Abstract
The genetic heterogeneity of HIV-1 poses a major obstacle to vaccine development. Although most horizontally acquired HIV-1 infections are initiated by a single homogeneous virus, marked genetic diversification and evolution occur following transmission. The relative contribution of the antiviral immune response to intrahost viral evolution remains controversial, in part because the sequence of the transmitted virus and the array of T-cell epitopes targeted by both donor and recipient are seldom known. We directly compared predominant viral sequences derived from 52 mother-child transmission pairs following vertical infection and identified 1,475 sites of mother-infant amino acid divergence within Nef, Gag, and Pol. The cumulative number of mutations away from the consensus subtype B sequence increased linearly with time since transmission, whereas reversions toward the consensus sequence accumulated more slowly with increasing duration of infection. Comprehensive mapping of T-cell epitopes targeted by these mothers and infants revealed that 14% of nonsynonymous mutations away from the consensus sequence were located within regions targeted by the infant, whereas 24% of nonsynonymous mutations toward the consensus sequence were located in regions targeted by the mother. On the basis of analysis of optimal epitopes listed in the HIV Molecular Immunology Database, fewer than 10% of epitopes containing maternal escape mutations reverted to the consensus sequence following transmission to an infant lacking the restricting HLA allele. This surprisingly low reversion rate of mutated epitopes following transmission suggests that the fitness cost associated with many CD8 epitope mutations may be modest.
HIV-1 displays a striking degree of heterogeneity both within and among individuals. Due to its extremely high replication rate and error-prone reverse transcription, it has been estimated that every possible nucleotide substitution arises at each site of the HIV-1 genome daily (9). Evolutionary pressure from the host immune response selects for HIV-1 mutations, resulting in amino acid changes that allow the virus to evade cellular and humoral immune responses. Published estimates of the proportion of amino acid sequence changes arising during early HIV-1 and simian immunodeficiency virus infection that are attributable to selection pressure from virus-specific T cells have ranged widely, from 13.5% (4) to over 60% (2, 20). Some well-characterized CD8-escape mutations frequently revert to the consensus sequence following transmission to a new host who lacks the restricting HLA (3, 13, 18), while others do not (10, 17, 23). The extent, kinetics, and determinants of CD8 T-cell-driven viral escape and reversion remain unclear. Epitopes that rapidly revert to wild type following transmission represent attractive vaccine targets due to the presumed fitness cost associated with escape, as well as the likelihood that they will continue to propagate in the population as the virus evolves.
Efforts to define the kinetics of intrahost viral evolution during early infection and the relative contribution of HIV-specific immunity to early selection pressure have been hampered by the fact that the transmitted HIV-1 sequence responsible for establishing infection is seldom known. Recently, new experimental approaches such as single-genome amplification (SGA) followed by direct sequencing and advanced phylogenetic modeling have enabled inference of transmitted founder sequences, revealing that 70 to 80% of cases of sexually acquired HIV infection result from transmission of a single virus (16, 21). This approach has recently been extended to full-length HIV-1 genomes, permitting characterization of early changes in non-env sequences (14, 22). Despite these advances, phylogenetic inference of the transmitted sequences is inexact, particularly when multiple variants are transmitted or when samples are obtained at later time points of infection. Moreover, without data regarding the HLA haplotype and virus-specific T-cell responses present in both transmission partners, it remains difficult to estimate the proportion of sequence evolution within an individual that is immune driven.
Direct comparison of viral sequences derived from vertically infected infants and their mothers enables detection of early escape and reversion events in subjects whose duration of infection is known with relatively high precision. Moreover, because infants share at least 3 class I HLA molecules with their mothers, viral evolution within epitopes restricted by shared and unshared alleles can be directly compared. Vertical transmission pairs therefore provide a powerful model for analyzing the impact of immune pressure on viral evolution following transmission. We performed population sequencing of HIV-1 isolates from 52 mother-child pairs in order to characterize early intrahost viral evolution and to estimate the proportion of mutations that are selected in response to HIV-specific CD8 T-cell immune pressure. HIV-1 Nef, Gag, and Pol were sequenced, as they are major targets of the CD8 T-cell response and are not subject to significant humoral immune selection pressure. Viral sequence data were supplemented by screening for HIV-specific T-cell responses in both mothers and infants. We hypothesized that the majority of nonsynonymous mutations would occur within T-cell epitopes and that reversion toward the consensus sequence would predominate during early infection, particularly in structurally constrained proteins, such as p24-Gag.
MATERIALS AND METHODS
Study cohort.
Fifty-two perinatally HIV-infected children and their mothers were enrolled in collaboration with the Jamaica Pediatric, Perinatal, and Adolescent HIV/AIDS Program (7, 8). All of the children were either antiretroviral therapy naïve at the time of analysis (n = 39; median age, 1.1 years) or had received antiretroviral therapy for less than 3 months (n = 13; median age, 2.9 years). Samples from the mothers were drawn concurrently with those from their children. The majority of subjects had been exposed to either peripartum nevirapine or zidovudine to prevent mother-to-child transmission. The study was approved by the Institutional Review Board of the Massachusetts General Hospital and the Ethics Committee of the University Hospital of the West Indies in Kingston, Jamaica. A parent or legal guardian of each subject provided written informed consent prior to participation.
