Abstract
DDX3 belongs to the DEAD box family of RNA helicases, but the details of its biological function remain largely unclear. Here we show that knockdown of DDX3 expression impedes G1/S-phase transition of the cell cycle. To know how DDX3 may act in cell cycle control, we screened for cellular mRNA targets of DDX3. Many of the identified DDX3 targets encoded cell cycle regulators, including G1/S-specific cyclin E1. DDX3 depletion specifically downregulates translation of cyclin E1 mRNA. Moreover, our data suggest that DDX3 participates in translation initiation of targeted mRNAs as well as in cell growth control via its RNA helicase activity. Consistent with these findings, we show that in the temperature-sensitive DDX3 mutant hamster cell line tsET24, cyclin E1 expression is downregulated at a nonpermissive temperature that inactivates mutant DDX3. Taken together, our results indicate that DDX3 is critical for translation of cyclin E1 mRNA, which provides an alternative mechanism for regulating cyclin E1 expression during the cell cycle.
The DEAD box family of RNA helicases plays diverse roles in eukaryotic gene expression, including transcriptional and posttranscriptional regulation (8, 40). These helicases contain a highly conserved catalytic core domain that mediates ATP binding, in addition to their ATPase and helicase activities. They are presumed to function in unwinding RNA duplexes or remodeling RNA-protein complexes in an ATP-dependent manner. However, their cellular functions and localization may be defined largely by the divergent sequences flanking the catalytic core domain.
Human DDX3 is a DEAD box RNA helicase that participates in various aspects of mRNA metabolism, including translation. The role of DDX3 in translational control is phylogenetically conserved (47). The DDX3 homolog in Saccharomyces cerevisiae, Ded1, participates in translation initiation (6, 9). Analogously, in the fission yeast Schizosaccharomyces pombe, Ded1 is implicated in translational control of two B-type cyclin genes, Cig2 and Cdc13, whose mRNAs contain a complex structure in the 5′ untranslated region (5′ UTR) (16). Indeed, Ded1 facilitates efficient RNA duplex unwinding, thus supporting its role in ribosome scanning at the translation initiation step (32). We recently reported that although DDX3 is dispensable for general mRNA translation, it is required for efficient translation of mRNAs that contain a long or structured 5′ UTR (25). How the biochemical activity of DDX3 contributes to its function, however, remains unclear.
In this study, we found that DDX3 targets the mRNA encoding the cell cycle regulator cyclin E1. The eukaryotic cell cycle is driven by a series of cyclin-dependent kinases (Cdks), which are activated via association with their respective cyclin partners (31). Cyclins D and E are specific for cell cycle progression from G1 to S phase. In early G1 phase, activated cyclin D-Cdk4/6 phosphorylates the retinoblastoma protein (pRb), which in turn releases E2F from the E2F-pRb complex and stimulates E2F-mediated transcription of cell cycle genes, including E-type cyclin genes. Cyclin E-Cdk2 further activates downstream genes involved in S-phase entry and DNA replication. Cyclin E-Cdk2 also phosphorylates pRb, which thus leads to positive-feedback regulation between E2F and cyclin E (20, 35). Furthermore, cyclin E promotes DNA replication, and even oncogenic transformation, in a Cdk-independent manner (14, 15, 33). Levels of both monomeric cyclin E and the cyclin E-Cdk2 complex are tightly regulated by proteolysis via the ubiquitin-proteasome system (7, 34, 44). Deregulation of cyclin E not only perturbs cell cycle progression but also may cause genomic instability and centrosome amplification (28, 45). Recent studies have revealed that cyclin E is frequently overexpressed in various human tumors, due primarily to disruption of its proteolysis or the upregulated transcription of its corresponding gene (20, 35, 46). Therefore, cyclin E is critical for normal cell growth, and thus its level must be controlled precisely.
We found that DDX3 plays a role in cell cycle control. A screen for mRNA targets of DDX3 identified cyclin E1 mRNA as a candidate. We demonstrate that DDX3 indeed modulates translation initiation of the cyclin E1 mRNA.
MATERIALS AND METHODS
Plasmids.
pSilencer-derived vectors expressing short hairpin RNAs (shRNAs) targeting human DDX3 have been described previously (25). An shRNA targeting eIF4A1 coding region nucleotides (nt) 226 to 244 was also constructed in the pSilencer 1.0-U6 vector (Ambion). Expression vectors encoding wild-type FLAG-tagged DDX3, shRNA1-resistant FLAG-DDX3, FLAG-S382L mutant DDX3, and glutathione S-transferase (GST)-DDX3 have also been described (25). To generate antisense probes for RNase protection assays (RPAs), cDNA fragments for human cyclin E1 (nucleotides 375 to 730 of the protein coding region) and cyclin D1 (nucleotides 184 to 424 of the protein coding region) were cloned into the pGEM-T vector (Promega). The control reporter pRL-SV40 (Promega), used for in vivo translation assays, was described previously (25). pFL-SV40-E1 was constructed by inserting the human cyclin E1 5′ UTR sequence upstream of the firefly luciferase coding region in pFL-SV40 (25). A cDNA fragment carrying the human cyclin E1 open reading frame was cloned into hemagglutinin (HA) tag-containing pCEP4 (Invitrogen), generating the pCEP4-E1-HA plasmid. Afterward, cDNA fragments carrying the human cyclin E1 and D1 5′ UTRs were inserted into the pCEP4-E1-HA plasmid, using HindIII, to generate EUE and DUE reporters, respectively.
Cell culture, transfection, and generation of stable clones.
HeLa and HEK-293 cell culture was carried out essentially as we described previously (24). H1299 and A549 cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco), and U2OS cells were cultured in McCoy's 5A medium (Gibco) at 37°C; both media were supplemented with 10% fetal bovine serum (FBS). Hamster tsET24 cells were kindly given by Takeshi Sekiguchi (Kyushu, Japan) and were cultured essentially the same as HeLa cells, except that tsET24 cells were maintained in 10% CO2 and at 33.5°C (12). To induce the temperature-sensitive (TS) phenotype, tsET24 cells were shifted to 39.5°C (12). Cell transfection was performed using Lipofectamine 2000 (Invitrogen) essentially according to the manufacturer's instructions. To measure the cell growth rate, HeLa cells were transfected with an shRNA expression vector for 48 h. Transfected cells were then seeded in 6-well culture dishes (2 × 105 cells/well) and cultured for another 1 to 3 days. Cells were harvested by trypsinization and counted with a hemocytometer. For cyclin E1 rescue assay, 2 μg each of the cyclin E1 expression vector (pCEP4-E1-HA) and the DDX3 shRNA1 expression vector was cotransfected into HeLa cells. To establish DDX3-overexpressing or knockdown clones, HEK-293 cells were transfected with vectors for expression of FLAG-tagged DDX3 or DDX3 shRNA1. Transfected cells were incubated under selection with 400 μg/ml G418 for 2 weeks. G418-resistant colonies were picked, incubated in 24-well culture dishes, and further screened by immunoblotting with anti-DDX3.
Sucrose gradient fractionation.
