Abstract
Type I interferon (IFN-α/β) induction upon viral infection contributes to the early antiviral host defense and ensures survival until the onset of adaptive immunity. Many viral infections lead to an acute, transient IFN expression which peaks a few hours after infection and reverts to initial levels after 24 to 36 h. Robust IFN expression often is conferred by specialized plasmacytoid dendritic cells (pDC) and may depend on positive-feedback amplification via the type I IFN receptor (IFNAR). Here, we show that mice infected with Thogoto virus (THOV), which is an influenza virus-like orthomyxovirus transmitted by ticks, mounted sustained IFN responses that persisted up to 72 h after infection. For this purpose, we used a variant of THOV lacking its IFN-antagonistic protein ML, an elongated version of the matrix (M) protein [THOV(ΔML)]. Of note, large amounts of type I IFN were also found in the serum of mice lacking the IFNAR. Early IFN-α expression seemed to depend on Toll-like receptor (TLR) signaling, whereas prolonged IFN-α responses strictly depended on RIG-I-like helicase (RLH) signaling. Unexpectedly, THOV(ΔML)-infected bone marrow-derived pDC (BM-pDC) produced only moderate IFN levels, whereas myeloid DC (BM-mDC) showed massive IFN induction that was IPS-1-dependent, suggesting that BM-mDC are involved in the massive, sustained IFN production in THOV(ΔML)-infected animals. Thus, our data are compatible with the model that THOV(ΔML) infection is sensed in the acute phase via TLR and RLH systems, whereas at later time points only RLH signaling is responsible for the induction of sustained IFN responses.
Type I interferons (IFNs) are the first line of defense against many viral infections and contribute to initial survival of the host until the onset of adaptive immunity. In mice, type I IFNs comprise 14 IFN-α isoforms (42) and one IFN-β which all bind one common type I IFN receptor (IFNAR) (35). Although systemic type I IFN can be detected in the absence of the IFNAR (4), robust type I IFN responses upon infection critically involve positive-feedback signaling via the IFNAR (35, 39, 40).
Mice deficient in a functional type I IFN system are highly susceptible to infections with a broad range of different viruses. IFNAR-deficient mice die after inoculation with as little as 50 PFU of the negative-strand RNA-encoded vesicular stomatitis virus (VSV), whereas wild-type (WT) mice eliminate 2 × 106 PFU by day 3 after infection (29). Infections of IFNAR-deficient mice with DNA-encoded vaccinia virus (VACV) strain Western Reserve result in about 103-fold higher virus titers than in WT mice, and knockout animals succumb to the infection (41).
Most, if not all, viral infections can be sensed by dendritic cells (DC) via distinct pattern recognition receptor (PRR) systems such as Toll-like receptors (TLRs) located at the cell surface and within endosomes or the retinoic acid-inducible gene I (RIG-I)-like helicases (RLHs) detecting nucleic acids within the cytosol (32). The different PRR systems are used differentially by certain DC subsets, resulting in the release of distinct cytokine patterns, including type I IFNs (20, 24). Two major types of DC are termed myeloid DC (mDC; also called conventional DC), which play a crucial role in antigen presentation but show rather limited capacities in type I IFN production, and plasmacytoid DC (pDC), which can produce large amounts of type I IFNs upon appropriate stimulation (3, 7, 8, 39). Although most cell types can produce type I IFN, pDC are the major type I IFN producers in response to infections caused by many different viruses and produce up to 100- to 1,000-times more IFN than other cell types (reviewed in reference 12). Usually, pathogen-induced type I IFN responses are tightly regulated: appropriate stimulation leads to an acute but transient expression of IFN, which peaks only a few hours after stimulation and reverts to basal levels within 1 to 2 days (19, 44-46). The downregulation of type I IFN responses is of importance because constantly elevated levels of serum IFN-α can contribute to chronic inflammation and autoimmune or autoinflammatory diseases (5).
Thogoto virus (THOV) is the prototype of tick-transmitted orthomyxoviruses (33). Its genome consists of six single-stranded RNA (ssRNA) segments of negative polarity, each coding for a structural protein. Members of the genus THOV are structurally and genetically similar to influenza viruses (13). Dhori virus, a member of the genus THOV, induces disease and cytokine response patterns upon infection of mice which are similar to those of highly pathogenic influenza virus infections in humans (23). In contrast to its close relative influenza virus, which is usually restricted to the respiratory system, THOV as a tick-transmitted virus is systemically distributed.
THOV was shown to be sensitive to the IFN-induced host protein Mx1, which acts against orthomyxoviruses by blocking primary transcription of the viral RNA genome in the nucleus of infected cells (30). Consequently, Mx1-deficient mice are more susceptible to infection with THOV than Mx1-competent animals (14). Like many viruses such as VACV, influenza viruses A and B, herpes simplex virus I, HIV, and Epstein-Barr virus (2, 15), THOV evolved an evasion strategy to prevent induction of IFNs and/or IFNAR signaling. The THOV sixth segment encodes two different transcripts: a spliced transcript that is translated into the matrix (M) protein and an unspliced transcript coding for an elongated form of M called ML. ML was shown to suppress the induction of type I IFN in vitro by blocking the action of IFN-regulatory factors (6, 18) and by directly interacting with general transcription factor TFIIB (43). Thus, recombinant THOV lacking the capacity to express ML, THOV(ΔML), strongly induces IFN synthesis in vivo (31).
Here, we show that some general rules of virus-induced type I IFN responses do not seem to apply to infection with THOV. We observed sustained, systemic expression of IFN in mice infected with THOV(ΔML) persisting up to 72 h after infection. Surprisingly, this prolonged IFN response was dependent on IPS-1 (IFN-β promoter stimulator 1) but not on TLR signaling. Moreover, mDC but not pDC turned out to be the major type I IFN producers upon THOV(ΔML) infection. Collectively, THOV(ΔML) infection induces an unexpected kind of type I IFN response and thus serves as a model to obtain new insights into virus-induced innate immune responses.