Viral sequencing.
Population sequencing of HIV-1 gag, pol, and nef from plasma was performed using a QIAamp viral RNA minikit (Qiagen) following amplification by nested PCR (the inner and outer primer sets and PCR conditions were described previously [11]). The PCR products of the secondary reaction were purified and sequenced directly using an ABI 3100 DNA analyzer from Applied Biosystems.
Sequence analysis.
Sequencher software (Gene Codes Corp., Ann Arbor, MI) and MacVector software (version 4.1; Oxford Molecular) were used to edit and align sequences. Nucleotide mixtures were assigned if the secondary peak height exceeded 25% of the dominant peak height. Paired mother and infant sequences were aligned with the HIV-1 subtype B consensus sequence (http://www.hiv.lanl.gov/) for Gag, Pol, and Nef (data not shown). Insertions relative to the consensus sequence were deleted, and all sites of amino acid discordance between mother and child were identified. Changes both from a single residue to a mixed residue and from a mixed residue to a single residue were considered mutations. Ten subjects exhibited known antiretroviral drug resistance mutations (mostly K103N or M184V in Pol), but these represented only 1.1% of total mutations. Sensitivity analyses were performed to assess their influence on all statistical measures (see “Statistical analysis” below).
IFN-γ ELISPOT assay screening.
Subjects were screened by enzyme-linked immunospot (ELISPOT) assay for responses to overlapping peptides (18-mers with a 10-amino-acid [aa] overlap) spanning HIV-1 Gag, Nef, and Pol arrayed in a matrix format, with confirmation of individual responses being performed on the following day, as described previously (11). Peptide sequences were based on the HIV-1 consensus sequences for clade B. Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by Ficoll-Hypaque density gradient centrifugation and were plated at 50,000 to 100,000 cells/well in 96-well polyvinylidene difluoride-backed plates (Millipore, Bedford, MA) precoated with 2 μg/ml anti-gamma interferon (anti-IFN-γ) monoclonal antibody (Mabtech). Peptides were added at a final concentration of 20 μg/ml, and the plates were incubated overnight and processed by standard methods. Three negative-control wells contained cells and medium alone, and a positive-control well contained phytohemagglutinin. Individual IFN-γ-secreting cells were counted using an AID ELISPOT reader system (Cell Technology, Inc.). Results were calculated as the number of spot-forming cells (SFCs) per million input cells after subtraction of the background response (mean number of SFCs of all no-antigen wells, which in all cases was ≤20 SFCs/million PBMCs). A response was considered positive if it was both >50 SFCs/million PBMCs and >3 standard deviations above the average of the negative-control wells.
HIV-1 optimal epitopes.
HIV-1 optimal epitopes listed in the 2006 HIV Molecular Immunology Database (http://www.hiv.lanl.gov/content/immunology) were used to analyze maternal transmission of wild-type versus variant optimal sequences.
Shannon entropy scores.
Shannon entropy scores at each amino acid position were calculated on the basis of all full-length clade B Gag, Pol, and Nef protein sequences in the Los Alamos National Laboratory HIV Sequence Database using its online Entropy tool (24).
Statistical analysis.
Statistical analysis was performed using Stata statistical software, release 8.0 (Stata Corp., College Station, TX). Two-group comparisons were performed using the Wilcoxon rank-sum test, and categorical outcomes were compared using Fisher's exact test. Negative binomial regression was used to evaluate the relationship of age to accumulation of forward and reverse mutations. The final model selected by stepwise regression included age and its second-order polynomial as independent variables. The correlation of mutation frequencies at each residue with Shannon entropy values, which followed a nonnormal distribution, was assessed using the Spearman rank correlation coefficient. Sensitivity analyses performed for all statistical comparisons revealed that inclusion of the antiretroviral drug mutations did not alter the interpretation of any statistical measures; therefore, all mutations were included in the final analyses. All statistical tests were two-tailed, with P values of <0.05 being considered significant.
RESULTS
HIV-1 sequence divergence between mother and child.
We performed population sequencing of HIV-1 gag, nef, and pol from plasma RNA samples obtained from 52 vertically HIV-infected children and their mothers. At the time of analysis, the median age of the children, and thus their approximate duration of infection, was 1.3 years (interquartile range, 0.8 to 2.8 years) and their median viral load was 232,500 RNA copies/ml (range, 2,670 to >1,000,000). Full-length sequences were successfully generated from both mother and child for 48 pairs for nef, 39 pairs for gag, 40 pairs for pol, and 34 pairs for all three viral genes (Table 1). Viral sequences derived from mother-infant pairs were aligned to identify sites of amino acid discordance. A total of 1,475 discordant sites were identified.
TABLE 1.