HeLa cells (∼1 × 107) were transfected with an expression vector encoding a DDX3 shRNA for 72 h. Cells were collected in phosphate-buffered saline (PBS) containing 100 μg/ml cycloheximide. All subsequent steps were performed at 4°C. Cell pellets were resuspended in RSB-150 (10 mM Tris-HCl, pH 7.4, 3 mM MgCl2, and 150 mM NaCl) containing 100 μg/ml cycloheximide, 40 μg/ml digitonin (Calbiochem), 20 U/ml RNasin (Promega), and Complete protease inhibitor mixture (Roche). After incubation on ice for 5 min, cells were disrupted by passage five times through a 26-gauge needle. The cell lysates were collected by centrifugation in a microcentrifuge at 3,000 × g for 2 min and were clarified by further centrifugation at 11,000 × g for 15 min. The samples were loaded on a linear gradient of 15 to 40% sucrose and centrifuged at 178,000 × g at 4°C for 3 h in a Beckman SW41 rotor. After centrifugation, the polysome profile was monitored at 254 nm, using a fractionation system (ISCO, Lincoln, NE). Total RNA was extracted from each fraction for gel electrophoresis and RPA. RPA was performed as described previously (26), using half of the RNA isolated from gradient fractions and 1 × 105 cpm of antisense probes.
cDNA microarray analysis.
For microarray analysis, the procedure was provided by the manufacturer (Affymetrix). In brief, the lysate of ∼4 × 107 HeLa cells was subjected to sucrose gradient sedimentation. Total RNA was isolated from the 40S ribosome-containing fractions by use of TRIzol LS reagent (Invitrogen) and was cleaned up with an RNeasy Mini kit (Qiagen). Approximately 3 μg of RNA was reverse transcribed into cDNA, using an oligo(dT) primer that contained the T7 RNA polymerase promoter sequence at the 3′ end. Biotin-labeled cRNA was produced by in vitro transcription followed by metal-induced hydrolysis at 94°C. Subsequently, 11 μg of fragmented cRNA was hybridized to a U133 Plus 2.0 human genome array at 45°C for 16.5 h. Subsequent washing and staining were performed with a model 450 fluidic station, and GeneChips were scanned with an Affymetrix GeneChip 7G scanner. Microarray data were analyzed with GeneSpring GX 10 software (Silicon Genetics).
Computational analysis.
In duplicate experiments, 375 genes reproducibly showed a ≥2.0-fold increase in expression in translation initiation complexes upon DDX3 knockdown. By using the programs provided at the DAVID 2008 website (http://david.abcc.ncifcrf.gov), we converted the Affymetrix chip ID to a GenBank Entrez (http://www.ncbi.nlm.nih.gov) gene ID and gene symbol. Using the reference sequence (RefSeq) database in GenBank, we eliminated 20 genes that had no or a short (<20 nt) 5′ UTR or lacked an annotated open reading frame in the subsequent analysis. To examine whether the remaining 332 DDX3 target candidates had any distinctive features in their 5′ UTRs, we randomly selected two sets of genes for comparison by using the program Randomness (version 1.5.2; http://www.merenbach.com). After removal of unqualified genes as described above, two sets, of 327 and 338 genes, were used as controls. We analyzed the features of the 5′ UTRs, including their nucleotide length, GC percentage and number, minimum free energy, and RNA secondary structure, by using the program RNAfold (version 1.8.4) from Vienna RNA WebServers (http://rna.tbi.univie.ac.at) with default parameter settings. The data were subjected to statistical analysis.
Immunoblotting.
The primary antibodies used included polyclonal antibodies against DDX3 (25), cyclin D1 (Santa Cruz Biotechnology), and eIF4A1 (Abcam) and monoclonal antibodies against α-tubulin (NeoMarkers), cyclin A (BD Transduction Laboratories), cyclin E, cyclin E2, cyclin B1 (the last three from Santa Cruz Biotechnology), and the HA epitope (a generous gift from S.-C. Cheng, Academia Sinica, Taiwan). The secondary antibodies and the chemiluminescence procedure were as described previously (25).
FACScan analysis.
HeLa cells were synchronized at the G2/M phase by thymidine-nocodazole treatment (18). Cells were treated with 2 mM thymidine for 16 h and subsequently with 100 ng/ml nocodazole for 12 h. Synchronized cells were then transferred to fresh medium and collected at various times. Cells were harvested by trypsinization and fixed with cold (−20°C) methanol for at least 30 min. Fixed cells were rehydrated with cold PBS on ice for 30 min and subsequently stained with 60 μg/ml propidium iodide (Sigma) in PBS containing 50 μg/ml RNase A for 30 min at ambient temperature. DNA content was analyzed with a FACSCalibur flow cytometer (BD Biosciences), and the data were acquired and analyzed using software provided by the manufacturer.
BrdU incorporation assay.
For flow cytometry, a bromodeoxyuridine (BrdU) incorporation assay was performed essentially as described previously (17). Synchronized HeLa cells were released from the G2/M phase and pulse labeled with 10 μM BrdU at 37°C for 30 min. Cells were harvested and subsequently fixed with ice-cold 100% ethanol for at least 30 min. Fixed cells were rehydrated with PBS and then treated with 2 N HCl containing 0.2 mg/ml pepsin at 37°C for 20 min. Subsequently, cells were incubated in PBS containing 0.05% Triton X-100, 0.5% bovine serum albumin (BSA), and 0.5% FBS for 20 min and then in the same buffer additionally containing fluorescence isothiocyanate (FITC)-conjugated anti-BrdU (BD Pharmingen) for 30 min at ambient temperature. Finally, cells were stained with propidium iodide. The data were acquired and analyzed as described above.
For immunofluorescence assay, HeLa cells were mock transfected or transfected with the shRNA1-expressing vector for 72 h. Transfected cells were seeded on coverslips and cultured at 37°C overnight. The procedure for G2/M-phase synchronization and subsequent release from the block was described above. Nonsynchronized or synchronized cells were pulse labeled with 10 μM BrdU at 37°C for 30 min and then fixed with cold (−20°C) methanol for 30 min. Fixed cells were rehydrated with PBS and treated twice with 4 N HCl at room temperature for 15 min each. After being washed with PBS, cells were incubated with FITC-conjugated anti-BrdU (6 μg/ml; Abcam) for 1 h. Images were obtained using a fluorescence microscope (Axiovert 200M; Zeiss) equipped with a charge-coupled device (CCD) camera (Coolsnap HQ; Photometrics) and supported with a Metamorph software platform (Universal Imaging Corporation).
Colony formation assay.
HeLa cells were cotransfected with pTK-Hyg (Clontech) and the DDX3 shRNA1 or empty expression vector. Cells were then cultured under selection with 200 μg/ml hygromycin for 2 to 3 weeks. Colonies were fixed and stained with 1% crystal violet in 30% ethanol for 30 min and washed with H2O.
Pulse labeling and immunoprecipitation.
For pulse labeling, ∼5 × 106 cells grown in 10-cm culture dishes were washed and preincubated with methionine- and cysteine-free DMEM (Gibco) for 30 min and then labeled with 100 μCi/ml [35S]methionine-cysteine (35S-ProMix; GE Healthcare) for 30 min. Cell lysates were prepared as described previously (29) and subjected to immunoprecipitation using mouse anti-cyclin E (Santa Cruz Biotechnology) or rabbit anti-cyclin D1 (Santa Cruz Biotechnology). Bound proteins were resolved by SDS-PAGE and then visualized by autoradiography.
Translation assays.