MATERIALS AND METHODS
Mice.
MyD88-deficient (MyD88−/−) mice were provided by Shizuo Akira (1), TRIF-deficient (TRIF−/−) mice were from Bruce Beutler (16), and IPS-1-deficient (IPS-1−/−) mice were provided by Jürg Tschopp (27). For breeding of MyD88/TRIF double knockout mice, animals were treated as already described (45). All mice were backcrossed at least 10 times on the C57BL/6 background. Type I IFN receptor-deficient mice (IFNAR−/−) (29) were backcrossed 20 times on the C57BL/6 background. All mice were bred under specific-pathogen-free (SPF) conditions at the Zentrale Tierhaltung of the Paul-Ehrlich-Institut. Unmutated C57BL/6 mice were purchased from Harlan. Mouse experimental work was carried out using 8- to 12-week-old mice in compliance with regulations of German animal welfare. For infection, mice were anesthetized using Isofluran (CP-Pharma) and infected by the intraperitoneal (i.p.) or intravenous (i.v.) route with a total maximum volume of 200 μl of virus in phosphate-buffered saline (PBS). Animals were euthanized if severe symptoms developed. To determine cytokine levels, peripheral blood was taken retro-orbitally upon anesthetization using Isofluran (CP-Pharma), and serum was prepared. IFN levels in serum were analyzed using enzyme-linked immunosorbent assay (ELISA) kits, allowing the determination of mouse IFN-α or mouse IFN-β (PBL Biomedical Laboratories).
Viruses.
Recombinant Thogoto virus lacking the ML open reading frame, THOV(ΔML), was propagated on BHK-21 cells and titrated on Vero cells as described previously (43). Modified vaccinia virus Ankara (MVA; cloned isolate F6 from the 584th passage in chick embryo fibroblasts [CEF]) (26, 38) was kindly provided by Gerd Sutter (Ludwig Maximilians University, Munich, Germany). MVA was propagated and titrated on chicken embryo fibroblasts and purified by centrifugation through sucrose using standard methodologies (37). Vesicular stomatitis virus (VSV) Indiana, Mudd-Summers isolate, was originally obtained from D. Kolakofsky, University of Geneva, Geneva, Switzerland. VSV was grown on BHK-21 cells and titrated on Vero cells. To analyze virus growth upon THOV infection in vivo, organs were homogenized in medium using lysing matrix tubes D (MP Biomedicals), and virus titers were determined by plaque assays.
Cell isolation and culture.
Bone marrow (BM) cells were isolated by flushing femur and tibia of mice with RPMI medium supplemented with 10% fetal calf serum (FCS). Upon red blood cell lysis, cells were washed and seeded at a density of 1 × 106 cells/ml or 2 × 106 cells/ml in medium supplemented with granulocyte-macrophage colony-stimulating factor ([GM-CSF] 100 ng/ml; R&D Systems) or Flt3-L (100 ng/ml; R&D systems), respectively. Flt3-L-supplemented cultures (BM-pDC) were cultivated for 8 days with one medium change at day 4, whereas medium of GM-CSF-supplemented cultures (BM-mDC) was changed every 2 days, depending on the status of cultures, by replacing half of the medium with fresh cytokine-supplemented medium.
In vitro stimulation and quantification of cytokine production.
For stimulation, in vitro differentiated DC were seeded at 1 × 106 cells/well in 24-well culture plates in 1 ml of medium. Only for VSV infections (see Fig. 3C), DC were seeded at 5 × 106 cells/well in 24-well culture plates in 1 ml of medium (46). CpG-containing oligodeoxynucleotide 2216 (ggGGGACGATCGTCgggggG, where lowercase letters indicate phosphorothioate-modified nucleotides; Sigma-ARK) was used at a concentration of 5 μg/ml. For transfection of 2 μg of poly(I:C) (Sigma-Aldrich), the reagent Fugene (Roche) was used according to the manufacturer's instructions. For UV irradiation of virus, a UV irradiation chamber (Herolab) was used. Irradiation with 75 mJ/cm2 took approximately 10 s. After stimulation of DC cultures, cell-free supernatant was collected and analyzed with ELISA kits allowing the determination of mouse IFN-α or mouse IFN-β (PBL Biomedical Laboratories).
RT-PCR.
Total RNA was prepared using Trizol reagent (Invitrogen) according to the manufacturer's instructions. RNA was incubated with DNase I (Roche) for 15 min at 37°C, and cDNA was prepared by using SuperScript II (Invitrogen) according to the manufacturer's instructions. PCR was performed by using primer pairs for cDNA of THOV segment 5 encoding nucleoprotein (GenBank accession number X96872; primers from positions 467 to 486 and 1396 to 1373) and mouse β-actin (GenBank accession number X03672; primers from positions 1374 to 1396 and 1585 to 1564). Reverse transcription-PCR (RT-PCR) products were separated by agarose gel electrophoresis, stained with ethidium bromide, and visualized under UV light. PCR with RNA as a template confirmed the absence of genomic DNA within all samples, and controls with no template confirmed specificity.
RESULTS
THOV(ΔML) infection of mice induces sustained type I IFN responses even in the absence of IFNAR signaling.