Viral regions for which paired mother-child sequences were generated
| Region | No. of subject pairs | Median (range) no. of mutations | Protein length (aa) | Median no. of mutations per aa |
|---|---|---|---|---|
| Gag | 39 | 10 (1-35) | 501 | 0.020 |
| Pol | 40 | 12 (0-46) | 1003 | 0.012 |
| Nef | 48 | 9 (0-34) | 206 | 0.043 |
| All 3 | 34 | 30 (12-94) | 0.018 |
Among subject pairs with data for all 3 proteins, there were a median of 30 amino acid sequence differences between mother and child (range, 12 to 94). The distribution of these nonsynonymous mutations was nearly equal among the 3 proteins, with medians of 10 in Gag, 12 in Pol, and 9 in Nef (Table 1). However, following adjustment for the length of the protein, the frequency of mutations was the highest in Nef, at 4.3% of all amino acids, compared to 2.0% in Gag and 1.2% in Pol (Table 1; P = 0.1566, Kruskal-Wallis test). These results are in agreement with those of two earlier studies of horizontal HIV infection (4, 19), which found that after adjustment for length, the nonstructural protein Nef accumulated more nonsynonymous mutations during early infection than the products of the relatively conserved genes pol and gag.
We next examined the relationship between the number of discordant amino acids and the age of the child, which roughly equates with time since infection. Among the 34 mother-child pairs for whom sequences of all three genes were obtained, there was a strong linear correlation between age and the cumulative number of amino acid changes (P < 0.0001; R2 = 0.398) (Fig. 1). The overall degree of mother-child divergence during the first 2 years of life was 1.74% per amino acid per year (95% confidence interval, 1.34 to 2.15%). When viral proteins were analyzed individually, a linear correlation was observed between age and the numbers of Gag mutations (P < 0.0001; R2 = 0.375) and Pol mutations (P < 0.0001; R2 = 0.450) but not the number of Nef mutations (P = 0.287; R2 = 0.025), perhaps due to the rapid early accumulation of mutations in this nonstructural protein.
FIG. 1.
Accumulation of viral mutations with age (in years). The relationship between age and the cumulative number of discordant amino acids in mother-child sequences is shown for Gag (a; n = 39), Nef (b; n = 48), Pol (c; n = 40), and all three proteins combined (d; n = 34). There was a strong linear correlation with age for all except Nef.
Mutations toward and away from the HIV-1 consensus sequence.
We next compared the amino acid residues at sites of mother-child discordance to the HIV-1 subtype B consensus sequence, a curated alignment of the amino acid most frequently observed at each site (www.hiv.lanl.gov), in order to discriminate between forward mutations away from the consensus sequence, which represent candidate escape mutations, and reverse mutations toward the consensus sequence, which represent candidate reversions. “Forward” mutations were defined as sites where the mother's amino acid matched the consensus sequence but the child's did not. “Reverse” mutations were defined as sites where the child's amino acid matched the consensus sequence but the mother's did not. “Lateral” mutations were defined as sites of amino acid discordance at which the amino acid from neither the mother nor the child matched the consensus sequence. The ratios of forward/reverse mutations were 1.3 for Gag, 1.0 for Pol, and 0.93 for Nef. Overall, the proportions of cumulative forward (47%) and reverse (42%) mutations observed in the cohort were nearly equal, indicating that positive selection due to immune and nonimmune pressures was nearly balanced by purifying selection pressures to preserve optimal viral fitness in the new host.
We hypothesized that escape and reversion may be subject to different kinetics, with a greater number of reversions occurring in early infection, upon removal of immune pressure mediated by maternal HLA, and a more gradual accumulation of escape mutations occurring in response to the emergence of infant T-cell responses over time. We therefore assessed the relationship between age and the cumulative number of forward and reverse mutations using negative binomial regression. Reverse mutations fit a second-order polynomial distribution, with rapid accumulation occurring during infancy, which leveled off after early childhood (P < 0.0001) (Fig. 2). In the case of forward mutations, the data best-fit model was nearly linear, with progressive accumulation of mutations away from the consensus sequence occurring throughout childhood (P = 0.0014; the polynomial term is nonsignificant). Thus, while overall the accumulation of forward and reverse mutations was nearly balanced, the kinetics of their emergence were distinct. These data support and extend an earlier observation by Li et al. that reverse mutations predominate during the first 6 months after horizontal infection but that forward mutations predominate thereafter (19).
FIG. 2.
Temporal emergence of forward and reverse mutations. The cumulative number of forward (red) and reverse (blue) mutations is shown according to age (in years). Curve fits were generated using negative binomial regression.
The relationship of viral load to the cumulative number of forward and reverse mutations was assessed for each protein using multiple linear regression. The number of reverse mutations in Gag showed a weak positive correlation with HIV RNA levels following adjustment for age (P = 0.048), but this association was not significant following Bonferroni correction for multiple comparisons. The numbers of forward and reverse mutations in other proteins were not associated with viral load.
Contribution of HIV-specific CD8 T-cell responses to early intrahost viral evolution.