For in vivo translation assay, ∼5 × 105 HeLa cells were transfected with 0.2 μg each of the pFL-SV40-E1 and pRL-SV40 reporters as well as 1 μg of the DDX3 shRNA-expressing vector. To express shRNA-resistant DDX3, 0.6 μg of expression vector was used for transfection. A dual-luciferase activity assay was carried out as described previously (25). For EUE or DUE reporter assay, 0.5 μg of each reporter and 1.5 μg of the DDX3 shRNA-expressing vector were cotransfected into ∼5 × 105 HeLa cells. The in vitro translation assay was performed as described previously (25), except that the cyclin E1 5′ UTR-containing firefly luciferase (FL) transcript was used as a template. Purification of recombinant GST-DDX3 was performed as described previously (25).
Biotinylated RNA binding assay.
Riboprobes were transcribed in vitro in the presence of biotinylated UTP. The pGEM RNA of ∼60 nt was transcribed from SpeI-digested pGEM-T (Promega) by use of T7 RNA polymerase. The cyclin E1 5′ UTR and its antisense RNAs were ∼180 nt long. Biotinylated riboprobes were incubated with streptavidin-agarose (Sigma) in a buffer containing 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.05% NP-40 at 4°C for 30 min. The HEK-293 cell lysate was then added to riboprobe-bound beads for further incubation at 4°C for 2 h. After being washed with the same buffer, bound proteins were recovered and analyzed by immunoblotting.
RESULTS
Knockdown of DDX3 suppresses cell proliferation.
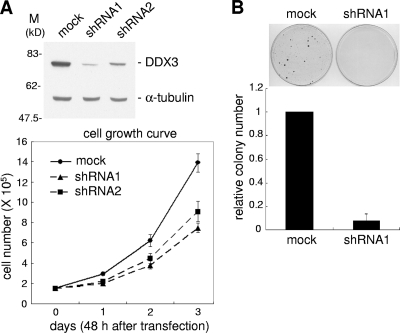
Several lines of evidence have suggested that DDX3 is involved in cell viability (5, 12, 16, 22, 27, 41), but its underlying molecular mechanism remains elusive. To address this issue, we knocked down DDX3 expression in HeLa cells by using the shRNA strategy. Immunoblotting showed that the DDX3 protein level was reduced substantially by two different DDX3-targeting shRNAs (Fig. 1 A, top panel; 80% and 60% reductions by shRNA1 and shRNA2, respectively). shRNA-mediated transient knockdown of DDX3 did indeed impair cell growth (Fig. 1A, bottom panel). In this regard, shRNA1 was more effective than shRNA2, which correlates with their relative efficiencies of depleting DDX3 (Fig. 1A). This result suggested that DDX3 is indeed required for cell growth (12, 16, 27). We further assessed the effect of DDX3 depletion on cell viability by using a colony formation assay. HeLa cells were transfected with the shRNA1-expressing vector, followed by a 2-week selection with hygromycin. DDX3 knockdown cells showed markedly reduced colony formation compared with mock-treated cells (Fig. 1B), reinforcing the potential role for DDX3 in control of cell viability.
FIG. 1.
Knockdown of DDX3 inhibits cell growth. (A) HeLa cells were mock transfected or transfected with a vector expressing a short hairpin RNA (DDX3-targeting shRNA1 or shRNA2) for 48 h. Immunoblotting was performed using anti-DDX3 and anti-α-tubulin (top). Cell growth was assessed by counting cells over 3 days (bottom). (B) HeLa cells were cotransfected with pTK-Hyg, carrying the hygromycin resistance gene, and an empty (mock) or shRNA1-expressing vector. Hygromycin-resistant clones are shown in the top panel. The bottom panel shows the clone number for DDX3 knockdown cells relative to that for mock-treated cells. The data reflect averages for three independent experiments.
Knockdown of DDX3 delays the G1/S transition.
To investigate the molecular mechanisms underlying DDX3 function in cell growth control, we performed a cell cycle analysis of DDX3-deficient HeLa cells. As described above, transient transfection of HeLa cells with the shRNA1 expression vector knocked down DDX3 protein expression by 80 to 90% (data not shown). DDX3-depleted cells were then synchronized at the G2/M phase by a thymidine-nocodazole block and subsequently released for analysis of cell cycle progression. Fluorescence-activated cell sorter (FACS) analysis showed that ∼12 h after blockade release, DDX3 knockdown cells had larger G1-phase and smaller S-phase populations than those in control cells (Fig. 2 A, 9, 12, and 15 h). Our data apparently suggested that knockdown of DDX3 decelerated cell cycle progression by preventing entry into S phase. Nevertheless, nearly 20% of DDX3 knockdown cells had shifted to G2 phase 18 h after release (Fig. 2A), indicating that overall cell viability was still retained after the block.
FIG. 2.
Knockdown of DDX3 slows the G1/S transition and inhibits DNA synthesis. (A) HeLa cells were mock transfected or transfected with the shRNA1 expression vector and then synchronized at the G2/M phase. After cell release, FACS analysis was performed at the indicated time points. The percentage of cells in each stage is shown. The data were averaged for three independent experiments. (B) HeLa cell transfection and synchronization were performed as for panel A. After cell release, BrdU incorporation was measured to determine the amount of DNA synthesis in the cells. The numbers indicate percentages of BrdU-positive cells. Nonsynchronized (asynchronous) and mock-transfected cells were used for comparison. The experiments in panels A and B were each reproduced at least three times, and representative data are shown.
To examine whether DDX3 is indeed important for S-phase entry, we performed a BrdU incorporation assay. Immunofluorescence showed that DDX3 knockdown slightly reduced BrdU incorporation into nonsynchronized HeLa cells, but such a reduction was more significant 12 h after cell release from the G2/M block (see Fig. S1 in the supplemental material). Using flow cytometry to examine G2/M-arrested cells at different time points after release, we confirmed that DDX3 depletion delayed S-phase entry (Fig. 2B). This result, consistent with previous genetic observations in fission yeast and hamster tsET24 cells (12, 16), indicated that DDX3 plays a role in regulating cell cycle progression, particularly at the G1/S transition.
Depletion of DDX3 may attenuate translation of a set of cell cycle factors.
DDX3 is a multifunctional RNA helicase. We previously reported that DDX3 facilitates translation initiation of reporter mRNAs containing a complex 5′ UTR (25). Therefore, we postulated that DDX3 is involved in translation of a select set of cellular mRNAs and that DDX3 depletion may cause the accumulation of those mRNAs in translation initiation complexes. Perhaps some of the DDX3 targets encode cell cycle regulators and their aberrant expression thus results in cell growth defects. We thus exploited sucrose gradient sedimentation in conjunction with cDNA microarray analysis to screen for mRNA substrates of DDX3. We prepared cytoplasmic extracts from DDX3-depleted and mock-treated cells and fractionated cytoplasmic mRNPs by centrifugation through a 15 to 40% sucrose gradient. DDX3 depletion showed no considerable change in polysome profile (see Fig. S2 in the supplemental material), consistent with the previous conclusion that DDX3 is not critical for global translation (12, 25).
Based on our hypothesis that DDX3 depletion would result in cessation of translation of certain mRNAs, we isolated mRNAs in fractions containing the ∼40S initiation complexes and analyzed them with a cDNA microarray containing ∼54,000 spots representing ∼38,000 genes. Duplicate experiments showed that the mRNAs of 375 genes were enriched >2-fold in the ∼40S complexes in DDX3-depleted cells compared to those in control cells (data not shown). To exclude the possibility that expression of these genes was upregulated transcriptionally or that their mRNAs were particularly stabilized, we also profiled global gene expression of DDX3-depleted cells. Among the 375 genes we identified by microarray analysis, only 23 genes showed an increased mRNA level (data not shown). We eliminated these 23 genes as well as 20 genes lacking definite coding sequences, leaving 332 genes whose transcripts might represent targets of DDX3.
