Abstract
Increased risk of preterm labor has been linked to cervicovaginal infection with Ureaplasma urealyticum and group B streptococci. Although various experimental models have been developed to study the role of amniochorion infection in preterm labor, they typically exclude the initial interaction between intrauterine leukocytes (recruited from decidual vessels into the avascular fetal membranes) and infecting bacteria. In this work, we ascertained whether inflammatory molecules secreted by bacterium-activated intrauterine leukocytes stimulate the amniochorion production of mediators involved in human labor. Using a two-step process beginning with placental circulating leukocytes as a proxy for intrauterine leukocytes, we found that coincubation of amniochorion explants with plasma from placental whole blood preincubated with group B streptococci resulted in a significant increase in tumor necrosis factor alpha (TNF-α) and matrix metalloproteinase 9 (MMP-9) levels in tissue. Extensive changes in the connective tissue arrangement and a decrease in collagen content demonstrated the degradation of the extracellular matrix following this treatment. In contrast, plasma from blood preconditioned with U. urealyticum induced a highly significant secretion of interleukin-1β (IL-1β) and prostaglandin E2 (PGE2) by the amniochorion without changes in the extracellular matrix organization or content. These data demonstrate that group B streptococci induce degradation of the amniochorion as a result of MMP-9 production, probably via TNF-α, whereas U. urealyticum stimulates the secretion of PGE2, probably via IL-1β, potentially stimulating myometrial contraction. Our study provides novel evidence that the immunological cells circulating within the uterine microenvironment respond differentially to an infectious agent, triggering alternative molecular signaling pathways leading to human labor.
Colonization of the cervicovaginal tract with pathogenic microorganisms, such as group B streptococci (GBS) and Ureaplasma urealyticum, during pregnancy has been associated with premature rupture of the fetal membranes (14, 32, 41). Bacteria colonizing the lower genital tract may ascend to the gestational tissues, triggering an inflammatory response mediated primarily by interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), and IL-6 (14, 25, 41). These cytokines stimulate the secretion of additional cytokines and other mediators, including uterotonic compounds, such as prostaglandin E2 (PGE2) (9, 27, 42) and matrix metalloproteinases (MMPs) (3, 33, 37), which participate in the weakening of the fetal membranes and their consequent rupture (41, 47). However, the interactions between these microorganisms and the fetal membranes that lead to the secretion of these biochemical mediators, and their cellular origins, are poorly understood.
Most in vitro experiments that have explored the effects of microorganisms on the fetal membranes involve the exposure of ex vivo explants of these tissues to whole bacteria or their products (5, 21, 36). However, none of these experimental models considered the possible contribution of other local cells that are components of the choriodecidual microenvironment. This perspective may be critical to an understanding of the rupture of the membranes in the presence of infection, because several leukocyte populations are accumulated selectively in the gestational tissues (6, 31, 46). There, they interact directly with the avascular fetal membranes, leading to further leukocyte recruitment, especially under infectious conditions (18, 44, 52). Additionally, we recently demonstrated that the soluble products of leukocytes circulating in the placental blood induce collagenolysis in the fetal membranes (13). Further characterization indicated that these leukocytes are phenotypically and functionally different than those in the peripheral circulation (49), and because they are sources of activated matrix metalloproteinase 9 (MMP-9), PGE2 and the proinflammatory cytokines IL-1β and TNF-α (49) may play prominent roles in the process that leads to the rupture of the fetal membranes.
To define the role of local leukocytes in the infection-mediated rupture of the fetal membranes, in this study, using an in vitro model, we first explored whether direct contact between two distinct pathogenic bacteria and leukocytes circulating in the placental blood induced the differential secretion of mediators responsible for the initiation of an inflammatory response. Second, we examined the subsequent effects of the products derived from this initial interaction on the amniochorion. Our results showed that GBS induce the production of TNF-α and MMP-9, promoting degradation of the amniochorion, whereas U. urealyticum induces secretion of IL-1β and PGE2 potentially stimulating myometrial contraction. These findings suggest that local leukocytes are able to respond specifically to the infectious agents triggering alternative molecular signaling cascades leading to human labor.
MATERIALS AND METHODS
The study protocol was approved by the Instituto Nacional de Perinatologia Internal Review Board (authorization number 212250-02081).
Amniochorion culture.
A previously described in vitro model was used to expose fetal membranes to various treatment conditions (13). Fetal membranes were obtained from healthy women with normal pregnancies at term who delivered by cesarean section with no evidence of labor. Amniochorion swabs were rolled onto blood agar plates and cultured under aerobic and anaerobic conditions to ensure that the tissues were free from infection or contamination. An additional swab was inoculated with Urea-Arginine LYO 2 broth (bioMérieux, France) to preclude the presence of amniochorion infection due to mycoplasma species. The explants (1-cm diameter) were allowed to equilibrate overnight at 37°C in 5% CO2 in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 1% sodium pyruvate, and 1% antimycotic antibiotic as the preincubation medium. The explants were incubated for an additional 24 h with DMEM supplemented with 166.5 g/liter lactalbumin hydrolysate. The media were then removed, and fresh media were added before the explants were stimulated with plasma. Tissue culture reagents were purchased from Invitrogen (Grand Island, NY). During all experimental procedures, the viability of the amniochorion explants was tested using the XTT {sodium 3′-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro) benzene sulfonic acid hydrate} reduction method (Boehringer-Manheim, Germany).
Preparation of bacteria.