We next investigated the extent to which the observed forward and reverse amino acid mutations could be explained by CD8 T-cell responses, using two complementary approaches. In the first approach, which is similar to that used in prior studies of horizontal transmission (2, 4, 15), we determined whether observed mutations were located within defined optimal epitopes listed in the 2006 HIV Molecular Immunology Database. Forward mutations were considered to be potentially CD8 associated if they were located within or immediately adjacent to a previously defined CD8 epitope restricted by HLA expressed by the child, and reverse mutations were considered to be potentially CD8 associated if they were located within or immediately adjacent to a previously defined CD8 epitope restricted by HLA expressed by the mother. This analysis revealed that, overall, 11.8% of forward mutations were potentially associated with CD8 responses. When proteins were analyzed individually, 13% of forward mutations in Gag, 16% of forward mutations in Nef, and 7% of forward mutations in Pol mapped to previously defined epitopes (Fig. 3). For reverse mutations, the overall proportion potentially attributable to CD8 T-cell responses was 13% (13% in Gag, 16% in Nef, and 10% in Pol).
FIG. 3.
Proportion of nonsynonymous mutations attributable to CD8 T-cell pressure. The proportion of forward (red) and reverse (blue) mutations in Gag, Nef, and Pol that were potentially attributable to cytotoxic T-lymphocyte immune pressure were determined using two different criteria: location within a published optimal HIV epitope (left) and location within an overlapping peptide eliciting a demonstrable T-cell response (right).
To complement the above approach, we conducted an IFN-γ ELISPOT assay screen with overlapping peptides spanning each HIV protein to comprehensively catalog all of the T-cell responses present in both mother and child and assessed the proportion of amino acid changes lying within these targeted peptides. In this analysis, forward mutations were defined as “potentially CD8 associated” if they were within an overlapping HIV peptide that was recognized by the child. Reverse mutations were considered to be “potentially CD8 associated” if they were within an overlapping HIV peptide that was recognized by the mother. Screening for demonstrable T-cell responses resulted in a substantially higher estimate of the proportion of mutations that were potentially attributable to CD8 escape and reversion (14% of forward mutations and 24% of reverse mutations; Fig. 3). There was a substantial degree of overlap between the two analyses, as the vast majority of mutations within optimal epitopes predicted on the basis of HLA were confirmed by ELISPOT assay. However, the converse was not true, as many mutations located within peptides recognized by ELISPOT assay did not contain epitopes that could be predicted on the basis of HLA. Combining the two approaches, we found that 15% of forward mutations and 25% of reverse mutations could be attributed to T-cell activity by at least one of the criteria.
Mutational hot spots coincide predominantly with high-entropy codons.
We determined the frequency of forward and reverse mutations at each codon to identify hot spots of viral evolution following transmission. The highest density of mutations was observed in Nef, where nonsynonymous mutations were observed at some codons in nearly a third of all subjects (Fig. 4). Within Gag, the majority of mutations were concentrated outside p24-encoding regions. We next compared the mutation frequency at each codon to its Shannon entropy score, generated using all clade B HIV-1 sequences in the Los Alamos National Laboratory HIV Sequence Database and the online Entropy tool (24). The median entropy values generated for codons in Gag, Pol, and Nef were 0.08, 0.05, and 0.23, respectively. The entropy scores for codons where forward mutations (n = 381) and reverse mutations (n = 342) were observed did not differ (P = 0.461, Wilcoxon rank-sum test). There was a strong positive association between the overall frequency of mutations observed at individual residues and entropy (P < 0.0001; Spearman's rho = 0.629). A similar relationship was observed when forward mutations (P < 0.0001; Spearman's rho = 0.387) and reverse mutations (P < 0.0001; Spearman's rho = 0.376) were considered separately.
FIG. 4.
Distribution of mutations in Nef, Gag, and Pol. The proportion of mother-child pairs exhibiting nonsynonymous mutations is shown individually for each codon in Nef, Gag, and Pol. The contributions of forward mutations (red), reverse mutations (blue), and lateral mutations (green) are indicated by the stacked segments. Brackets indicate the boundaries of sequences encoding subproteins of Gag (p17, p24, p2p7p1p6) and Pol (protease, reverse transcriptase [RT], and integrase [int]).
Proportion of defined optimal epitopes that develop forward or reverse mutations.