To evaluate whether the potential DDX3 target mRNAs had any unusual features in their 5′ UTRs, we randomly selected two sets of ∼400 mRNAs as a negative control for comparison. Bioinformatic analysis showed that the 5′ UTRs of the DDX3 substrate candidates (∼275 nucleotides, on average) were slightly longer than those of the control mRNAs (∼246 nt) and had a relatively higher GC content (69.8% versus 63.0%) (Table 1). This observation might somewhat support our previous assumption that DDX3 substrates have a preference for complex 5′ UTR-containing mRNAs.
TABLE 1.
Bioinformatic analysis of potential DDX3 target mRNAs
| Target mRNAs | No. of genes | No. of matches in RefSeq | Length (nt) of 5′ UTR | 5′ UTR ΔG (kcal/mol) | 5′ UTR GC content (%)b |
|---|---|---|---|---|---|
| DDX3 | 332 | 453 | 274.8 | −125.42 | 69.79 ± 12.42 |
| Reference set 1a | 327 | 417 | 244.3 | −94.08 | 62.72 ± 11.05 |
| Reference set 2a | 338 | 419 | 248 | −99.34 | 63.34 ± 11.75 |
Reference sets represent ∼400 randomly selected mRNAs for comparison with the DDX3 targets.
Averages and standard deviations were calculated for the number of genes given in the table.
DDX3 modulates cyclin E1 expression.
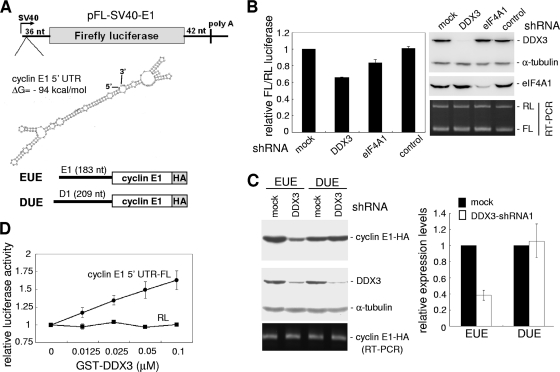
Among the set of putative DDX3 targets identified above, ∼30 mRNAs encoded cell cycle regulators (see Table S1 in the supplemental material). Because one of these regulators, cyclin E1, plays a key role in the G1/S transition, we next examined whether its expression is specifically regulated by DDX3. We first evaluated whether the expression of cyclin E1 was impeded by knockdown of DDX3. Immunoblotting analysis showed that the levels of cyclin E1 and its low-molecular-weight isoform (39) significantly declined in DDX3-depleted HeLa cells, whereas the levels of α-tubulin and another G1/S-specific cyclin, cyclin D1, were not affected (Fig. 3 A, top panel). An RNase protection assay revealed that the level of cyclin E1 mRNA was only minimally reduced in DDX3-depleted cells (Fig. 3A, bottom panel), which might result from a positive-feedback control loop between cyclin E1 and E2F (20, 35, 36). Attenuated cyclin E1 protein expression caused by DDX3 knockdown was also observed in HEK-293 cells and various cancer cells, such as H1299, A549, and U2OS cells (see Fig. S3 in the supplemental material). Nevertheless, knockdown of eIF4A1, a DEAD box RNA helicase involved in translation initiation, did not specifically reduce cyclin E1 expression (Fig. 3B).
FIG. 3.
Knockdown of DDX3 inhibits cyclin E1 mRNA translation. (A) Cell transfection was performed as described in the legend to Fig. 1A, followed by immunoblotting using antibodies specific to the indicated proteins (top). The bottom panel shows cyclin E1 and D1 mRNAs in mock-treated and DDX3 knockdown cells, detected by RNase protection assay. The levels of cyclin E1 and D1 proteins (top) and mRNAs (bottom) in DDX3 knockdown cells were first compared to those in the mock-treated cells. The values shown below the gels are relative levels of cyclin E1 to D1. The asterisk denotes a low-molecular-weight isoform of cyclin E1. (B) To knock down eIF4A1, HeLa cells were transiently transfected with a vector expressing an eIF4A1-targeting shRNA. Immunoblotting was performed using specific antibodies as indicated. (C) Cellular proteins were pulse labeled with [35S]methionine-cysteine. The cell lysate was prepared for immunoprecipitation with antibodies against cyclin E1 and cyclin D1. Asterisks denote bands that nonspecifically cross-reacted with the antibody. The bar graph shows relative cyclin E1 and D1 levels of DDX3 knockdown cells and mock-treated cells. Average values ± standard deviations (SD) were obtained from three independent experiments. (D) Immunoblotting was performed with mock-treated, DDX3-overexpressing, and DDX3-depleted stable HEK-293 cells, using antibodies specific to the indicated proteins.
The above data showing severely impaired cyclin E1 protein but not mRNA expression in DDX3-depleted cells indicated that DDX3 might control cyclin E1 mRNA translation. Next, we performed pulse labeling of DDX3 knockdown and mock-treated HeLa cells, using [35S]methionine-cysteine. Immunoprecipitation using respective specific antibodies revealed a significant reduction of newly synthesized cyclin E1, but not cyclin D1, in DDX3-depleted cells (Fig. 3C).
To further determine whether cyclin E1 abundance is specifically regulated by DDX3, we established stable clones of HEK-293 cells in which DDX3 was either overexpressed ectopically up to 2-fold or downregulated by ∼80% (Fig. 3D). Immunoblotting detection of cyclins revealed that the expression level of cyclin E1, but not that of cyclin A, B1, or even E2, closely correlated with that of DDX3 (Fig. 3D). This result reinforced the specific role of DDX3 in cyclin E1 protein expression and indicated that delay of S-phase entry and DNA synthesis in DDX3-depleted cells is likely caused by downregulation of cyclin E1 expression.
DDX3 facilitates translation initiation of cyclin E1 mRNA via its 5′ UTR.
To evaluate whether reduced cyclin E1 synthesis was a consequence of a blockade of translation initiation, we examined the polysome profile of cyclin E1 mRNA in DDX3-depleted and mock-treated HeLa cells. Sucrose density gradient fractionation revealed that a large portion of cyclin E1 mRNA cosedimented with polysomes in control cells, whereas knockdown of DDX3 resulted in an ∼50% shift of polysome-associated cyclin E1 mRNA to the ∼40S fractions that represented the initiation complex (Fig. 4, left panel, lane 3). In contrast, the distributions of cyclin D1 mRNA in polysomes were similar between DDX3-depleted and mock-treated cells (Fig. 4). This result reflected the fact that the absence of DDX3 may cause a block of translation initiation of cyclin E1 mRNA.
FIG. 4.