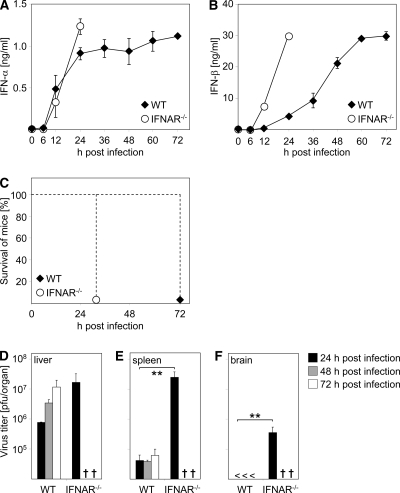
To gain insight into IFN induction upon THOV infection in vivo, mice were infected intraperitoneally (i.p.) with 2 × 104 PFU of THOV. Since THOV-encoded ML protein was shown to suppress activation of an IFN-β reporter construct and thus was described as a potent IFN antagonist (13), THOV devoid of the ML open reading frame, THOV(ΔML), was used throughout our study. Serum IFN-α levels reached a plateau at 24 h after infection. Unexpectedly, IFN-α responses were sustained until termination of the experiment at 72 h after infection (Fig. 1A, filled diamonds). Comparable sustained, elevated levels were observed for IFN-β until 72 h after infection (Fig. 1B, filled diamonds). Similar results were obtained when THOV(ΔML) was applied via the intravenous route (data not shown). To determine a possible role of the IFNAR, IFNAR−/− mice were infected with THOV(ΔML), and serum type I IFN levels were analyzed at the time points indicated in Fig. 1A and B. IFNAR−/− mice mounted even higher and/or faster IFN-α (Fig. 1A) and IFN-β (Fig. 1B) responses, respectively, than WT animals. Similarly, IFN-β deficiency did not reduce IFN-α responses upon THOV(ΔML) infection (data not shown). Of note, type I IFN levels induced upon THOV(ΔML) infection had only a limited protective capacity due to the lack of a functional IFN-induced Mx1 gene in C57BL/6 mice (36). Therefore, all WT animals uniformly succumbed to infection at 72 h after infection, and experiments had to be discontinued (Fig. 1C). Nevertheless, absence of the IFNAR advanced the onset of signs of disease, and IFNAR−/− mice had to be killed already at 30 h postinfection (Fig. 1C), a difference which indicates the presence of IFN-induced antiviral activities in WT animals. Thus, THOV(ΔML) infection induces high, sustained type I IFN responses in vivo even in the absence of feedback signaling via the IFNAR. Of note, it cannot be excluded that WT animals, sufficient for the IFNAR, use the receptor for positive-feedback amplification of type I IFN responses upon THOV infection.
FIG. 1.
IFNAR-independent induction of sustained type I IFN production upon THOV(ΔML) infection in vivo. C57BL/6 mice (WT) and IFNAR−/− mice were inoculated i.p. with 2 × 104 PFU of THOV(ΔML). Serum was collected at the indicated time points after infection and analyzed for IFN-α (A) and IFN-β (B) by ELISA (n = 6). (C) In parallel, survival of mice was monitored. (D) At the indicated time points mice (n = 2 to 6) were sacrificed, organs were homogenized, and virus titers were determined by plaque assays on Vero cells. <, not detectable. Data shown are representative of two to five independent experiments. Error bars indicate standard deviations. **, P < 0.01 ≥ 0.001 by unpaired two-tailed t test.
To compare replication of THOV(ΔML) in the tested mouse strains, we analyzed viral loads in different organs of WT and IFNAR−/− mice. As expected, and recapitulating the hepatotropism of THOV, the highest virus titers were detected in liver (Fig. 1D) (11, 14). Compared to levels in WT mice, virus load in livers of IFNAR−/− animals was increased 10-fold already at 24 h after infection; comparable virus levels (about 107 PFU/organ) were reached in WT animals at the time point at which they had to be sacrificed (Fig. 1D). Interestingly, IFNAR−/− mice showed more than 100-fold higher virus titers in the spleen than WT mice, which showed only low levels of THOV(ΔML) replication in this organ (Fig. 1E). Moreover, THOV(ΔML) could be detected in the brain exclusively in IFNAR−/− mice (Fig. 1F). This enhanced replication of THOV(ΔML) might explain the strong and early systemic IFN synthesis in IFNAR−/− mice (Fig. 1A and B). Taken together, high levels of IFNs produced in WT animals are able to decelerate viral replication and restrict viral tissue tropism but confer only limited protection against a fatal outcome of the infection.
Sustained type I IFN production upon THOV(ΔML) infection in vivo is dependent on IPS-1.
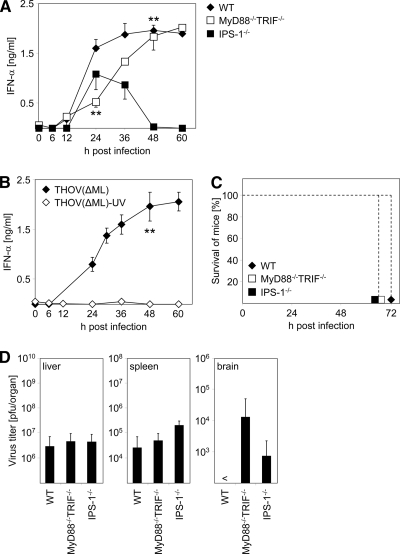
We next wanted to gain insight into the molecular mechanism involved in sensing THOV(ΔML) infection in vivo. Therefore, we infected WT mice, MyD88−/− TRIF−/− mice that are devoid of any TLR signaling, and IPS-1−/− mice lacking the RLH adaptor molecule IFN-β promoter stimulator-1 (also known as MAVS, VISA or Cardif) i.p. with 2 × 104 PFU of THOV(ΔML). As shown in Fig. 2A, THOV(ΔML)-induced IFN-α responses in mice devoid of the TLR adaptors MyD88 and TRIF were delayed by 24 to 36 h after infection and reached normal levels after 48 to 60 h (Fig. 2A). Importantly, when IPS-1-deficient mice were infected, animals mounted early IFN-α responses, whereas at later time points no IFN-α was detected (Fig. 2A). These data suggest a role for TLR signaling in the induction of early IFN-α responses, whereas IPS-1 contributes to sustained IFN-α secretion upon THOV(ΔML) infection.
FIG. 2.