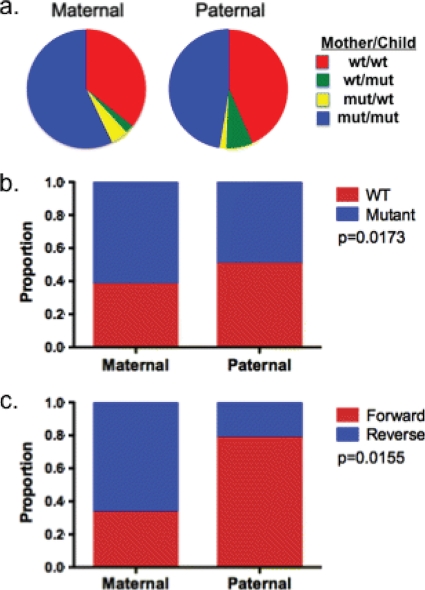
Using the maternal plasma RNA population sequence as an approximation of the virus that was transmitted to the child, we estimated the proportion of optimal epitopes transmitted intact (i.e., with a sequence matching the published sequence of the epitope) that escape following transmission and the proportion of optimal epitopes transmitted mutated that revert following transmission (Fig. 5). We restricted this analysis to 24 mother-child pairs from whom full sequences for Gag, Nef, and Pol were available and for whom optimal epitopes restricted by both maternally and paternally inherited alleles were listed in the HIV Molecular Immunology Database. A total of 406 defined optimal epitopes were restricted by HLA expressed in the children (median, 16.5 per child; range, 6 to 32). Among 174 defined optimal epitopes that were transmitted intact to a child expressing the restricting HLA, 9.8% acquired mutations following transmission. To determine reversion rates, sequence variations within epitopes restricted by HLA alleles that were expressed by the mother but not by the child were examined. Among 236 optimal epitopes restricted by HLA present in the mother but not the child, 9.6% of variant epitopes (12 of 125) reverted to the wild-type optimal sequence following transmission to the child. Reversion to the wild type was also observed, although at a lower frequency, within epitopes restricted by alleles that were shared by the child and the child's mother (7.7%) or father (3.9%). The surprisingly low rate of reversion of mutated epitopes following transmission to a new host who lacks the restricting HLA allele, despite a median of 1.3 years of clinical follow-up, suggests that the fitness cost associated with many CD8 epitope mutations may be modest.
FIG. 5.
Escape and reversion within defined optimal HIV-1 epitopes. (a) The proportion of all optimal epitopes restricted by child-expressed HLA (total, 406) is displayed according to whether the autologous sequences matched the published epitope sequence. Data are shown separately for optimal epitopes restricted by HLA alleles that were inherited maternally (n = 252, left) versus paternally (n = 154, right). The proportion of these epitopes for which maternal and child sequence were both wild type (wt or WT) relative to the optimal sequence is shown in red, the proportion for which maternal and child sequences were both mutated (mut) relative to the published sequence is shown in blue, and the proportion of epitopes which developed mutations toward or away from the published optimal sequence are shown in yellow and green, respectively. (b) The proportion of optimal epitopes whose sequence was transmitted intact (wild type, in red) versus mutated (blue) is shown separately, according to the parental inheritance of the restricting HLA allele (P = 0.0173). (c) Among epitopes that were observed to mutate, the proportions of forward and reverse mutations are shown according to parental inheritance, with forward mutations predominating in paternally restricted epitopes and reverse mutations predominating in maternally restricted epitopes (P = 0.0155).
Also notable in this analysis was the overall high degree of mismatch between the viral sequences observed in our subjects and the sequences of defined optimal epitopes in the HIV Molecular Immunology Database. Of the 406 defined optimal epitopes restricted by the children's HLA alleles, the sequences of only 174 (42.8%) of these matched the predominant viral sequence observed in the corresponding mother. The optimal sequence was more likely to be intact if the epitope was restricted by paternally inherited HLA (50.6%) than by maternally inherited HLA (38.1%) (P = 0.0173, Fisher's exact test) (Fig. 5b), indicating that maternal immune selection pressure was responsible for some of the sequence mismatch. Nonetheless, only half of the epitopes restricted by paternal alleles, which had not been subject to immune pressure in the mother prior to transmission, were intact in the transmitted sequences. While sequence variation within epitopes does not always preclude presentation and recognition of the variant (12), these data underscore the limitations of published epitope databases for predicting the epitopes targeted by an infected individual.
DISCUSSION
At the population level, HIV-1 evolution is characterized by repeated cycles of transmission, which imposes a severe genetic bottleneck, followed by dramatic intrahost diversification to yield a complex and heterogeneous population of viral quasispecies. The selective forces responsible for both the genetic bottleneck and intrahost evolution are only now beginning to be understood (1). Our data indicate that the overall degree of early mother-child divergence is 1.74% of all amino acids per year. This represents a maximal estimate of the degree of sequence evolution in the newly infected child, as some of this divergence is likely due to continued viral evolution in the mother, despite chronic infection. This estimate is quite similar to the mutation frequency rate of 1.66% per amino acid per year during early infection among horizontally infected adults reported by Li et al. (19). Mutations toward and away from the consensus sequence were nearly balanced but differed slightly in their kinetics.
Because viral sequence and HLA data are rarely available from both members of a horizontal transmission pair, prior studies that examined HIV-1 escape and reversion used the earliest available sample from an acutely HIV-infected subject, rather than samples derived from the infecting partner, as a baseline for comparison to sequences obtained later in infection (2, 4, 19). This approach may fail to detect the very early escape or reversion events that have been shown to arise within 4 to 6 weeks of infection, before the majority of acute HIV infections are diagnosed (22). Likely as a result of this difference in approach, our estimates of the mutation frequency per amino acid in Nef, Gag, and Pol were 66 to 80% higher than those observed by Li et al. during the first year following horizontal infection in adults (19). The longer duration of infection in our cohort (median, 1.3 years) and continued evolution of the maternal virus following transmission may also have contributed to these higher estimates. Still, the ratio of forward and reverse mutations observed in our study was nearly identical to that reported by Li et al., who found that 42% of nonsynonymous mutations represented changes toward the consensus sequence. These findings contrast with the 3:1 ratio of forward to reverse mutations observed in chronic infection (2), providing further evidence that the kinetics of escape and reversion following transmission differ between acute and chronic infection, with a predominance of reversions toward the consensus sequence occurring in the early stages. In agreement with prior studies (4, 19), we observed that Nef developed more nonsynonymous mutations per amino acid than either Gag or Pol, both of which are structural proteins and therefore perhaps less able to accommodate amino acid variation.