Knockdown of DDX3 perturbs translation of cyclin E1 mRNA at the initiation step. The cytoplasmic extract prepared from mock-transfected or DDX3-depleted (shRNA1) HeLa cells was subjected to 15 to 40% sucrose gradient sedimentation. RNAs extracted from the gradient fractions were analyzed by RNase protection assay, using antisense riboprobes complementary to cyclin E1 and D1 mRNAs (top two panels on left). RNAs were also separated in an agarose gel, followed by ethidium bromide staining (bottom panel on left). The bar graph shows the change in the ratio of polysome-associated cyclin E1 or D1 mRNAs (fractions 7 to 11) to total mRNA (all fractions) for DDX3 knockdown cells relative to that for mock-transfected cells. The level of mRNAs was measured in arbitrary units by a PhosphorImager with ImageQuant software. Average values ± SD were obtained from three independent experiments.
To assess the mechanism by which DDX3 facilitates translation initiation of cyclin E1 mRNA, we established an FL reporter containing the 183-nt 5′ UTR of human cyclin E1 mRNA. This 5′ UTR contains 82.5% GC and may contain a stable secondary structure with an estimated free energy of −94 kcal/mol (Fig. 5 A). A Renilla luciferase (RL) reporter plasmid containing an unstructured 5′ UTR was used as a reference. Although depletion of eIF4A1 had no significant effect on the steady-state level of cyclin E1 protein, it could somewhat impair transient expression of the cyclin E1 5′ UTR-containing FL reporter (Fig. 5B). A more significant reduction of FL expression was observed with DDX3 knockdown (Fig. 5B), suggesting that DDX3 participates in cyclin E1 translation, at least in part, via its 5′ UTR. To examine gene-specific effects of DDX3, we constructed a cyclin E1 reporter containing the 5′ UTR and open reading frame (Fig. 5A, reporter EUE) and also replaced the 5′ UTR of this reporter with that of cyclin D1 (DUE). DDX3 knockdown impaired the translation of EUE but not DUE (Fig. 5C). This result, consistent with the observation above, indicated that DDX3-mediated translation regulation was specific to the cyclin E1 5′ UTR.
FIG. 5.
DDX3 facilitates cyclin E1 mRNA translation, likely by its RNA helicase activity. (A) The FL reporter plasmid pFL-SV40-E1 contains the human cyclin E1 5′ UTR (183 nt), for which the predicted secondary structure is shown. The HA-tagged cyclin E1 reporters contained the cyclin E1 (EUE) and cyclin D1 (DUE) 5′ UTRs, respectively. (B) The pFL-SV40-E1 reporter and the control pRL-SV40 vector encoding RL were cotransfected with an empty vector (mock) or a vector expressing DDX3- or eIF4A1-targeting shRNA into HeLa cells. The control shRNA targets TNPO3, which encodes transportin-SR (25). For each transfectant, the FL activity was first normalized to that of the RL control. The normalized FL activity of shRNA transfectants was then compared to that of the mock-treated cells. Relative activities are shown in the bar graph. Immunoblotting was performed using antibodies against DDX3, eIF4A1, and α-tubulin. FL and RL mRNAs were analyzed by RT-PCR and visualized by agarose gel electrophoresis. (C) The EUE and DUE reporters were mock transfected or cotransfected with DDX3 shRNA1. The cyclin E1-HA protein was detected with anti-HA. (D) The in vitro translation assay was performed using in vitro-transcribed cyclin E1 5′ UTR-containing FL mRNA or control RL mRNA as a template in rabbit reticulocyte lysate supplemented with different amounts of recombinant GST-DDX3. The graph shows the FL or RL activity of individual reactions relative to that of the corresponding reaction without addition of DDX3. The data were obtained from three independent experiments.
We tested whether DDX3 might have a preference for binding cyclin E1 mRNA. We immunoprecipitated FLAG-tagged DDX3 and analyzed DDX3-bound mRNAs by reverse transcription-PCR (RT-PCR). However, the result showed that DDX3 bound to cyclin E1 mRNA as well as to other mRNAs, to different extents (see Fig. S4A in the supplemental material). A similar result was obtained by incubating the HEK-293 cell lysate with various biotinylated RNA transcripts, followed by affinity selection using streptavidin-agarose. As a result, neither DDX3 nor eIF4A1 had specificity for the tested RNA fragments (see Fig. S4B in the supplemental material). Therefore, we assumed that DDX3 has no RNA binding preference, but it can still facilitate translation of specific types of mRNA via an unknown mechanism.
We next evaluated whether DDX3 by itself promotes translation initiation of cyclin E1 via its 5′ UTR. Using the above FL reporter mRNA as a substrate, we performed an in vitro translation assay. Addition of increasing amounts of purified recombinant GST-DDX3 revealed a dose-dependent enhancement of FL but not RL translation (Fig. 5D), suggesting that DDX3 has a direct role in translation of an mRNA containing the cyclin E1 5′ UTR. In total, all of our experiments indicated that translation initiation of cyclin E1 mRNA can be facilitated by the RNA helicase DDX3.
RNA helicase activity is important for the function of DDX3 in translation and cell growth control.
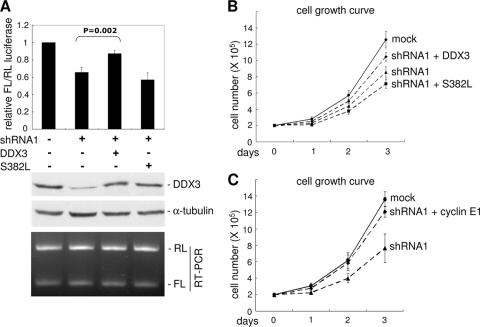
Next, we evaluated whether the RNA helicase activity of DDX3 contributes to translation activation of cyclin E1 mRNA. A vector expressing shRNA-resistant DDX3 was cotransfected with the pFL-SV40-E1 reporter in DDX3 knockdown cells. The luciferase reporter assay showed that wild-type DDX3, but not a helicase-defective mutant (S382L) (48), rescued FL translation (Fig. 6 A). This result indicated that the RNA helicase activity of DDX3 is important for translation of cyclin E1, perhaps by facilitating ribosome scanning during translation initiation. We next examined whether helicase activity is also essential for the capability of DDX3 to promote cell growth. Consistent with the above observation, knockdown of DDX3 in HeLa cells slowed cell proliferation (Fig. 6B). The cell growth rate of DDX3-depleted cells was partially recovered upon supplementation with wild-type DDX3 (Fig. 6B) and cyclin E1 (Fig. 6C) but not with the S382L mutant of DDX3 (Fig. 6B). Therefore, the RNA helicase activity of DDX3 might be required for its function in cell growth control, probably via promoting cyclin E1 translation.
FIG. 6.
RNA helicase activity of DDX3 is important for translation and cell growth control. (A) HeLa cells were transiently transfected with the mock or shRNA1 vector or together with an shRNA1-resistant DDX3-expressing vector (wild type or S382L mutant). Relative FL activity determination, immunoblotting, and RT-PCR were performed as described in the legend to Fig. 5B. (B) HeLa cells were mock transfected or transfected with a vector expressing shRNA1 alone or together with an shRNA1-resistant DDX3-expressing vector (wild type or S382L). (C) HeLa cells were mock transfected or transfected with the shRNA1-expressing vector or both shRNA1- and cyclin E1-expressing vectors. For panels B and C, cell growth was measured as described in the legend to Fig. 1A. The data were averaged for three and four independent experiments, respectively.
Cyclin E1 mRNA translation is compromised in a DDX3 mutant cell line.