The microorganisms chosen for this study were GBS, U. urealyticum serovar 3, and Lactobacillus acidophilus, which were isolated from amniochorionic swabs of women with clinical evidence of premature rupture of the fetal membranes. The bacterial strains were grown on blood agar plates (GBS) or Man, Rogosa, Sharp (MRS) agar slopes (L. acidophilus) in an aerobic atmosphere at 37°C (17, 24, 45). The bacterial colonies were harvested after 24 h, washed three times in physiological saline solution, and resuspended at a density of 3 × 108 CFU/ml. The U. urealyticum strain was cultured in Urea-Arginine LYO 2 broth for 24 h at 37°C, harvested by centrifugation at 30,000 × g for 30 min at 4°C, washed three times by resuspension in phosphate-buffered saline (PBS), and centrifuged as described above for 30 min. After a final wash, the number of color-changing units (CCU) was determined in duplicate by 10-fold titration in the same broth (1). One sample of each bacterial suspension was heat inactivated at 70°C for 10 min in a water bath to explore whether an inflammatory response was triggered by secreted microbial products or by the cell wall components (or the outer plasma membrane for U. urealyticum).
Recovery of plasma from placental blood.
Ten milliliters of placental intervillous space blood from women with spontaneous labor at term was collected in the labor room within 10 min after delivery in heparinized tubes by draining placental cotyledons, as we reported previously (13). Labor was documented by cervical dilation (≥4 cm) and contractility of the myometrium (≥3 contractions of 40 s in 10 min by tocodynamometer) in the presence of membrane rupture. The leukocyte numbers (10,300 ± 312 per ml) and phenotypes (54% ± 6% lymphocytes, 21% ± 3% monocytes, and 33% ± 5% granulocytes) between samples were not significantly different.
Placental-blood treatments.
One-milliliter samples were treated as follows. Two were used to inoculate two Pedi-BacT blood culture bottles (Organon Teknika Corp., Durham, NC) and one each for aerobic and anaerobic conditions. The cultures were mechanically agitated and continuously monitored for growth (BacT/Alert automated system) (19). Subculture of the aerobic blood cultures onto Agar A7 (bioMérieux, France) was used to detect mycoplasma blood infection (30). Negative blood cultures were discarded after a 7-day incubation; samples with a positive culture were excluded from the study. Another placental blood sample was stimulated with 106 CFU of GBS or L. acidophilus or 106 CCU of U. urealyticum (at a ratio of 100 bacteria/leukocyte), the corresponding heat-inactivated bacteria were added to a third sample, and a fourth sample was used as the unstimulated control. All blood samples were incubated for 16 h at 37°C, and the plasma was then separated by centrifugation for 10 min at 2,000 × g. To remove GBS or L. acidophilus, the plasma samples were filtered through a 0.22-μm-pore-size membrane. To eliminate U. urealyticum, the plasma samples were ultracentrifuged at 12,000 × g for 20 min at 4°C. Finally, the plasma samples were tested microbiologically on blood agar plates or MRS agar slopes and in Urea-Arginine LYO 2 broth to ensure that no bacteria were present before coculture with the explants. The protein concentrations in plasma samples were determined by the method of Bradford (8).
Coculture of amniochorion explants and placental plasma.
Amniochorion explants were stimulated with 100 μl of plasma in 900 μl of DMEM supplemented with lactalbumin hydrolysate and cocultured for 12 h at 37°C in 5% CO2. The plasma concentration was chosen after various concentrations were tested (data not shown) and represented a proportion of 1,000 leukocytes per amniochorion explant. Untreated amniochorion explants incubated for the same period as under the coculture conditions were included as a control to rule out preexisting inflammation or other spontaneous release of cytokines. Following incubation, the supernatants were recovered and the total protein concentrations were determined as described above.
Cytokine quantification.
The amounts of IL-1β, TNF-α, and IL-6 in the plasma samples and the supernatants were quantified by enzyme-linked immunosorbent assay (ELISA). Antibodies and recombinant cytokines were purchased from R&D Systems (Minneapolis, MN). Capture monoclonal antibodies were used in combination with biotinylated polyclonal detection antibodies according to the manufacturer's instructions.
Prostaglandin assay.
Concentrations of PGE2 were measured in the plasma samples and the supernatants using a commercially available specific enzyme immunoassay (Amersham Life Science, Buckinghamshire, United Kingdom) according to the manufacturer's instructions.
MMP-9 quantification.
The presence of MMP-9 was tested in the plasma samples and the supernatants by SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) using gelatin zymography, incorporating 1 mg/ml porcine gelatin (Sigma Chemical Co., St. Louis, MO) as the substrate, using a technique described elsewhere (47). Samples of the placental plasma and the supernatants containing 0.5 μg of protein were applied to the gelatin substrate gels under nondenaturing conditions. A standard for MMP-9 gelatinolytic activity obtained from U-937 promyelocyte cell medium (29) was included in each gel. The relative intensities of the lysis bands were quantified by densitometry (NIH Image software). Additionally, total MMP-9 was quantified in the same samples by ELISA (GE Healthcare, Buckinghamshire, United Kingdom). Since this commercial ELISA kit detected both the proenzyme and the active form, the specific quantification of active MMP-9 was assessed using an activity-assay system (GE Healthcare, Buckinghamshire, United Kingdom).
Ultrastructural collagen arrangement.
After coculture, the amniochorion explants were fixed, postfixed, dehydrated, and embedded following standard microscopy procedures to analyze any changes in the collagen fibril distribution. Ultrathin sections (60 to 90 nm) were treated with uranyl acetate and lead citrate to enhance contrast and viewed with a Zeiss EM10 electron microscope. Micrographs were taken using conventional procedures.
Collagen content.
Duplicates from each amniochorion explant of approximately 40 mg were hydrolyzed in 6 N hydrochloric acid at 110°C for 24 h and assayed for hydroxyproline content after the hydrochloric acid was removed (34). A factor of 7.42 was used to convert hydroxyproline to total collagen. The results were expressed as micrograms of collagen per milligram dry tissue.
MMP-9 activity inhibition assay.
Following subtraction of a sample for analysis, the effect of MMP-9 inhibition on amniochorion explants treated with plasma containing live GBS was evaluated by adding 0.1 μg/ml of tissue inhibitor of matrix metalloproteinases 1 (TIMP-1) (R&D Systems, Minneapolis, MN) to cocultures under the experimental conditions previously described. The explants were then processed for electron microscopy, and the supernatants were assayed for total and active MMP-9 as described above.