Several prior studies of horizontal transmission have attempted to estimate the extent to which intrahost sequence diversification can be attributed to immune selection pressure, most often by determining the proportion of observed mutations that could be mapped onto defined optimal epitopes in the Los Alamos National Laboratory HIV Molecular Immunology Database. Two such studies of adults with acute HIV-1 infection found that 8 to 13.5% of non-Env mutations mapped within or adjacent to known CD8 epitopes restricted by the subjects’ class I HLA (4, 19), comparable to our estimates (11.8% of forward mutations and 13% of reverse mutations) obtained using similar criteria. However, there are several limitations to this approach, including the high degree of mismatch between the sequences of circulating viruses and the sequences published in epitope databases and the increasing density of published epitope maps, which could result in erroneous attribution of CD8 selection pressure. Moreover, estimating the proportion of mutations that represent reversions is particularly problematic in the absence of HLA information for the transmitter. Prior efforts have designated mutations CD8 associated if they lie within any epitope restricted by HLA not expressed by the host (2, 19), but these criteria are quite liberal, given the current density of epitope maps, where few codons are not encompassed by at least one epitope. To circumvent these shortcomings, we used the complementary approach of screening for demonstrable T-cell responses in the child (at sites of forward mutations) and mother (at sites of reverse mutations). On the basis of this analysis, we found that an unprecedented 24% of reverse mutations and more than one-third of those in Nef occurred in regions eliciting a demonstrable response by maternal T cells. The increased yield of CD8-attributable responses was less marked for forward mutations, which may be due in part to the fact that the frequencies of CD8 T cells targeting epitopes that have escaped often decline to levels that are undetectable ex vivo (5, 14).
The primary limitations of our study arise from its cross-sectional design, which limited our ability to define the kinetics of viral evolution precisely, and our reliance on the sequence of the maternal virus as an approximation of the sequence of the transmitted virus. While the study of paired mother-child sequences presents several advantages, including the ability to detect hyperacute escape and reversion and the ability to determine the HLA type and T-cell responses of the transmitting partner, it is likely that the predominant sequence in the mother does not precisely reflect the transmitted species, which may be a minor variant of the circulating viral population in the infecting partner at the time of transmission. It is not currently known how many HIV-1 species are typically transmitted from a mother to her infant, nor is it known whether distinct bottleneck pressures influence perinatal transmission. While recent studies employing single-genome amplification and direct sequencing have shed light on the molecular details of horizontal transmission, a similarly detailed molecular understanding of mother-to-child transmission and early viral evolution is lacking.
While our data suggest that a substantial proportion of early viral mutations were attributable to CD8 immune pressure, the majority were not, indicating that early positive selection events are driven by other forces, in addition to CD8 T cells. These may include compensatory mutations (6, 18) and transmission bottleneck pressures, including biological constraints for transmitted virus to establish infection via the mucosal route (22), or nonimmune selection pressures acting subsequent to transmission. Our data support those from recent studies that identified many early positive selection events that could not be explained by CD8, neutralizing antibodies, or reversion (14). It is likely that viral evolution is driven by a composite of both immune (including potentially CD4/NK/humoral) and nonimmune pressures, including biological constraints which characterize transmitted founder sequences, such as C-C chemokine receptor type 5 (CCR5) tropism and concealment of coreceptor binding bridging sheet and V3 loop structures, and which may facilitate mucosal transmission and efficient replication in CD4+ T lymphocytes (22). Further studies are needed to elucidate the unique determinants and biological mechanisms underlying HIV-1 transmission from mother to child.
Acknowledgments
We thank all of the study subjects and their families for participating in this study. We are grateful to Otto Yang for his assistance with calculating Shannon entropy scores from clade B sequences and to the UCSF Clinical and Translational Science Institute (CTSI) Biostatistics Consulting Unit for assistance with regression analyses.
This work was supported by the Elizabeth Glaser Pediatric AIDS Foundation (M.E.F. and C.D.C.), Jewelers for Children, the Charles H. Hood Foundation (M.E.F.), and the NIAID/NIH (grant R01-AI068497 to M.E.F.). C.D.C. is a recipient of an International Leadership Award from the Elizabeth Glaser Pediatric AIDS Foundation. M.E.F. is the recipient of the Jewelers for Children Elizabeth Glaser Scientist Award.
Footnotes
Published ahead of print on 22 September 2010.