Sustained type I IFN production upon THOV(ΔML) infection in vivo is dependent on IPS-1. (A) C57BL/6 mice (WT), MyD88−/− TRIF−/− mice, and IPS-1−/− mice were inoculated i.p. with 2 × 104 PFU of THOV(ΔML). Serum was collected at the indicated time points after immunization and analyzed for IFN-α by ELISA (n = 3 to 5). (B) C57BL/6 mice were i.p. inoculated with 2 × 104 PFU of THOV(ΔML) or 2 × 104 PFU of THOV(ΔML) which was UV irradiated (150 mJ/cm2) prior to infection. Serum was collected at the indicated time points after immunization and analyzed for IFN-α by ELISA (n = 3). (C) In parallel, survival of mice was monitored. (D) At 60 h postinfection mice were sacrificed, organs were homogenized, and virus titers were determined by plaque assays on Vero cells. <, not detectable. Data shown are representative of three independent experiments. Error bars indicate standard deviations. **, P < 0.01 ≥ 0.001 by one-factorial analysis of variance (WT compared to MyD88−/− TRIF−/− mice at 24 h postinfection and WT compared to IPS-1−/− mice at 48 h after infection) and by a unpaired two-tailed t test (for panel B).
In order to dissect whether active THOV(ΔML) replication was necessary for IFN induction, we used untreated and UV-irradiated THOV(ΔML) for infection of WT mice. Even at a low dose of UV irradiation (75 mJ/cm2), no further viral replication could be detected in an in vitro assay (data not shown). As shown in Fig. 2B, IFN-α responses were abrogated upon infection with UV-irradiated THOV(ΔML). Thus, high-level and sustained type I IFN production upon THOV infection in vivo is dependent on replication-competent virus.
As shown in Fig. 1, sensing of type I IFNs via the IFNAR contributes to reduced viral replication and delayed onset of disease upon THOV(ΔML) infection. Thus, we explored whether the modulation of IFN responses as observed in MyD88−/− TRIF−/− and IPS-1−/− mice had any biological consequences. Indeed, the delayed and/or reduced systemic production of IFN in MyD88−/− TRIF−/− and IPS-1−/− mice, respectively, promoted development of severe disease in the animals (uniformly at 60 h after infection) compared to WT animals, which had to be sacrificed 72 h after infection (Fig. 2C). Furthermore, MyD88−/− TRIF−/− and IPS-1−/− mice showed THOV(ΔML) infection of the brain, which was not observed upon infection of WT animals, whereas viral load in liver and spleen of MyD88−/− TRIF−/− and IPS-1−/− mice was comparable to that of WT animals (Fig. 2D). Whether elevated viral titers observed in brains of knockout animals were related to the earlier onset of disease remains unclear. We used protein kinase R-deficient mice (PKR−/−) for infection as well. However, type I IFN levels upon THOV(ΔML) infection were comparable to levels in strain-matched WT control animals (mice on the SV129 background) (data not shown).
Robust type I IFN expression by BM-mDC but not BM-pDC upon THOV(ΔML) infection in vitro.
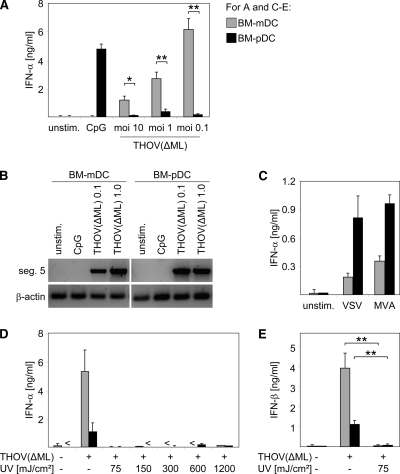
It is well accepted that upon infection with most viruses, pDC are the main source of type I IFNs and are responsible for systemic type I IFN responses in vivo (8, 12, 39). Surprisingly, upon THOV(ΔML) infection, BM-mDC elicited robust IFN-α responses, whereas BM-pDC produced only low IFN-α levels (Fig. 3A). Of note, both BM-mDC and BM-pDC were successfully infected by THOV(ΔML), as indicated by RT-PCR amplification of transcripts of THOV segment 5 (Fig. 3B). Moreover, BM-pDC secreted interleukin-10 (IL-10) upon THOV(ΔML) infection (data not shown), indicating that BM-pDC were susceptible to THOV(ΔML) infection. Interestingly, in BM-mDC IFN-α levels were highest at the lowest multiplicity of infection (MOI) tested (Fig. 3A), most probably due to cytopathic effects observed upon infection with an MOI of 10 (data not shown). As a control, the TLR9 ligand double-stranded CpG-containing oligonucleotide 2216 was used to induce IFN responses by BM-pDC but not by BM-mDC (Fig. 3A). Infection with ssRNA-encoded VSV or double-stranded DNA (dsDNA)-encoded modified vaccinia virus Ankara (MVA) confirmed that BM-pDC but not BM-mDC were the main type I IFN producers in response to these viral infections (Fig. 3C), as described previously (45, 46). To further analyze conditions required for THOV(ΔML)-mediated IFN production, cells were infected with virus irradiated with graded doses of UV light prior to infection. Reminiscent of the data obtained in vivo, IFN secretion upon THOV(ΔML) infection was abrogated when UV-irradiated virus was used, demonstrating that viral replication was necessary for both IFN-α (Fig. 3D) and IFN-β (Fig. 3E) induction in BM-DC. Thus, we showed that upon infection with ssRNA-encoded THOV(ΔML) BM-mDC, but not BM-pDC, are the main source for type I IFNs and that these BM-mDC-derived IFN responses were critically dependent on viral replication.
FIG. 3.