Statistical analysis.
Five independent experiments (using five different membranes with five different plasma samples) were performed in duplicate (n = 5). The results are expressed as means and standard deviations (SD) and were compared using Friedman's test with the Bonferroni correction for multiple comparisons. A P value of ≤0.05 was considered statistically significant.
RESULTS
Stimulation of placental blood with GBS induces an increase in TNF-α and IL-6 plasma levels, whereas U. urealyticum increases IL-1β and PGE2.
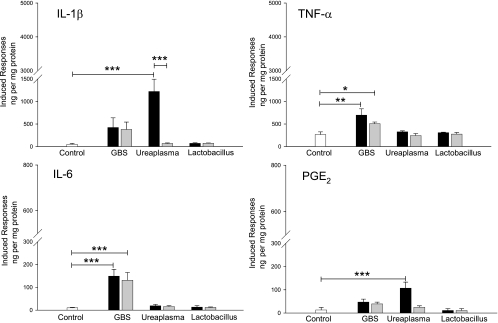
To study whether intrauterine leukocytes might trigger differential secretion of mediators depending on the infecting microorganism, we first evaluated the specific response of placental leukocytes to GBS and U. urealyticum. The concentrations of IL-1β, TNF-α, IL-6, and PGE2 in the plasma after infection of the placental blood are shown in Fig. 1. Basal levels of all markers were found in untreated plasma samples (before bacterial stimulus), which increased after incubation with the pathogenic bacteria. Whereas live or heat-inactivated GBS induced TNF-α (P = 0.001 and P = 0.05, respectively) and IL-6 (P < 0.001) levels significantly higher than those of the control, incubation with live U. urealyticum stimulated significant increases in IL-1β (P < 0.001) and PGE2 (P < 0.001), which were not observed when the bacteria had been previously inactivated. Plasma samples derived from stimulation with L. acidophilus showed no differences in the levels of these cytokines compared with the basal levels.
FIG. 1.
Pathogenic bacteria induce differential secretion of inflammatory mediators by leukocytes circulating in placental blood. Stimulation with live or heat-inactivated GBS significantly increased TNF-α and IL-6 in plasma from placental blood, while live U. urealyticum significantly increased IL-1β and PGE2. The bars represent the concentration of each marker in plasma from nonstimulated placental blood (white) or plasma from placental blood stimulated with live (black) or heat-inactivated (gray) bacteria. The data presented are means and SD of duplicate measurements from five separate experiments (***, P < 0.001; **, P = 0.001; *, P = 0.05).
Plasma from placental blood stimulated with pathogenic bacteria induces an additional inflammatory response by the amniochorion.
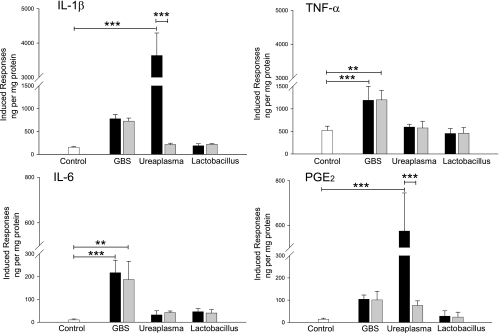
We next studied whether inflammatory molecules secreted by bacterium-activated placental leukocytes stimulate amniochorion production of mediators involved in human labor. Figure 2 shows the concentrations of IL-1β, TNF-α, IL-6, and PGE2 in supernatants of amniochorion explants cocultured with plasma from placental blood previously stimulated with bacteria. Plasma from blood stimulated with both live and heat-inactivated GBS induced a significant increase in TNF-α (P < 0.001 and P = 0.005). Similarly, both live and heat-inactivated bacteria significantly increased the IL-6 production of amniochorion explants about 20-fold (P < 0.001 and P = 0.001, respectively).
FIG. 2.
Plasma from placental blood stimulated with pathogenic bacteria induces an additional inflammatory response by the amniochorion. Coculture with plasmas from placental blood stimulated with live or heat-inactivated GBS significantly increased secretion of TNF-α and IL-6 by amniochorion explants, while live U. urealyticum significantly increased IL-1β and PGE2. The bars represent the concentration of each marker in supernatants of amniochorion explants cocultured with 10% plasma from nonstimulated placental blood (white) or plasma from placental blood stimulated with live (black) or heat-inactivated (gray) bacteria. The final values were calculated by subtracting the original quantities in the plasma and untreated explants; thus, the bars reflect only the induced responses in the explants activated with the corresponding plasma. The data presented are means and SD of duplicate measurements from five separate experiments (***, P < 0.001; **, P ≤ 0.005).
Interestingly, explants treated with plasma from placental blood stimulated with live U. urealyticum produced the greatest amount of IL-1β among the bacterial treatments. Whereas U. urealyticum induced a 24-fold increase compared to the control (P < 0.001), GBS and L. acidophilus induced only 5- and 1.2-fold increases, respectively. Response to U. urealyticum decreased significantly (to just 1.4-fold) (P < 0.001) when the bacteria were heat inactivated. A similar situation was observed for PGE2, where plasma conditioned with live U. urealyticum induced the highest secretion (41-fold) (P < 0.001) and bacterial inactivation significantly reduced this response (to 5.4-fold) (P < 0.001).
The amniochorion secretes MMP-9 in response to the addition of plasma conditioned with GBS.
