-370. [DOI] [PubMed] [Google Scholar]
- 17.Lutz, M. B., N. Kukutsch, A. L. Ogilvie, S. Rossner, F. Koch, N. Romani, and G. Schuler. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223:77-92. [DOI] [PubMed] [Google Scholar]
- 18.Miller, J. F., C. Kurts, J. Allison, H. Kosaka, F. Carbone, and W. R. Heath. 1998. Induction of peripheral CD8+ T-cell tolerance by cross-presentation of self antigens. Immunol. Rev. 165:267-277. [DOI] [PubMed] [Google Scholar]
- 19.Nishikawa, H., E. Sato, G. Briones, L. M. Chen, M. Matsuo, Y. Nagata, G. Ritter, E. Jager, H. Nomura, S. Kondo, I. Tawara, T. Kato, H. Shiku, L. J. Old, J. E. Galan, and S. Gnjatic. 2006. In vivo antigen delivery by a Salmonella typhimurium type III secretion system for therapeutic cancer vaccines. J. Clin. Invest. 116:1946-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panthel, K., K. M. Meinel, V. E. Sevil Domenech, K. Trülzsch, and H. Rüssmann. 2008. Salmonella type III-mediated heterologous antigen delivery: a versatile oral vaccination strategy to induce cellular immunity against infectious agents and tumors. Int. J. Med. Microbiol. 298:99-103. [DOI] [PubMed] [Google Scholar]
- 21.Petrovska, L., R. J. Aspinall, L. Barber, S. Clare, C. P. Simmons, R. Stratford, S. A. Khan, N. R. Lemoine, G. Frankel, D. W. Holden, and G. Dougan. 2004. Salmonella enterica serovar Typhimurium interaction with dendritic cells: impact of the sifA gene. Cell. Microbiol. 6:1071-1084. [DOI] [PubMed] [Google Scholar]
- 22.Rüssmann, H., H. Shams, F. Poblete, Y. Fu, J. E. Galan, and R. O. Donis. 1998. Delivery of epitopes by the Salmonella type III secretion system for vaccine development. Science 281:565-568. [DOI] [PubMed] [Google Scholar]
- 23.Shastri, N., and F. Gonzalez. 1993. Endogenous generation and presentation of the ovalbumin peptide/Kb complex to T cells. J. Immunol. 150:2724-2736. [PubMed] [Google Scholar]
- 24.Xiong, G., M. I. Husseiny, L. Song, A. Erdreich-Epstein, G. M. Shackleford, R. C. Seeger, D. Jäckel, M. Hensel, and L. S. Metelitsa. 2010. Novel cancer vaccine based on genes of Salmonella pathogenicity island 2. Int. J. Cancer 126:2622-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu, X., and M. Hensel. 2010. Systematic analysis of the SsrAB virulon of Salmonella enterica. Infect. Immun. 78:49-58. [DOI] [PMC free article] [PubMed] [Google Scholar]