Robust type I IFN expression by BM-mDC but not BM-pDC upon THOV(ΔML) infection in vitro. (A) BM-mDC and BM-pDC were infected with THOV(ΔML) at MOIs of 10, 1, and 0.1. Untreated and CpG-stimulated cells served as controls. At 24 h after treatment supernatants were analyzed for IFN-α by ELISA. (B) BM-mDC and BM-pDC were infected with THOV(ΔML) at an MOI of 0.1 or 1. Untreated and CpG-stimulated cells served as controls. At 24 h after treatment RNA was extracted and analyzed by RT-PCR for transcripts of segment 5 of THOV(ΔML) and of mouse β-actin. RT-PCR products were visualized in an ethidium bromide-stained agarose gel under UV light. (C) BM-mDC and BM-pDC were infected with VSV at an MOI of 1 or with MVA with at an MOI of 1. Untreated (unstim.) cells served as controls. At 24 h after treatment supernatants were analyzed for IFN-α by ELISA. (D) BM-mDC and BM-pDC were infected with THOV(ΔML) (MOI of 0.1) or with THOV(ΔML) (MOI of 0.1) that was irradiated with UV light (at the indicated dosages in mJ/cm2) prior to infection. Control cells were left untreated. At 24 h after infection supernatants were analyzed for IFN-α and IFN-β (E) by ELISA. <, not detectable. Data shown are representative of two to four independent experiments. Error bars indicate standard deviations. *, P < 0.05 ≥ 0.01; **, P < 0.01 ≥ 0.001 by unpaired two-tailed t test.
Type I IFN production by BM-mDC upon THOV infection is dependent on IPS-1.
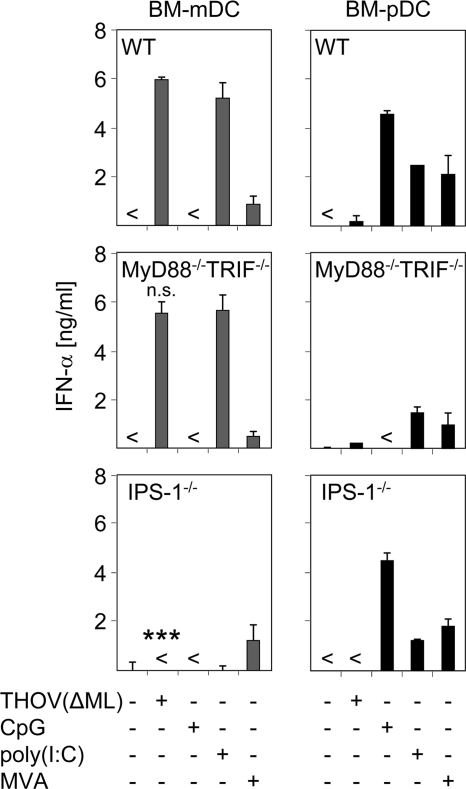
As indicated by the data shown in Fig. 3, BM-mDC produced large amounts of type I IFN upon THOV(ΔML) infection. To test whether the in vivo IPS-1 dependence of THOV(ΔML)-induced IFN-α is also found in specialized DC subsets, BM-mDC and BM-pDC were generated from WT, MyD88−/− TRIF−/−, and IPS-1−/− mice. The total numbers of cells derived from the different knockout mice were comparable to levels in WT mice. Reminiscent of the results obtained in vivo, THOV(ΔML)-induced IFN-α production by BM-mDC was dependent on IPS-1-adapted signaling. Furthermore, IFN-α levels in supernatants of infected MyD88−/− TRIF−/− DC were comparable with those elicited by WT DC (Fig. 4). Controls such as TLR9 ligand CpG (inducing IFN-α responses by BM-pDC but not BM-mDC in a strictly TLR-dependent manner), IFN induction by synthetic dsRNA poly(I:C) (being dependent on IPS-1-adapted RLH signaling in BM-mDC and dependent on TLR signaling in BM-pDC) (20, 22, 25, 28), and infection with the dsDNA-encoded MVA (inducing IFN responses in both DC subsets) confirmed specificity and functionality of DC subsets (Fig. 4). Collectively, data presented here show that BM-mDC but not highly specialized BM-pDC are the main type I IFN producers upon THOV(ΔML) infection in a strictly IPS-1-dependent manner.
FIG. 4.
Type I IFN production by BM-mDC upon THOV(ΔML) infection is dependent on IPS-1. (A) BM-mDC and BM-pDC were generated from C57BL/6 mice (WT), MyD88−/− TRIF−/− mice, and IPS-1−/− mice and infected with THOV(ΔML) (MOI of 0.1). Untreated, CpG-stimulated, poly(I:C)-transfected, and MVA-infected (MOI of 1) cells served as controls. At 24 h after treatment supernatants were analyzed for IFN-α by ELISA. <, not detectable. Data shown are representative of three to four independent experiments. Error bars indicate standard deviations. ***, P < 0.001 by unpaired two-tailed t test [for THOV(ΔML)-infected WT compared to IPS-1−/− mDC]; n.s., not significant [for THOV(ΔML)-infected WT compared to MyD88−/− TRIF−/− mDC].
DISCUSSION
THOV is the prototype of tick-transmitted orthomyxoviruses and shares structural and genetic similarities with its relative, influenza virus. In contrast to influenza virus infections which in situ are mediated via the respiratory system and thus are acting locally, THOV, as a tick-mediated virus, is acting systemically per se. Moreover, for THOV but not influenza virus, mice are an important natural host (9, 11). Thus, THOV is a suitable model that avoids certain drawbacks that may play a role when influenza virus is used for systemic experimental infection studies. We previously showed that THOV induces type I IFN responses in cell lines and mouse embryonic fibroblasts (6, 13). However, basically nothing was known about THOV-mediated induction of type I IFN responses in vivo and about cell types specialized for high IFN production upon THOV infection or about the pattern recognition receptor systems involved.