REFERENCES
- 1.Abrahams, M. R., J. A. Anderson, E. E. Giorgi, C. Seoighe, K. Mlisana, L. H. Ping, G. S. Athreya, F. K. Treurnicht, B. F. Keele, N. Wood, J. F. Salazar-Gonzalez, T. Bhattacharya, H. Chu, I. Hoffman, S. Galvin, C. Mapanje, P. Kazembe, R. Thebus, S. Fiscus, W. Hide, M. S. Cohen, S. A. Karim, B. F. Haynes, G. M. Shaw, B. H. Hahn, B. T. Korber, R. Swanstrom, and C. Williamson. 2009. Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-Poisson distribution of transmitted variants. J. Virol. 83:3556-3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, T. M., M. Altfeld, S. C. Geer, E. T. Kalife, C. Moore, K. M. O'Sullivan, I. Desouza, M. E. Feeney, R. L. Eldridge, E. L. Maier, D. E. Kaufmann, M. P. Lahaie, L. Reyor, G. Tanzi, M. N. Johnston, C. Brander, R. Draenert, J. K. Rockstroh, H. Jessen, E. S. Rosenberg, S. A. Mallal, and B. D. Walker. 2005. Selective escape from CD8+ T-cell responses represents a major driving force of human immunodeficiency virus type 1 (HIV-1) sequence diversity and reveals constraints on HIV-1 evolution. J. Virol. 79:13239-13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barouch, D. H., J. Powers, D. M. Truitt, M. G. Kishko, J. C. Arthur, F. W. Peyerl, M. J. Kuroda, D. A. Gorgone, M. A. Lifton, C. I. Lord, V. M. Hirsch, D. C. Montefiori, A. Carville, K. G. Mansfield, K. J. Kunstman, S. M. Wolinsky, and N. L. Letvin. 2005. Dynamic immune responses maintain cytotoxic T lymphocyte epitope mutations in transmitted simian immunodeficiency virus variants. Nat. Immunol. 6:247-252. [DOI] [PubMed] [Google Scholar]
- 4.Bernardin, F., D. Kong, L. Peddada, L. A. Baxter-Lowe, and E. Delwart. 2005. Human immunodeficiency virus mutations during the first month of infection are preferentially found in known cytotoxic T-lymphocyte epitopes. J. Virol. 79:11523-11528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrow, P., H. Lewicki, X. Wei, M. S. Horwitz, N. Peffer, H. Meyers, J. A. Nelson, J. E. Gairin, B. H. Hahn, M. B. Oldstone, and G. M. Shaw. 1997. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 3:205-211. [DOI] [PubMed] [Google Scholar]
- 6.Carlson, J. M., Z. L. Brumme, C. M. Rousseau, C. J. Brumme, P. Matthews, C. Kadie, J. I. Mullins, B. D. Walker, P. R. Harrigan, P. J. Goulder, and D. Heckerman. 2008. Phylogenetic dependency networks: inferring patterns of CTL escape and codon covariation in HIV-1 Gag. PLoS Comput. Biol. 4:e1000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christie, C. D. 2008. Pediatric and perinatal HIV/AIDS in Jamaica. West Indian Med. J. Special Issue 57:187-320. [Google Scholar]
- 8.Christie, C. D. 2004. Pediatric and Perinatal HIV/AIDS in Jamaica. West Indian Med. J. Special Issue 53:271-365. [Google Scholar]
- 9.Coffin, J. M. 1995. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science 267:483-489. [DOI] [PubMed] [Google Scholar]
- 10.Crawford, H., J. G. Prado, A. Leslie, S. Hue, I. Honeyborne, S. Reddy, M. van der Stok, Z. Mncube, C. Brander, C. Rousseau, J. I. Mullins, R. Kaslow, P. Goepfert, S. Allen, E. Hunter, J. Mulenga, P. Kiepiela, B. D. Walker, and P. J. Goulder. 2007. Compensatory mutation partially restores fitness and delays reversion of escape mutation within the immunodominant HLA-B*5703-restricted Gag epitope in chronic human immunodeficiency virus type 1 infection. J. Virol. 81:8346-8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feeney, M. E., K. A. Roosevelt, Y. Tang, K. J. Pfafferott, K. McIntosh, S. K. Burchett, C. Mao, B. D. Walker, and P. J. Goulder. 2003. Comprehensive screening reveals strong and broadly directed human immunodeficiency virus type 1-specific CD8 responses in perinatally infected children. J. Virol. 77:7492-7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feeney, M. E., Y. Tang, K. Pfafferott, K. A. Roosevelt, R. Draenert, A. Trocha, X. G. Yu, C. Verrill, T. Allen, C. Moore, S. Mallal, S. Burchett, K. McIntosh, S. I. Pelton, M. A. St. John, R. Hazra, P. Klenerman, M. Altfeld, B. D. Walker, and P. J. Goulder. 2005. HIV-1 viral escape in infancy followed by emergence of a variant-specific CTL response. J. Immunol. 174:7524-7530. [DOI] [PubMed] [Google Scholar]