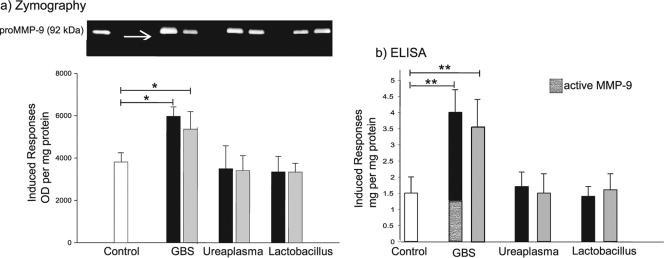
Elevation of MMP-9 in amniotic fluid and the amniochorion has been implicated in both physiologic and pathological rupture of the fetal membranes (22, 47, 48). We evaluated if plasma samples derived from the initial interaction between bacteria and placental blood stimulated the secretion of MMP-9 by the amniochorion. The enzyme was undetectable by gelatin zymography and ELISA in plasma samples from noninfected and infected placental blood. Zymography (Fig. 3a) showed that membranes incubated with plasma from placental blood stimulated with live or inactivated GBS produced significantly more pro-MMP-9 than did the control (P = 0.03 and P = 0.05, respectively). In addition, the active form of the enzyme was observed only after treatment with live GBS. Quantification of total and active MMP-9 in the supernatants confirmed the zymography findings (Fig. 3b). Plasma conditioned with U. urealyticum or L. acidophilus did not induce an increase in pro-MMP-9 activity.
FIG. 3.
The amniochorion secretes MMP-9 in response to plasma from placental blood stimulated with GBS. (a) Representative zymography gel (0.5 μg of protein per lane). The bars represent the OD (optical densities) of pro-MMP-9 in supernatants of amniochorion explants cocultured with 10% plasma from nonstimulated placental blood (white) or plasma from placental blood stimulated with live (black) or heat-inactivated (gray) bacteria. (b) The bars represent the total MMP-9 (proenzyme and active enzyme) quantified by ELISA in the same supernatants. Coculture with plasma from placental blood stimulated with live or inactivated GBS significantly increased secretion of MMP-9 by the amniochorion explants (**, P ≤ 0.005; *, P ≤ 0.05). Active MMP-9 was detected only after treatment with plasma conditioned with live GBS in both experiments (arrow on the zymography gel in panel a). For both experiments, the final values were calculated by subtracting the original quantity secreted by untreated explants; thus, the bars reflect only the induced responses in the explants. Five independent experiments in duplicate are expressed as means and SD.
The amniotic compact layer shows a decrease in collagen content and extensive ultrastructural collagen damage only after incubation with plasma conditioned with GBS.
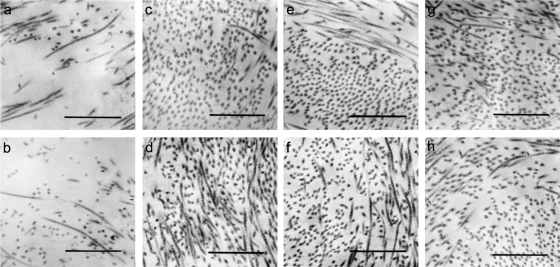
Rupture of the fetal membranes is accompanied by alterations in amniotic collagen structure (13, 32). Since collagen degradation is mediated primarily by MMPs, we investigated if the increase in MMP-9 results in a controlled degradation of collagen within the fetal membranes. Electron microscopy (Fig. 4) showed that the collagen fibrils in the amniotic compact layer of explants incubated with control plasma had a homogeneous distribution, occurring in compact wavy bundles with a defined orientation (Fig. 4g). However, extensive connective tissue damage was observed when the explants were treated with plasma from placental blood stimulated with GBS, either live (4a) or heat inactivated (4b), where a loss of collagen fibrils with amorphous material between them was observed. Moreover, weaker staining of these fibrils was indicative of damage to the three-dimensional structure of the collagen. These changes in collagen arrangement were correlated with a significant decrease in collagen content in the amniochorion explants (P < 0.001) (Table 1). No changes in collagen content (Table 1) or structure were observed in the explants treated with plasma conditioned with U. urealyticum (Fig. 4c and d) or L. acidophilus (Fig. 4e and f), regardless of whether the bacteria were live or heat inactivated.
FIG. 4.
Plasma from placental blood stimulated with GBS induced extracellular matrix degradation in the amniochorion. Micrographs of the amniotic compact layer of the explants exposed to 10% plasma from placental blood stimulated with live (a) or heat-inactivated (b) GBS exhibit a drastic loss of collagen fibrils with amorphous material between them after 12 h of treatment. These alterations were inhibited by the addition of 0.1 μg/ml TIMP-1 (h), an MMP-9 inhibitor. Collagen fibrils appeared normally organized in bundles with specific orientation in explants treated with plasma from placental blood stimulated with live (c) or heat-inactivated (d) U. urealyticum and live (e) or heat-inactivated (f) L. acidophilus, as well as in control explants (g). Magnification, ×60,000. Bars, 0.0332 μm.
TABLE 1.
Collagen contents of amniochorion explants after coculture with plasma samples obtained from infected placental blood
| Plasma | Collagen content (μg/mg) in explanta | P value (treatment vs. control) |
|---|---|---|
| Controlb | 12.20 ± 2.13 | |
| Live GBS | 2.84 ± 1.05 | <0.001 |
| Heat-inactivated GBS | 3.02 ± 1.70 | <0.001 |
| Live GBS + TIMP-1 | 11.30 ± 3.05 | NSc |
| Live U. urealyticum | 12.44 ± 1.78 | NS |
| Heat-inactivated U. urealyticum | 12.28 ± 1.62 | NS |
| Live L. acidophilus | 12.50 ± 1.09 | NS |
| Heat-inactivated L. acidophilus | 11.82 ± 2.00 | NS |
The values are means ± SD (5 samples per group in duplicate).
The control was plasma from noninfected placental blood.
NS, not significant.
TIMP-1 inhibits collagen degradation and MMP-9 activation in explants treated with plasma conditioned with GBS.