Here, we show that THOV(ΔML) infection in vivo induced a robust type I IFN response that was sustained until the experiments had to be discontinued at 72 h after infection (Fig. 1A). Unexpectedly, strong IFN-α responses upon THOV(ΔML) infection were also detected in IFNAR−/− mice, i.e., in the absence of positive-feedback amplification via the IFNAR (Fig. 1A). It has been shown already that some viruses, such as VSV or herpes simplex virus 2, or artificial stimuli, such as synthetic dsRNA poly(I:C), can induce IFN-α responses in the absence of the IFNAR. However, in these studies levels induced in IFNAR−/− animals were reduced by more than 50-fold compared to levels in WT animals (4). In particular for pDC, IFNAR-independent type I IFN production was indicated (17, 21). However, recent studies showed that pDC also use IFNAR feedback signaling for amplification of type I IFN secretion (reviewed in reference 39).
In our experimental setting, IFN-α levels induced in IFNAR- and IFN-β-deficient mice were not reduced compared to levels in WT animals and showed no delay in time kinetics (Fig. 1A and data not shown). However, these IFN-α levels might possibly be boosted by the enhanced virus replication in IFNAR−/− mice (Fig. 1D). Of note, because of the early death of the IFNAR−/− mice upon THOV(ΔML) infection, our experimental setup does not exclude a role for positive-feedback amplification in IFN induction at later time points during infection of WT animals. The sustained, robust IFN response could hint at an interplay of certain cell types in vivo that produce type I IFNs. Our in vitro experiments suggest that mDC play an important role. However, nonmyeloid cells may secrete type I IFNs as well and, thus, may contribute to systemic type I IFN production upon THOV(ΔML) infection in vivo.
We found that the RLH adaptor molecule IPS-1 is crucial for robust and sustained type I IFN induction (Fig. 2A and 4). Interestingly, Kato et al. demonstrated that RIG-I is essential for induction of IFN production after RNA virus infection of myeloid cells and fibroblasts, whereas pDC use the TLR system rather than RIG-I for detection of viral infections (20). Hence, the clear dependency on IPS-1 in THOV(ΔML)-induced IFN responses is consistent since we show that BM-mDC account for the main type I IFN responses upon THOV(ΔML) infection. By using UV-irradiated and, thus, replication-incompetent virus, we could show that for induction of IFN-α both in vivo (Fig. 2B) and in vitro (Fig. 3D), virus replication is crucial upon infection with THOV(ΔML). In line with these findings, using influenza virus A and Sendai virus, Rehwinkel et al. just recently showed that RIG-I agonists are exclusively generated by the process of virus replication (34).
Interestingly, we found some reduced, transient IFN-α production in THOV(ΔML)-infected IPS-1−/− mice that could reflect TLR-dependent THOV-induced IFN responses. This assumption goes with the delayed IFN-α response in MyD88−/− TRIF−/− mice (Fig. 2A). Accordingly, IPS-1−/− BM-mDC did not mount any IFN responses upon THOV(ΔML) infection (Fig. 4). Thus far, it is unclear which cell type accounts for this early IPS-1-independent, possibly TLR-dependent, response in vivo. Preliminary experiments show that cells such as macrophages do not seem to play a major role since peritoneal exudate cells, containing many macrophages, do not produce high levels of IFN upon THOV(ΔML) stimulation in an IPS-1-independent manner. Moreover, IFN levels upon THOV(ΔML) infection of total BM or total spleen cells were too low to allow clear discrimination between IPS-1-competent and -deficient preparations (data not shown).
Unexpectedly, our experiments using BM-derived DC subsets clearly indicated that BM-mDC but not BM-pDC were the main type I IFN-producing cell type upon THOV(ΔML) infection (Fig. 3A). We along with others showed that mDC can produce IFN upon viral infection, mainly after infections with attenuated viruses such as MVA (45), VSV-M2 (46), and lymphocytic choriomeningitis virus (LCMV) clone 13 (10). However, to our knowledge, IFN levels secreted by mDC upon viral infection were significantly below those secreted by pDC (also reviewed in reference 12).
In the present study, we used THOV devoid of the ML open reading frame. THOV-encoded ML protein was shown to suppress activation of an IFN-β reporter construct und thus was described as a potent IFN antagonist (13). Of note, we used both THOV variants, THOV(ΔML) and THOV encoding ML, and both were able to induce IFN responses by BM-mDC in an IPS-1-dependent manner (data not shown). Levels of IFN induced by BM-mDC were comparable when cells were infected with these two viruses, indicating that BM-mDC might have developed strategies to circumvent viral escape mediated by the ML protein, which is effective in other cell types such as fibroblasts or pDC (6). Recent studies suggest the involvement of the TFIIB transcription factor in the ML-mediated suppression of IFN induction (43). A unique transcription factor repertoire and its involvement in IFN expression by mDC might represent a possible mechanism to circumvent viral escape as mediated by ML.
We observed some protective capacity of type I IFNs in vivo since blocking of IFN signaling in IFNAR−/− mice strongly decreased the survival of the animals (Fig. 1C). Additionally, IFN expression contributed to decelerate viral replication and to limit viral spread (Fig. 1D to F). Of note, we used naturally Mx1-deficient mouse strains in our study to investigate induction of IFN by THOV(ΔML). IFN-dependent induction of Mx1 in THOV-infected adult animals efficiently suppresses THOV replication to undetectable levels (14), and therefore these animals are not appropriate for studying the long-term effects of viral replication on IFN induction. However, the high IFN-inducing capacity of THOV(ΔML) has been detected in newborn Mx1-positive mice despite severely reduced viral titers (31). It will be a matter of future investigation to analyze whether sustained type I IFN production as observed in our study is applicable to Mx1-positive mice as well and, hence, to what extent virus replication is involved.
Collectively, THOV(ΔML) infection of mice leads to an unexpected strong and long-lasting mode of type I IFN expression that is most likely dominated by IPS-1-dependent IFN production of infected mDC but not pDC. This system will serve as an ideal model to obtain novel insights in virus-induced innate immune responses.
Acknowledgments
We thank Dorothea Kreuz and Simone Gruber for expert technical assistance, Yasemin Süzer for provision of MVA preparations, and Kay-Martin Hanschmann for statistical analyses.