Finally, we examined cyclin E1 expression in a hamster TS mutant cell line, tsET24, which has a point mutation in the Ddx3x gene (12). At the nonpermissive temperature (39.5°C), at which DDX3 is inactivated, tsET24 cells are arrested in G1 phase and show delayed entry into S phase (12). Indeed, we observed that ectopic expression of human wild-type DDX3, but not the S382L mutant, rescued the cell growth defect of tsET24 cells (data not shown), consistent with the previous report (12). However, the observation that the levels of cyclins E1 and D1 were elevated 1 day after the temperature shift to 39.5°C was somewhat unexpected, as it was contradictory to cell growth arrest (Fig. 7 A). We reasoned that the initial cell cycle block of tsET24 cells after the temperature rise was due to a reduction of the S-phase cyclin A mRNA level (12). Nevertheless, prolonged incubation of tsET24 cells at 39.5°C caused a gradual decline in the cyclin E1 protein expression level, in parallel with the decrease of the DDX3 protein level (Fig. 7B). No change was observed for either cyclin D1 protein or mRNA (Fig. 7B). Therefore, consistent with the above results, cyclin E1 expression was likely regulated by DDX3 at the translational level, and its inactivation or downregulation might account for G1 arrest of tsET24 cells.
FIG. 7.
Cyclin E1 translation is compromised in a DDX3 mutant cell line. (A) Hamster tsET24 cells were maintained at 33.5°C and then incubated at the same temperature or at the nonpermissive temperature of 39.5°C for 24 h. Immunoblotting was performed as described in the legend to Fig. 3A. (B) The experiment was essentially analogous to that for panel A, except that cells were cultured for 5 days after the temperature shift. Immunoblotting was as performed as for panel A. Cyclin E1 and D1 mRNAs were detected by RT-PCR. The experiment was reproduced at least three times, and a representative figure is shown. The graphs compare the DDX3 protein levels measured at 33.5°C and 39.5°C (top) and show relative cyclin E1 mRNA and protein levels at 33.5°C (middle) and 39.5°C (bottom).
DISCUSSION
Role of DDX3 in translation of cyclin E1.
Human DDX3 and its homologs in other species are multifunctional RNA helicases, and all can participate in translational control (3, 6, 9, 16, 25, 27). However, the exact role of mammalian DDX3 in translation has been disputed (41, 47). We observed in our previous and present studies that knockdown of DDX3 by RNA interference in HeLa cells neither impaired de novo global protein synthesis (25) nor affected polysome profiles (see Fig. S2 in the supplemental material). These results are in line with the observation in tsET24 cells that global translation is not significantly affected at the nonpermissive temperature that inactivates mutant DDX3 (12). We therefore concluded that human DDX3 is not critical for general translation. In contrast, the yeast DDX3 homolog, Ded1, is essential for global mRNA translation (6, 9). Ded1 may also participate in translation initiation of specific mRNAs, such as the mRNA encoding brome mosaic virus RNA2 (38). Notably, fission yeast Ded1 also selectively promotes translation of the Cig2 and Cdc13 mRNAs and thus has been implicated in translation of mRNAs that harbor either a long or complex 5′ UTR (16). Analogously, by using reporter assays, we previously observed that human DDX3 facilitates translation initiation of mRNAs with long or complex 5′ UTRs (25). In this study, the potential substrates of DDX3 that we identified appeared to have a slightly longer 5′ UTR and higher GC content than average (Table 1). Therefore, it is possible that DDX3 affects the translation of only a specific set of mRNAs.
The 5′ UTR of cyclin E1 mRNA is GC rich and perhaps highly structured. We demonstrated that DDX3 has a profound effect on the translation of cyclin E1 mRNA by promoting association of cyclin E1 mRNA with polysomes (Fig. 3, 4, and 7). Because DDX3, like eIF4A1, appeared to have no particular substrate binding specificity (see Fig. S4 in the supplemental material), we assumed that its function of facilitating translation of certain mRNAs requires other cofactors. Nevertheless, our data showing that the RNA helicase activity of DDX3 was essential for efficient translation of the cyclin E1 5′ UTR-containing reporter suggested that DDX3 functions in the translation initiation step (Fig. 5 and 6). Biochemical studies have indicated that yeast Ded1 is more potent than eIF4A with regard to RNA unwinding activity, and therefore Ded1 may enable the 40S ribosomal subunit to achieve higher processivity while scanning for the initiation codon (3, 32). DDX3 may likewise participate in such a step. Based on our data, we inferred that DDX3 facilitates translation initiation of target mRNAs by resolving secondary structures in their 5′ UTRs during ribosome scanning.
Role of DDX3 in cell growth.
In fission yeast, inactivation of Ded1 results in cell cycle arrest in G1, likely by downregulating the levels of the B-type cyclins Cig2 and Cdc13 (16). Defective cell growth has also been observed with knockdown of DDX3 in both human and Drosophila cells (27). Notably, hamster tsET24 cells show G1/S-phase arrest under nonpermissive conditions due to inactivation of a temperature-sensitive mutant of DDX3 (12). Our present data also provide evidence that depletion of DDX3 delays entry into the S phase and inhibits colony formation in HeLa cells (Fig. 1 and 2). We attribute such DDX3 depletion-induced G1/S-phase arrest to downregulation of cyclin E1. Therefore, DDX3 likely plays an evolutionarily conserved role in facilitating the G1/S transition during the cell cycle.
In contrast to the above conclusions, however, one observation has shown that DDX3 knockdown in NIH 3T3 cells leads to premature entry into the S phase and thus promotes cell proliferation (5). This discrepancy might be a consequence of the multiple functions of DDX3 (41). We have now identified a number of mRNAs that encode cell cycle regulators as potential DDX3 targets (see Table S1 in the supplemental material). DDX3 may thus participate in a complex network of cell cycle control by regulating multiple targets. It is also notable that DDX3 facilitates translation of specific sets of mRNAs but, when overexpressed, suppresses general translation (43). Therefore, differential expression of DDX3 in cells may complicate the outcome of DDX3-mediated translation regulation. In this regard, it is not surprising that inconsistent results have been obtained among different cell types.
Biological functions of DDX3.
Several lines of evidence have indicated that DDX3 is critical for cell cycle progression and hence controls cell growth (12, 16). In line with this notion, increased expression of DDX3 has been observed in human breast epithelial MCF 10A cells and hepatocarcinomas, suggesting an oncogenic role of DDX3 (4, 19). We observed that DDX3 depletion caused cell cycle arrest by translational downregulation of cyclin E1, which is consistent with the underlying role of cyclin E in cell growth control. Cyclin E is a rate-limiting activator of Cdk2 kinase in late G1 phase and thereby controls the G1/S transition. The abundance of cyclin E is essentially controlled at the transcriptional level via the cyclin D-pRb-E2F pathway, and cyclin E can also be degraded by ubiquitin-mediated proteolysis (7, 34, 44). However, our observation of translational control of cyclin E1 is not unprecedented. A recent report showed that the Akt signaling pathway activates cyclin E translation by promoting the association of its mRNA with polysomes, ultimately resulting in liver growth (37). Therefore, it would be interesting to examine whether DDX3 is involved in Akt-mediated cyclin E translational control. In light of overexpression of cyclin E in many cancers (2, 10, 13, 30, 42), one could predict that DDX3 may also be upregulated in such cancers. Thus, DDX3 may constitute a potential therapeutic target for cancer treatment. It has been demonstrated that a number of cellular and viral RNA helicases, including DDX3, participate in viral production (1, 23, 48). Some of these RNA helicases have been selected as antiviral targets (23, 41). Therefore, ring-expanded nucleoside derivatives (49, 50) that can inhibit the ATPase activities of RNA helicases may be exploited in anticancer strategies to reduce DDX3 activity.