To further explore if the damage to the collagen network observed in the amniochorion was mediated specifically by MMP-9, we blocked the activity of the enzyme using TIMP-1, its natural inhibitor. The inhibition assay of MMP-9 activity showed that TIMP-1 treatment did not affect levels of MMP-9 released by the explants cocultured with plasma from placental blood stimulated with live GBS (4.0 ± 0.7 μg/mg protein without TIMP-1 versus 3.9 ± 0.9 μg/mg protein when the inhibitor was added; P > 0.05). However, active MMP-9 was reduced after TIMP-1 treatment from 1.2 ± 0.3 μg/mg protein to undetectable levels (P < 0.001). Although the use of this MMP-9 inhibitor in the cocultures with plasma conditioned with live GBS resulted in the preservation of the collagen fibrils, increased spacing between them was observed in some localized areas (Fig. 4h). However, the collagen content in these explants was not significantly different from that of the controls without treatment (Table 1), demonstrating that the collagenolytic activity was mediated almost completely by MMP-9.
DISCUSSION
Intrauterine infection by pathogenic bacteria and the consequent inflammatory response in the choriodecidual tissues have been implicated in preterm labor (14). Available in vitro experimental models have been used to evaluate the effects of direct contact between bacteria and the fetal membranes (20, 50, 51). However, no exploration of the complete choriodecidual microenvironment has been possible because of methodological difficulties. Uterine detachment of the membranes after cesarean section or labor necessarily destroys the choriodecidual compartment, and the integrated responses of all the cellular components at this site cannot be duplicated in vitro. The choriodecidual microenvironment is composed of maternal and fetal cells. In addition to the decidua and other uterine cells, the mother contributes a variable number of leukocytes, which invade the amnion and chorion as part of the maternal inflammatory response linked to normal labor or in increased quantities under intrauterine infection (44). Because the fetal membranes are predominantly avascular, the secretion of chemotactic signals may control the arrival of leukocytes in the choriodecidual compartment from the local circulation. Preliminary evidence of the existence of these signals has recently been demonstrated (15). These findings further support the existence of a specific microenvironment in the intrauterine circulating blood characterized by enrichment in specific subpopulations of leukocytes during labor, which are already programmed to induce biochemical changes in the reproductive tissues, such as degradation of the extracellular matrix in the amniochorion (13).
In this study, we explored the effects on the amniochorion of soluble products derived from placental blood cells (primary local candidates to infiltrate these tissues) that were previously stimulated with well-characterized pathogenic bacteria. This two-step experimental in vitro model was designed to resemble the response of the amniochorion to infection using a choriodecidual microenvironmental reconstruction, with the addition of local leukocytes as a third component. GBS and U. urealyticum were chosen for placental blood stimulation because they are implicated in the pathogenesis of the premature rupture of membranes (2, 16, 35). L. acidophilus, a nonpathogenic member of the normal vaginal flora, was used as the negative control.
The pathology of infection-induced rupture of the fetal membranes has been attributed to the secretion of proinflammatory cytokines, such as IL-1β and TNF-α, by gestational tissues in response to bacterial products (33). At the same time, these cytokines may stimulate the biosynthesis of IL-6 by decidual cells (12), which is considered a responsive indicator of intrauterine infection in pregnant women (7). We demonstrated the presence of IL-1β, TNF-α, IL-6, and PGE2 in the plasma of the placental blood from women in active labor in the absence of infection. However, the presence of the pathogenic microorganisms GBS and U. urealyticum stimulated greater secretion of these markers by circulating placental leukocytes. The secretion of these mediators by the explants also increased significantly after incubation with plasma conditioned by incubation of bacteria with leukocytes. We interpret this as evidence of the existence of a signaling network that involves the response of resident cells of the amniochorion to products secreted by activated leukocytes as a consequence of the natural immunological response to infection.
Although it is not clear which cytokine(s) is required, evidence suggests that the production of proinflammatory cytokines may be crucial in the final pathway toward the rupture of the fetal membranes (7, 27, 28). Their roles may include the upregulation of matrix metalloproteinase production (33, 43), principally MMP-9 (3), which is known to participate in the pathophysiological rupture of the amniochorion (47, 48). MMP-9 is also increased in the amniotic fluid of patients with cervicovaginal infection (22), making it a biochemical indicator of intra-amniotic infection and ultimately a predictive element in the development of labor or premature rupture of the membranes. In this study, we observed a significant increase in pro-MMP-9 activity and identified the active form only in the supernatants from explants incubated with plasma from placental blood stimulated with either live or heat-inactivated GBS. Since the amniotic compact layer in these explants showed collagen fibril degradation that was inhibited by the addition of TIMP-1, it is reasonable to infer that MMP-9 is responsible for this collagenolytic activity. The fact that the collagen explants were damaged after incubation with plasma exposed to heat-inactivated GBS suggests the existence of a virulence factor that maintains its activity independently of bacterial viability. These results support the proposition that even nonviable GBS mediate the inflammatory response via peptidoglycan and lipoteichoic acid, essential components of the cell wall (39).
The presence of proinflammatory cytokines leads to the production of PGE2 by the human amnion and decidual cells, which then causes cervical softening and uterine contractions (26, 38, 40). Our data indicate that microorganisms vary in their abilities to stimulate PGE2 production. In our in vitro model, the infection of placental blood with U. urealyticum induced PGE2 secretion by the explants, which greatly exceeded that caused by any other microorganism tested, suggesting that U. urealyticum has a particularly high capacity to stimulate the production of this mediator. Because this phenomenon was observed only when U. urealyticum had not been heat inactivated, we consider that the material responsible for this stimulation is secreted by the bacteria. The induction of PGE2 secretion is consistent with the increase in IL-1β and with reports that this cytokine stimulates prostaglandin production (27, 38). The activity of phospholipases secreted by U. urealyticum (10) produces free arachidonic acid, which can serve as a substrate for a variety of eicosanoid biosynthetic pathways, producing biologically active products leading to uterine contractions, cervical dilation, and the rupture of the fetal membrane (4).