This work was supported in part by Deutsche Forschungsgemeinschaft grants to G.K. (KO1579/3-7) and to Z.W. (WA 2873/1-1).
Footnotes
Published ahead of print on 22 September 2010.
REFERENCES
- 1.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. [DOI] [PubMed] [Google Scholar]
- 2.Alcami, A., and U. H. Koszinowski. 2000. Viral mechanisms of immune evasion. Immunol. Today 21:447-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asselin-Paturel, C., A. Boonstra, M. Dalod, I. Durand, N. Yessaad, C. Dezutter-Dambuyant, A. Vicari, A. O'Garra, C. Biron, F. Briere, and G. Trinchieri. 2001. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2:1144-1150. [DOI] [PubMed] [Google Scholar]
- 4.Barchet, W., M. Cella, B. Odermatt, C. Asselin-Paturel, M. Colonna, and U. Kalinke. 2002. Virus-induced interferon alpha production by a dendritic cell subset in the absence of feedback signaling in vivo. J. Exp. Med. 195:507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanco, P., A. K. Palucka, M. Gill, V. Pascual, and J. Banchereau. 2001. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science 294:1540-1543. [DOI] [PubMed] [Google Scholar]
- 6.Buettner, N., C. Vogt, L. Martinez-Sobrido, F. Weber, Z. Waibler, and G. Kochs. 2010. Thogoto virus ML protein is a potent inhibitor of the interferon regulatory factor-7 transcription factor. J. Gen. Virol. 91:220-227. [DOI] [PubMed] [Google Scholar]
- 7.Chehimi, J., S. E. Starr, H. Kawashima, D. S. Miller, G. Trinchieri, B. Perussia, and S. Bandyopadhyay. 1989. Dendritic cells and IFN-alpha-producing cells are two functionally distinct non-B, non-monocytic HLA-DR+ cell subsets in human peripheral blood. Immunology 68:486-490. [PMC free article] [PubMed] [Google Scholar]
- 8.Colonna, M., G. Trinchieri, and Y. J. Liu. 2004. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5:1219-1226. [DOI] [PubMed] [Google Scholar]
- 9.Darwish, M. A., H. Hoogstraal, and F. M. Omar. 1979. A serological survey for Thogoto virus in humans, domestic mammals, and rats in Egypt. J. Egypt. Public Health Assoc. 54:1-8. [PubMed] [Google Scholar]
- 10.Diebold, S. S., M. Montoya, H. Unger, L. Alexopoulou, P. Roy, L. E. Haswell, A. Al Shamkhani, R. Flavell, P. Borrow, and C. Reis e Sousa. 2003. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature 424:324-328. [DOI] [PubMed] [Google Scholar]
- 11.Filipe, A. R., M. C. Peleteiro, T. M. Monath, and E. H. Calisher. 1986. Pathological lesions in mice infected with Thogoto virus, a tick-borne orthomyxovirus. Acta Virol. 30:337-340. [PubMed] [Google Scholar]
- 12.Fitzgerald-Bocarsly, P., and D. Feng. 2007. The role of type I interferon production by dendritic cells in host defense. Biochimie 89:843-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagmaier, K., S. Jennings, J. Buse, F. Weber, and G. Kochs. 2003. Novel gene product of Thogoto virus segment 6 codes for an interferon antagonist. J. Virol. 77:2747-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haller, O., M. Frese, D. Rost, P. A. Nuttall, and G. Kochs. 1995. Tick-borne Thogoto virus infection in mice is inhibited by the orthomyxovirus resistance gene product Mx1. J. Virol. 69:2596-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haller, O., G. Kochs, and F. Weber. 2006. The interferon response circuit: induction and suppression by pathogenic viruses. Virology 344:119-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoebe, K., X. Du, P. Georgel, E. Janssen, K. Tabeta, S. O. Kim, J. Goode, P. Lin, N. Mann, S. Mudd, K. Crozat, S. Sovath, J. Han, and B. Beutler. 2003. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 424:743-748. [DOI] [PubMed] [Google Scholar]
- 17.Honda, K., H. Yanai, T. Mizutani, H. Negishi, N. Shimada, N. Suzuki, Y. Ohba, A. Takaoka, W. C. Yeh, and T. Taniguchi. 2004. Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signaling. Proc. Natl. Acad. Sci. U. S. A. 101:15416-15421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jennings, S., L. Martinez-Sobrido, A. Garcia-Sastre, F. Weber, and G. Kochs. 2005. Thogoto virus ML protein suppresses IRF3 function. Virology 331:63-72. [DOI] [PubMed] [Google Scholar]
- 19.Kamphuis, E., T. Junt, Z. Waibler, R. Forster, and U. Kalinke. 2006. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood 108:3253-3261. [DOI] [PubMed] [Google Scholar]
- 20.Kato, H., S. Sato, M. Yoneyama, M. Yamamoto, S. Uematsu, K. Matsui, T. Tsujimura, K. Takeda, T. Fujita, O. Takeuchi, and S. Akira. 2005. Cell type-specific involvement of RIG-I in antiviral response. Immunity 23:19-28. [DOI] [PubMed] [Google Scholar]
- 21.Kawai, T., S. Sato, K. J. Ishii, C. Coban, H. Hemmi, M. Yamamoto, K. Terai, M. Matsuda, J. Inoue, S. Uematsu, O. Takeuchi, and S. Akira. 2004. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 5:1061-1068. [DOI] [PubMed] [Google Scholar]
- 22.Kumar, H., S. Koyama, K. J. Ishii, T. Kawai, and S. Akira. 2008. Cutting edge: cooperation of IPS-1- and TRIF-dependent pathways in poly IC-enhanced antibody production and cytotoxic T cell responses. J. Immunol. 180:683-687. [DOI] [PubMed] [Google Scholar]
- 23.Li, G., N. Wang, H. Guzman, E. Sbrana, T. Yoshikawa, C. T. Tseng, R. B. Tesh, and S. Y. Xiao. 2008. Dhori virus (Orthomyxoviridae: Thogotovirus) infection of mice produces a disease and cytokine response pattern similar to that of highly virulent influenza A (H5N1) virus infection in humans. Am. J. Trop. Med. Hyg. 78:675-680. [PubMed] [Google Scholar]