We show in this study that ablation of DDX3 significantly reduces the level of cyclin E1. Notably, DDX3Y is frequently deleted in infertile males, and its absence results in oligozoospermia or azoospermia in humans, suggesting that DDX3Y is involved in spermatogenesis (11). It is interesting that a substantial fraction of postmeiotic mRNAs are translationally regulated during spermatogenesis (21). Therefore, it will be an exciting prospect to examine whether DDX3Y plays a role in modulating the translational status of these mRNAs.
Supplementary Material
Acknowledgments
We are grateful to T. Sekiguchi for hamster tsET24 cells.
This work was supported by the intramural fund of Academia Sinica and by an Academia Sinica investigator award to W.-Y. Tarn.
Footnotes
Published ahead of print on 13 September 2010.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Ariumi, Y., M. Kuroki, K. Abe, H. Dansako, M. Ikeda, T. Wakita, and N. Kato. 2007. DDX3 DEAD-box RNA helicase is required for hepatitis C virus RNA replication. J. Virol. 81:13922-13926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bani-Hani, K. E., N. M. Almasri, Y. S. Khader, F. M. Sheyab, and H. N. Karam. 2005. Combined evaluation of expressions of cyclin E and p53 proteins as prognostic factors for patients with gastric cancer. Clin. Cancer Res. 11:1447-1453. [DOI] [PubMed] [Google Scholar]
- 3.Berthelot, K., M. Muldoon, L. Rajkowitsch, J. Hughes, and J. E. McCarthy. 2004. Dynamics and processivity of 40S ribosome scanning on mRNA in yeast. Mol. Microbiol. 51:987-1001. [DOI] [PubMed] [Google Scholar]
- 4.Botlagunta, M., F. Vesuna, Y. Mironchik, A. Raman, A. Lisok, P. Winnard, Jr., S. Mukadam, P. Van Diest, J. H. Chen, P. Farabaugh, A. H. Patel, and V. Raman. 2008. Oncogenic role of DDX3 in breast cancer biogenesis. Oncogene 27:3912-3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, P. C., C. W. Chi, G. Y. Chau, F. Y. Li, Y. H. Tsai, J. C. Wu, and Y. H. Wu Lee. 2006. DDX3, a DEAD box RNA helicase, is deregulated in hepatitis virus-associated hepatocellular carcinoma and is involved in cell growth control. Oncogene 25:1991-2003. [DOI] [PubMed] [Google Scholar]
- 6.Chuang, R. Y., P. L. Weaver, Z. Liu, and T. H. Chang. 1997. Requirement of the DEAD-box protein Ded1p for messenger RNA translation. Science 275:1468-1471. [DOI] [PubMed] [Google Scholar]
- 7.Clurman, B. E., R. J. Sheaff, K. Thress, M. Groudine, and J. M. Roberts. 1996. Turnover of cyclin E by the ubiquitin-proteasome pathway is regulated by Cdk2 binding and cyclin phosphorylation. Genes Dev. 10:1979-1990. [DOI] [PubMed] [Google Scholar]
- 8.Cordin, O., J. Banroques, N. K. Tanner, and P. Linder. 2006. The DEAD-box protein family of RNA helicases. Gene 367:17-37. [DOI] [PubMed] [Google Scholar]
- 9.de la Cruz, J., I. Iost, D. Kressler, and P. Linder. 1997. The p20 and Ded1 proteins have antagonistic roles in eIF4E-dependent translation in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 94:5201-5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farley, J., L. M. Smith, K. M. Darcy, E. Sobel, D. O'Connor, B. Henderson, L. E. Morrison, and M. J. Birrer. 2003. Cyclin E expression is a significant predictor of survival in advanced, suboptimally debulked ovarian epithelial cancers: a Gynecologic Oncology Group study. Cancer Res. 63:1235-1241. [PubMed] [Google Scholar]
- 11.Foresta, C., A. Ferlin, and E. Moro. 2000. Deletion and expression analysis of AZFa genes on the human Y chromosome revealed a major role for DBY in male infertility. Hum. Mol. Genet. 9:1161-1169. [DOI] [PubMed] [Google Scholar]
- 12.Fukumura, J., E. Noguchi, T. Sekiguchi, and T. Nishimoto. 2003. A temperature-sensitive mutant of the mammalian RNA helicase, DEAD-BOX X isoform, DBX, defective in the transition from G1 to S phase. J. Biochem. 134:71-82. [DOI] [PubMed] [Google Scholar]
- 13.Fukuse, T., T. Hirata, H. Naiki, S. Hitomi, and H. Wada. 2000. Prognostic significance of cyclin E overexpression in resected non-small cell lung cancer. Cancer Res. 60:242-244. [PubMed] [Google Scholar]
- 14.Geisen, C., and T. Moroy. 2002. The oncogenic activity of cyclin E is not confined to Cdk2 activation alone but relies on several other, distinct functions of the protein. J. Biol. Chem. 277:39909-39918. [DOI] [PubMed] [Google Scholar]
- 15.Geng, Y., Y. M. Lee, M. Welcker, J. Swanger, A. Zagozdzon, J. D. Winer, J. M. Roberts, P. Kaldis, B. E. Clurman, and P. Sicinski. 2007. Kinase-independent function of cyclin E. Mol. Cell 25:127-139. [DOI] [PubMed] [Google Scholar]
- 16.Grallert, B., S. E. Kearsey, M. Lenhard, C. R. Carlson, P. Nurse, E. Boye, and K. Labib. 2000. A fission yeast general translation factor reveals links between protein synthesis and cell cycle controls. J. Cell Sci. 113:1447-1458. [DOI] [PubMed] [Google Scholar]
- 17.Hang, H., and M. H. Fox. 2004. Analysis of the mammalian cell cycle by flow cytometry. Methods Mol. Biol. 241:23-35. [DOI] [PubMed] [Google Scholar]
- 18.Harper, J. V. 2005. Synchronization of cell populations in G1/S and G2/M phases of the cell cycle. Methods Mol. Biol. 296:157-166. [DOI] [PubMed] [Google Scholar]
- 19.Huang, J. S., C. C. Chao, T. L. Su, S. H. Yeh, D. S. Chen, C. T. Chen, P. J. Chen, and Y. S. Jou. 2004. Diverse cellular transformation capability of overexpressed genes in human hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 315:950-958. [DOI] [PubMed] [Google Scholar]
- 20.Hwang, H. C., and B. E. Clurman. 2005. Cyclin E in normal and neoplastic cell cycles. Oncogene 24:2776-2786. [DOI] [PubMed] [Google Scholar]
- 21.Iguchi, N., J. W. Tobias, and N. B. Hecht. 2006. Expression profiling reveals meiotic male germ cell mRNAs that are translationally up- and down-regulated. Proc. Natl. Acad. Sci. U. S. A. 103:7712-7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnstone, O., R. Deuring, R. Bock, P. Linder, M. T. Fuller, and P. Lasko. 2005. Belle is a Drosophila DEAD-box protein required for viability and in the germ line. Dev. Biol. 277:92-101. [DOI] [PubMed] [Google Scholar]