The differences observed in the production of the cytokines PGE2 and MMP-9 by placental leukocytes suggest that the locally circulating cells are able to respond specifically to the infectious agents. Furthermore, in some cases, this differential response seems to be dependent on the viability of the microorganisms, because the secretion of effector molecules into the plasma was altered when the bacteria were inactivated.
Our results also suggest that, in the presence of infection, the mechanisms leading to the rupture of the fetal membranes appear to be dependent on the nature of the infecting microorganism. Whereas GBS may primarily induce the rupture of the membranes through the production of MMP-9, probably via TNF-α and IL-6, U. urealyticum may activate uterine contractions and cervical dilation by increasing PGE2 production, probably via IL-1β. This suggests that even though both can be considered pathogenic microorganisms, their specific recognition by leukocytes could trigger distinct transduction pathways, inducing the production of unique cytokines and different patterns of secondary effectors. It is known that GBS is the most provocative agent for triggering host cytokine cascades, in particular TNF-α (11). In contrast, U. urealyticum stimulates the production of several cytokines, but this stimulation apparently is different depending on the concentration of the microorganism and the type of infected cell, which suggests that effects of U. urealyticum may be cell type specific (23).
The model presented in this study is relevant because it suggests that the immunological cells circulating within the uterine microenvironment respond to an infectious stimulus with the production of inflammatory mediators and MMP-9. These not only stimulate other immunological cells, but also induce local tissue cells to secrete a second wave of molecular signals and effectors, including additional amounts of IL-1β, TNF-α, and MMP-9, leading to the induction of mechanisms that culminate in rupture. Better knowledge of the signaling processes between local tissue cells and those that arrive in the environment of the placenta and fetal membranes, as well as the mediators involved in such communication, will eventually allow the development of interventional therapies to control the premature rupture of the fetal membranes.
Acknowledgments
D.M.O. is supported by an Alberta Heritage Foundation for Medical Research Interdisciplinary Preterm Birth and Healthy Outcomes Team award. F. V.-O. was supported by SALUD-69353.
Editor: A. Camilli
Footnotes
Published ahead of print on 30 August 2010.
REFERENCES
- 1.Aaltonen, R., J. Heikkinen, T. Vahlberg, J. S. Jensen, and A. Alanen. 2007. Local inflammatory response in choriodecidua induced by Ureaplasma urealyticum. BJOG 114:1432-1435. [DOI] [PubMed] [Google Scholar]
- 2.Alger, L. S., J. C. Lovchik, J. R. Hebel, L. R. Blackmon, and M. C. Crenshaw. 1988. The association of Chlamydia trachomatis, Neisseria gonorrhoeae, and group B streptococci with preterm rupture of the membranes and pregnancy outcome. Am. J. Obstet Gynecol. 159:397-404. [DOI] [PubMed] [Google Scholar]
- 3.Arechavaleta-Velasco, F., D. Ogando, S. Parry, and F. Vadillo-Ortega. 2002. Production of matrix metalloproteinase-9 in lipopolysaccharide-stimulated human amnion occurs through an autocrine and paracrine proinflammatory cytokine-dependent system. Biol. Reprod. 67:1952-1958. [DOI] [PubMed] [Google Scholar]
- 4.Bejar, R., V. Curbelo, C. Davis, and L. Gluck. 1981. Premature labor. II. Bacterial sources of phospholipase. Obstet. Gynecol. 57:479-482. [PubMed] [Google Scholar]
- 5.Bennett, P. R., M. P. Rose, L. Myatt, and M. G. Elder. 1987. Preterm labor: stimulation of arachidonic acid metabolism in human amnion cells by bacterial products. Am. J. Obstet Gynecol. 156:649-655. [DOI] [PubMed] [Google Scholar]
- 6.Bokström, H., M. Brannstrom, M. Alexandersson, and A. Norstrom. 1997. Leukocyte subpopulations in the human uterine cervical stroma at early and term pregnancy. Hum. Reprod. 12:586-590. [DOI] [PubMed] [Google Scholar]
- 7.Bowen, J. M., L. Chamley, J. A. Keelan, and M. D. Mitchell. 2002. Cytokines of the placenta and extra-placental membranes: roles and regulation during human pregnancy and parturition. Placenta 23:257-273. [DOI] [PubMed] [Google Scholar]
- 8.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 9.Bry, K., M. Hallman, and U. Lappalainen. 1994. Cytokines released by granulocytes and mononuclear cells stimulate amnion cell prostaglandin E2 production. Prostaglandins 48:389-399. [DOI] [PubMed] [Google Scholar]
- 10.De Silva, N. S., and P. A. Quinn. 1991. Localization of endogenous activity of phospholipases A and C in Ureaplasma urealyticum. J. Clin. Microbiol. 29:1498-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doran, K. S., and V. Nizet. 2004. Molecular pathogenesis of neonatal group B streptococcal infection: no longer in its infancy. Mol. Microbiol. 54:23-31. [DOI] [PubMed] [Google Scholar]
- 12.Dudley, D. J., M. S. Trautman, B. A. Araneo, S. S. Edwin, and M. D. Mitchell. 1992. Decidual cell biosynthesis of interleukin-6: regulation by inflammatory cytokines. J. Clin. Endocrinol. Metab. 74:884-889. [DOI] [PubMed] [Google Scholar]