- 24.Luber, C. A., J. Cox, H. Lauterbach, B. Fancke, M. Selbach, J. Tschopp, S. Akira, M. Wiegand, H. Hochrein, M. O'Keeffe, and M. Mann. 2010. Quantitative proteomics reveals subset-specific viral recognition in dendritic cells. Immunity 32:279-289. [DOI] [PubMed] [Google Scholar]
- 25.McCartney, S., W. Vermi, S. Gilfillan, M. Cella, T. L. Murphy, R. D. Schreiber, K. M. Murphy, and M. Colonna. 2009. Distinct and complementary functions of MDA5 and TLR3 in poly(I:C)-mediated activation of mouse NK cells. J. Exp. Med. 206:2967-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer, H., G. Sutter, and A. Mayr. 1991. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J. Gen. Virol. 72:1031-1038. [DOI] [PubMed] [Google Scholar]
- 27.Michallet, M. C., E. Meylan, M. A. Ermolaeva, J. Vazquez, M. Rebsamen, J. Curran, H. Poeck, M. Bscheider, G. Hartmann, M. Konig, U. Kalinke, M. Pasparakis, and J. Tschopp. 2008. TRADD protein is an essential component of the RIG-like helicase antiviral pathway. Immunity 28:651-661. [DOI] [PubMed] [Google Scholar]
- 28.Miyake, T., Y. Kumagai, H. Kato, Z. Guo, K. Matsushita, T. Satoh, T. Kawagoe, H. Kumar, M. H. Jang, T. Kawai, T. Tani, O. Takeuchi, and S. Akira. 2009. Poly I:C-induced activation of NK cells by CD8α+ dendritic cells via the IPS-1 and TRIF-dependent pathways. J. Immunol. 183:2522-2528. [DOI] [PubMed] [Google Scholar]
- 29.Muller, U., U. Steinhoff, L. F. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918-1921. [DOI] [PubMed] [Google Scholar]
- 30.Pavlovic, J., O. Haller, and P. Staeheli. 1992. Human and mouse Mx proteins inhibit different steps of the influenza virus multiplication cycle. J. Virol. 66:2564-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pichlmair, A., J. Buse, S. Jennings, O. Haller, G. Kochs, and P. Staeheli. 2004. Thogoto virus lacking interferon-antagonistic protein ML is strongly attenuated in newborn Mx1-positive but not Mx1-negative mice. J. Virol. 78:11422-11424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pichlmair, A., and C. Reis e Sousa. 2007. Innate recognition of viruses. Immunity 27:370-383. [DOI] [PubMed] [Google Scholar]
- 33.Pringle, C. R. 1996. Virus taxonomy 1. Arch. Virol. 141:2251-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rehwinkel, J., C. P. Tan, D. Goubau, O. Schulz, A. Pichlmair, K. Bier, N. Robb, F. Vreede, W. Barclay, E. Fodor, and C. Reis e Sousa. 2010. RIG-I detects viral genomic RNA during negative-strand RNA virus infection. Cell 140:397-408. [DOI] [PubMed] [Google Scholar]
- 35.Samuel, C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14:778-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staeheli, P., R. Grob, E. Meier, J. G. Sutcliffe, and O. Haller. 1988. Influenza virus-susceptible mice carry Mx genes with a large deletion or a nonsense mutation. Mol. Cell. Biol. 8:4518-4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Staib, C., and G. Sutter. 2003. Live viral vectors: vaccinia virus. Methods Mol. Med. 87:51-68. [DOI] [PubMed] [Google Scholar]
- 38.Sutter, G., and B. Moss. 1992. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc. Natl. Acad. Sci. U. S. A. 89:10847-10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swiecki, M., and M. Colonna. 2010. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol. Rev. 234:142-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taniguchi, T., and A. Takaoka. 2001. A weak signal for strong responses: interferon-alpha/beta revisited. Nat. Rev. Mol. Cell Biol. 2:378-386. [DOI] [PubMed] [Google Scholar]
- 41.van den Broek, M. F., U. Muller, S. Huang, R. M. Zinkernagel, and M. Aguet. 1995. Immune defence in mice lacking type I and/or type II interferon receptors. Immunol. Rev. 148:5-18. [DOI] [PubMed] [Google Scholar]
- 42.van Pesch, V., H. Lanaya, J. C. Renauld, and T. Michiels. 2004. Characterization of the murine alpha interferon gene family. J. Virol. 78:8219-8228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vogt, C., E. Preuss, D. Mayer, F. Weber, M. Schwemmle, and G. Kochs. 2008. The interferon antagonist ML protein of Thogoto virus targets general transcription factor IIB. J. Virol. 82:11446-11453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waibler, Z., M. Anzaghe, T. Frenz, A. Schwantes, C. Pohlmann, H. Ludwig, M. Palomo-Otero, A. Alcami, G. Sutter, and U. Kalinke. 2009. Vaccinia virus-mediated inhibition of type I interferon responses is a multifactorial process involving the soluble type I interferon receptor B18 and intracellular components. J. Virol. 83:1563-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waibler, Z., M. Anzaghe, H. Ludwig, S. Akira, S. Weiss, G. Sutter, and U. Kalinke. 2007. Modified vaccinia virus Ankara induces Toll-like receptor-independent type I interferon responses. J. Virol. 81:12102-12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waibler, Z., C. N. Detje, J. C. Bell, and U. Kalinke. 2007. Matrix protein mediated shutdown of host cell metabolism limits vesicular stomatitis virus-induced interferon-alpha responses to plasmacytoid dendritic cells. Immunobiology 212:887-894. [DOI] [PubMed] [Google Scholar]