- 23.Kwong, A. D., B. G. Rao, and K. T. Jeang. 2005. Viral and cellular RNA helicases as antiviral targets. Nat. Rev. Drug Discov. 4:845-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai, M. C., R. I. Lin, and W. Y. Tarn. 2003. Differential effects of hyperphosphorylation on splicing factor SRp55. Biochem. J. 371:937-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai, M. C., Y. H. Lee, and W. Y. Tarn. 2008. The DEAD-box RNA helicase DDX3 associates with export messenger ribonucleoproteins as well as tip-associated protein and participates in translational control. Mol. Biol. Cell 19:3847-3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai, M. C., B. H. Teh, and W. Y. Tarn. 1999. A human papillomavirus E2 transcriptional activator. The interactions with cellular splicing factors and potential function in pre-mRNA processing. J. Biol. Chem. 274:11832-11841. [DOI] [PubMed] [Google Scholar]
- 27.Lee, C. S., A. P. Dias, M. Jedrychowski, A. H. Patel, J. L. Hsu, and R. Reed. 2008. Human DDX3 functions in translation and interacts with the translation initiation factor eIF3. Nucleic Acids Res. 36:4708-4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loeb, K. R., H. Kostner, E. Firpo, T. Norwood, D. Tsuchiya, B. E. Clurman, and J. M. Roberts. 2005. A mouse model for cyclin E-dependent genetic instability and tumorigenesis. Cancer Cell 8:35-47. [DOI] [PubMed] [Google Scholar]
- 29.Lykke-Andersen, J., M. D. Shu, and J. A. Steitz. 2001. Communication of the position of exon-exon junctions to the mRNA surveillance machinery by the protein RNPS1. Science 293:1836-1839. [DOI] [PubMed] [Google Scholar]
- 30.Ma, Y., S. Fiering, C. Black, X. Liu, Z. Yuan, V. A. Memoli, D. J. Robbins, H. A. Bentley, G. J. Tsongalis, E. Demidenko, S. J. Freemantle, and E. Dmitrovsky. 2007. Transgenic cyclin E triggers dysplasia and multiple pulmonary adenocarcinomas. Proc. Natl. Acad. Sci. U. S. A. 104:4089-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malumbres, M., and M. Barbacid. 2009. Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer 9:153-166. [DOI] [PubMed] [Google Scholar]
- 32.Marsden, S., M. Nardelli, P. Linder, and J. E. McCarthy. 2006. Unwinding single RNA molecules using helicases involved in eukaryotic translation initiation. J. Mol. Biol. 361:327-335. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto, Y., and J. L. Maller. 2004. A centrosomal localization signal in cyclin E required for Cdk2-independent S phase entry. Science 306:885-888. [DOI] [PubMed] [Google Scholar]
- 34.McEvoy, J. D., U. Kossatz, N. Malek, and J. D. Singer. 2007. Constitutive turnover of cyclin E by Cul3 maintains quiescence. Mol. Cell. Biol. 27:3651-3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moroy, T., and C. Geisen. 2004. Cyclin E. Int. J. Biochem. Cell Biol. 36:1424-1439. [DOI] [PubMed] [Google Scholar]
- 36.Morris, L., K. E. Allen, and N. B. La Thangue. 2000. Regulation of E2F transcription by cyclin E-Cdk2 kinase mediated through p300/CBP co-activators. Nat. Cell Biol. 2:232-239. [DOI] [PubMed] [Google Scholar]
- 37.Mullany, L. K., C. J. Nelsen, E. A. Hanse, M. M. Goggin, C. K. Anttila, M. Peterson, P. B. Bitterman, A. Raghavan, G. S. Crary, and J. H. Albrecht. 2007. Akt-mediated liver growth promotes induction of cyclin E through a novel translational mechanism and a p21-mediated cell cycle arrest. J. Biol. Chem. 282:21244-21252. [DOI] [PubMed] [Google Scholar]
- 38.Noueiry, A. O., J. Chen, and P. Ahlquist. 2000. A mutant allele of essential, general translation initiation factor DED1 selectively inhibits translation of a viral mRNA. Proc. Natl. Acad. Sci. U. S. A. 97:12985-12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porter, D. C., N. Zhang, C. Danes, M. J. McGahren, R. M. Harwell, S. Faruki, and K. Keyomarsi. 2001. Tumor-specific proteolytic processing of cyclin E generates hyperactive lower-molecular-weight forms. Mol. Cell. Biol. 21:6254-6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rocak, S., and P. Linder. 2004. DEAD-box proteins: the driving forces behind RNA metabolism. Nat. Rev. Mol. Cell. Biol. 5:232-241. [DOI] [PubMed] [Google Scholar]
- 41.Schroder, M. 2010. Human DEAD-box protein 3 has multiple functions in gene regulation and cell cycle control and is a prime target for viral manipulation. Biochem. Pharmacol. 79:297-306. [DOI] [PubMed] [Google Scholar]
- 42.Shaye, A., A. Sahin, Q. Hao, K. Hunt, K. Keyomarsi, and I. Bedrosian. 2009. Cyclin E deregulation is an early event in the development of breast cancer. Breast Cancer Res. Treat. 115:651-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shih, J. W., T. Y. Tsai, C. H. Chao, and Y. H. Wu Lee. 2008. Candidate tumor suppressor DDX3 RNA helicase specifically represses cap-dependent translation by acting as an eIF4E inhibitory protein. Oncogene 27:700-714. [DOI] [PubMed] [Google Scholar]
- 44.Singer, J. D., M. Gurian-West, B. Clurman, and J. M. Roberts. 1999. Cullin-3 targets cyclin E for ubiquitination and controls S phase in mammalian cells. Genes Dev. 13:2375-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spruck, C. H., K. A. Won, and S. I. Reed. 1999. Deregulated cyclin E induces chromosome instability. Nature 401:297-300. [DOI] [PubMed] [Google Scholar]
- 46.Strohmaier, H., C. H. Spruck, P. Kaiser, K. A. Won, O. Sangfelt, and S. I. Reed. 2001. Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature 413:316-322. [DOI] [PubMed] [Google Scholar]
- 47.Tarn, W. Y., and T. H. Chang. 2009. The current understanding of Ded1p/DDX3 homologs from yeast to human. RNA Biol. 6:17-20. [DOI] [PubMed] [Google Scholar]
- 48.Yedavalli, V. S., C. Neuveut, Y. H. Chi, L. Kleiman, and K. T. Jeang. 2004. Requirement of DDX3 DEAD box RNA helicase for HIV-1 Rev-RRE export function. Cell 119:381-392. [DOI] [PubMed] [Google Scholar]
- 49.Yedavalli, V. S., N. Zhang, H. Cai, P. Zhang, M. F. Starost, R. S. Hosmane, and K. T. Jeang. 2008. Ring expanded nucleoside analogues inhibit RNA helicase and intracellular human immunodeficiency virus type 1 replication. J. Med. Chem. 51:5043-5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang, N., H. M. Chen, V. Koch, H. Schmitz, M. Minczuk, P. Stepien, A. I. Fattom, R. B. Naso, K. Kalicharran, P. Borowski, and R. S. Hosmane. 2003. Potent inhibition of NTPase/helicase of the West Nile virus by ring-expanded (“fat”) nucleoside analogues. J. Med. Chem. 46:4776-4789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.