- 13.Estrada-Gutierrez, G., V. Zaga, M. A. Gonzalez-Jimenez, J. Beltran-Montoya, R. Maida-Claros, S. Giono-Cerezo, and F. Vadillo-Ortega. 2005. Initial characterization of the microenvironment that regulates connective tissue degradation in amniochorion during normal human labor. Matrix Biol. 24:306-312. [DOI] [PubMed] [Google Scholar]
- 14.Goldenberg, R. L., J. C. Hauth, and W. W. Andrews. 2000. Intrauterine infection and preterm delivery. N. Engl. J. Med. 342:1500-1507. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Lopez, N., G. Estrada-Gutierrez, L. Jimenez-Zamudio, R. Vega-Sanchez, and F. Vadillo-Ortega. 2009. Fetal membranes exhibit selective leukocyte chemotaxic activity during human labor. J. Reprod. Immunol. 80:122-131. [DOI] [PubMed] [Google Scholar]
- 16.Gravett, M. G., and D. A. Eschenbach. 1986. Possible role of Ureaplasma urealyticum in preterm premature rupture of the fetal membranes. Pediatr. Infect. Dis. 5:S253-S257. [DOI] [PubMed] [Google Scholar]
- 17.Henneke, P., O. Takeuchi, R. Malley, E. Lien, R. R. Ingalls, M. W. Freeman, T. Mayadas, V. Nizet, S. Akira, D. L. Kasper, and D. T. Golenbock. 2002. Cellular activation, phagocytosis, and bactericidal activity against group B streptococcus involve parallel myeloid differentiation factor 88-dependent and independent signaling pathways. J. Immunol. 169:3970-3977. [DOI] [PubMed] [Google Scholar]
- 18.Keski-Nisula, L. T., M. L. Aalto, P. P. Kirkinen, V. M. Kosma, and S. T. Heinonen. 2003. Myometrial inflammation in human delivery and its association with labor and infection. Am. J. Clin. Pathol. 120:217-224. [DOI] [PubMed] [Google Scholar]
- 19.Krisher, K. K., D. R. Whyburn, and F. E. Koepnick. 1993. Comparison of the BacT/Alert pediatric blood culture system, Pedi-BacT, with conventional culture using the 20-milliliter Becton-Dickinson supplemented peptone broth tube. J. Clin. Microbiol. 31:793-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamont, R. F., F. Anthony, L. Myatt, L. Booth, P. M. Furr, and D. Taylor-Robinson. 1990. Production of prostaglandin E2 by human amnion in vitro in response to addition of media conditioned by microorganisms associated with chorioamnionitis and preterm labor. Am. J. Obstet. Gynecol. 162:819-825. [DOI] [PubMed] [Google Scholar]
- 21.Lamont, R. F., M. Rose, and M. G. Elder. 1985. Effect of bacterial products on prostaglandin E production by amnion cells. Lancet ii:1331-1333. [DOI] [PubMed] [Google Scholar]
- 22.Locksmith, G. J., P. Clark, P. Duff, and G. S. Schultz. 1999. Amniotic fluid matrix metalloproteinase-9 levels in women with preterm labor and suspected intra-amniotic infection. Obstet. Gynecol. 94:1-6. [DOI] [PubMed] [Google Scholar]
- 23.Manimtim, W. M., J. D. Hasday, L. Hester, K. D. Fairchild, J. C. Lovchik, and R. M. Viscardi. 2001. Ureaplasma urealyticum modulates endotoxin-induced cytokine release by human monocytes derived from preterm and term newborns and adults. Infect. Immun. 69:3906-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLean, N. W., and I. J. Rosenstein. 2000. Characterisation and selection of a Lactobacillus species to re-colonise the vagina of women with recurrent bacterial vaginosis. J. Med. Microbiol. 49:543-552. [DOI] [PubMed] [Google Scholar]
- 25.Menon, R., K. F. Swan, T. W. Lyden, N. S. Rote, and S. J. Fortunato. 1995. Expression of inflammatory cytokines (interleukin-1 beta and interleukin-6) in amniochorionic membranes. Am. J. Obstet. Gynecol. 172:493-500. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell, M. D., S. Edwin, and R. J. Romero. 1990. Prostaglandin biosynthesis by human decidual cells: effects of inflammatory mediators. Prostaglandins Leukot. Essent. Fatty Acids 41:35-38. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell, M. D., S. S. Edwin, S. Lundin-Schiller, R. M. Silver, D. Smotkin, and M. S. Trautman. 1993. Mechanism of interleukin-1 beta stimulation of human amnion prostaglandin biosynthesis: mediation via a novel inducible cyclooxygenase. Placenta 14:615-625. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell, M. D., M. S. Trautman, and D. J. Dudley. 1993. Cytokine networking in the placenta. Placenta 14:249-275. [DOI] [PubMed] [Google Scholar]
- 29.Morodomi, T., Y. Ogata, Y. Sasaguri, M. Morimatsu, and H. Nagase. 1992. Purification and characterization of matrix metalloproteinase 9 from U937 monocytic leukaemia and HT1080 fibrosarcoma cells. Biochem. J. 285:603-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neman-Simha, V., H. Renaudin, B. de Barbeyrac, J. J. Leng, J. Horovitz, D. Dallay, C. Billeaud, and C. Bebear. 1992. Isolation of genital mycoplasmas from blood of febrile obstetrical-gynecologic patients and neonates. Scand. J. Infect. Dis. 24:317-321. [DOI] [PubMed] [Google Scholar]
- 31.Osman, I., A. Young, M. A. Ledingham, A. J. Thomson, F. Jordan, I. A. Greer, and J. E. Norman. 2003. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol. Hum. Reprod. 9:41-45. [DOI] [PubMed] [Google Scholar]
- 32.Parry, S., and J. F. Strauss III. 1998. Premature rupture of the fetal membranes. N. Engl. J. Med. 338:663-670. [DOI] [PubMed] [Google Scholar]
- 33.Peltier, M. R. 2003. Immunology of term and preterm labor. Reprod. Biol. Endocrinol. 1:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reddy, G. K., and C. S. Enwemeka. 1996. A simplified method for the analysis of hydroxyproline in biological tissues. Clin. Biochem. 29:225-229. [DOI] [PubMed] [Google Scholar]
- 35.Regan, J. A., S. Chao, and L. S. James. 1981. Premature rupture of membranes, preterm delivery, and group B streptococcal colonization of mothers. Am. J. Obstet. Gynecol. 141:184-186. [DOI] [PubMed] [Google Scholar]
- 36.Reisenberger, K., C. Egarter, M. Knofler, I. Schiebel, H. Gregor, A. M. Hirschl, G. Heinze, and P. Husslein. 1998. Cytokine and prostaglandin production by amnion cells in response to the addition of different bacteria. Am. J. Obstet. Gynecol. 178:50-53. [DOI] [PubMed] [Google Scholar]
- 37.Roh, C. R., W. J. Oh, B. K. Yoon, and J. H. Lee. 2000. Up-regulation of matrix metalloproteinase-9 in human myometrium during labour: a cytokine-mediated process in uterine smooth muscle cells. Mol. Hum. Reprod. 6:96-102. [DOI] [PubMed] [Google Scholar]
- 38.Romero, R., S. Durum, C. A. Dinarello, E. Oyarzun, J. C. Hobbins, and M. D. Mitchell. 1989. Interleukin-1 stimulates prostaglandin biosynthesis by human amnion. Prostaglandins 37:13-22. [DOI] [PubMed] [Google Scholar]
- 39.Romero, R., J. Espinoza, L. F. Goncalves, J. P. Kusanovic, L. Friel, and S. Hassan. 2007. The role of inflammation and infection in preterm birth. Semin. Reprod. Med. 25:21-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romero, R., M. Mazor, Y. K. Wu, C. Avila, E. Oyarzun, and M. D. Mitchell. 1989. Bacterial endotoxin and tumor necrosis factor stimulate prostaglandin production by human decidua. Prostaglandins Leukot. Essent. Fatty Acids 37:183-186. [DOI] [PubMed] [Google Scholar]
- 41.Romero, R., M. Mazor, Y. K. Wu, M. Sirtori, E. Oyarzun, M. D. Mitchell, and J. C. Hobbins. 1988. Infection in the pathogenesis of preterm labor. Semin. Perinatol. 12:262-279. [PubMed] [Google Scholar]
- 42.Sato, T. A., J. A. Keelan, and M. D. Mitchell. 2003. Critical paracrine interactions between TNF-alpha and IL-10 regulate lipopolysaccharide-stimulated human choriodecidual cytokine and prostaglandin E2 production. J. Immunol. 170:158-166. [DOI] [PubMed] [Google Scholar]
- 43.So, T., A. Ito, T. Sato, Y. Mori, and S. Hirakawa. 1992. Tumor necrosis factor-alpha stimulates the biosynthesis of matrix metalloproteinases and plasminogen activator in cultured human chorionic cells. Biol. Reprod. 46:772-778. [DOI] [PubMed] [Google Scholar]
- 44.Steel, J. H., K. O'Donoghue, N. L. Kennea, M. H. Sullivan, and A. D. Edwards. 2005. Maternal origin of inflammatory leukocytes in preterm fetal membranes, shown by fluorescence in situ hybridisation. Placenta 26:672-677. [DOI] [PubMed] [Google Scholar]
- 45.Tannock, G. W. 1999. Identification of lactobacilli and bifidobacteria. Curr. Issues Mol. Biol. 1:53-64. [PubMed] [Google Scholar]
- 46.Thomson, A. J., J. F. Telfer, A. Young, S. Campbell, C. J. Stewart, I. T. Cameron, I. A. Greer, and J. E. Norman. 1999. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum. Reprod. 14:229-236. [PubMed] [Google Scholar]
- 47.Vadillo-Ortega, F., G. Gonzalez-Avila, E. E. Furth, H. Lei, R. J. Muschel, W. G. Stetler-Stevenson, and J. F. Strauss III. 1995. 92-kd type IV collagenase (matrix metalloproteinase-9) activity in human amniochorion increases with labor. Am. J. Pathol. 146:148-156. [PMC free article] [PubMed] [Google Scholar]
- 48.Vadillo-Ortega, F., A. Hernandez, G. Gonzalez-Avila, L. Bermejo, K. Iwata, and J. F. Strauss III. 1996. Increased matrix metalloproteinase activity and reduced tissue inhibitor of metalloproteinases-1 levels in amniotic fluids from pregnancies complicated by premature rupture of membranes. Am. J. Obstet. Gynecol. 174:1371-1376. [DOI] [PubMed] [Google Scholar]
- 49.Vega-Sanchez, R., N. Gomez-Lopez, A. Flores-Pliego, S. Clemente-Galvan, G. Estrada-Gutierrez, A. Zentella-Dehesa, R. Maida-Claros, J. Beltran-Montoya, and F. Vadillo-Ortega. 2010. Placental blood leukocytes are functional and phenotypically different than peripheral leukocytes during human labor. J. Reprod. Immunol. 84:100-110. [DOI] [PubMed] [Google Scholar]
- 50.Zaga, V., G. Estrada-Gutierrez, J. Beltran-Montoya, R. Maida-Claros, R. Lopez-Vancell, and F. Vadillo-Ortega. 2004. Secretions of interleukin-1beta and tumor necrosis factor alpha by whole fetal membranes depend on initial interactions of amnion or choriodecidua with lipopolysaccharides or group B streptococci. Biol. Reprod. 71:1296-1302. [DOI] [PubMed] [Google Scholar]
- 51.Zaga-Clavellina, V., G. G. Lopez, G. Estrada-Gutierrez, A. Martinez-Flores, R. Maida-Claros, J. Beltran-Montoya, and F. Vadillo-Ortega. 2006. Incubation of human chorioamniotic membranes with Candida albicans induces differential synthesis and secretion of interleukin-1beta, interleukin-6, prostaglandin E, and 92 kDa type IV collagenase. Mycoses 49:6-13. [DOI] [PubMed] [Google Scholar]
- 52.Zlatnik, F. J., T. M. Gellhaus, J. A. Benda, F. P. Koontz, and L. F. Burmeister. 1990. Histologic chorioamnionitis, microbial infection, and prematurity. Obstet. Gynecol. 76:355-359. [PubMed] [Google Scholar]