- 13.Friedrich, T. C., E. J. Dodds, L. J. Yant, L. Vojnov, R. Rudersdorf, C. Cullen, D. T. Evans, R. C. Desrosiers, B. R. Mothe, J. Sidney, A. Sette, K. Kunstman, S. Wolinsky, M. Piatak, J. Lifson, A. L. Hughes, N. Wilson, D. H. O'Connor, and D. I. Watkins. 2004. Reversion of CTL escape-variant immunodeficiency viruses in vivo. Nat. Med. 10:275-281. [DOI] [PubMed] [Google Scholar]
- 14.Goonetilleke, N., M. K. Liu, J. F. Salazar-Gonzalez, G. Ferrari, E. Giorgi, V. V. Ganusov, B. F. Keele, G. H. Learn, E. L. Turnbull, M. G. Salazar, K. J. Weinhold, S. Moore, N. Letvin, B. F. Haynes, M. S. Cohen, P. Hraber, T. Bhattacharya, P. Borrow, A. S. Perelson, B. H. Hahn, G. M. Shaw, B. T. Korber, and A. J. McMichael. 2009. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J. Exp. Med. 206:1253-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kearney, M., F. Maldarelli, W. Shao, J. B. Margolick, E. S. Daar, J. W. Mellors, V. Rao, J. M. Coffin, and S. Palmer. 2009. Human immunodeficiency virus type 1 population genetics and adaptation in newly infected individuals. J. Virol. 83:2715-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keele, B. F., E. E. Giorgi, J. F. Salazar-Gonzalez, J. M. Decker, K. T. Pham, M. G. Salazar, C. Sun, T. Grayson, S. Wang, H. Li, X. Wei, C. Jiang, J. L. Kirchherr, F. Gao, J. A. Anderson, L. H. Ping, R. Swanstrom, G. D. Tomaras, W. A. Blattner, P. A. Goepfert, J. M. Kilby, M. S. Saag, E. L. Delwart, M. P. Busch, M. S. Cohen, D. C. Montefiori, B. F. Haynes, B. Gaschen, G. S. Athreya, H. Y. Lee, N. Wood, C. Seoighe, A. S. Perelson, T. Bhattacharya, B. T. Korber, B. H. Hahn, and G. M. Shaw. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 105:7552-7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leslie, A., D. Kavanagh, I. Honeyborne, K. Pfafferott, C. Edwards, T. Pillay, L. Hilton, C. Thobakgale, D. Ramduth, R. Draenert, S. Le Gall, G. Luzzi, A. Edwards, C. Brander, A. K. Sewell, S. Moore, J. Mullins, C. Moore, S. Mallal, N. Bhardwaj, K. Yusim, R. Phillips, P. Klenerman, B. Korber, P. Kiepiela, B. Walker, and P. Goulder. 2005. Transmission and accumulation of CTL escape variants drive negative associations between HIV polymorphisms and HLA. J. Exp. Med. 201:891-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leslie, A. J., K. J. Pfafferott, P. Chetty, R. Draenert, M. M. Addo, M. Feeney, Y. Tang, E. C. Holmes, T. Allen, J. G. Prado, M. Altfeld, C. Brander, C. Dixon, D. Ramduth, P. Jeena, S. A. Thomas, A. St. John, T. A. Roach, B. Kupfer, G. Luzzi, A. Edwards, G. Taylor, H. Lyall, G. Tudor-Williams, V. Novelli, J. Martinez-Picado, P. Kiepiela, B. D. Walker, and P. J. Goulder. 2004. HIV evolution: CTL escape mutation and reversion after transmission. Nat. Med. 10:282-289. [DOI] [PubMed] [Google Scholar]
- 19.Li, B., A. D. Gladden, M. Altfeld, J. M. Kaldor, D. A. Cooper, A. D. Kelleher, and T. M. Allen. 2007. Rapid reversion of sequence polymorphisms dominates early human immunodeficiency virus type 1 evolution. J. Virol. 81:193-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Connor, D. H., A. B. McDermott, K. C. Krebs, E. J. Dodds, J. E. Miller, E. J. Gonzalez, T. J. Jacoby, L. Yant, H. Piontkivska, R. Pantophlet, D. R. Burton, W. M. Rehrauer, N. Wilson, A. L. Hughes, and D. I. Watkins. 2004. A dominant role for CD8+-T-lymphocyte selection in simian immunodeficiency virus sequence variation. J. Virol. 78:14012-14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salazar-Gonzalez, J. F., E. Bailes, K. T. Pham, M. G. Salazar, M. B. Guffey, B. F. Keele, C. A. Derdeyn, P. Farmer, E. Hunter, S. Allen, O. Manigart, J. Mulenga, J. A. Anderson, R. Swanstrom, B. F. Haynes, G. S. Athreya, B. T. Korber, P. M. Sharp, G. M. Shaw, and B. H. Hahn. 2008. Deciphering human immunodeficiency virus type 1 transmission and early envelope diversification by single-genome amplification and sequencing. J. Virol. 82:3952-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salazar-Gonzalez, J. F., M. G. Salazar, B. F. Keele, G. H. Learn, E. E. Giorgi, H. Li, J. M. Decker, S. Wang, J. Baalwa, M. H. Kraus, N. F. Parrish, K. S. Shaw, M. B. Guffey, K. J. Bar, K. L. Davis, C. Ochsenbauer-Jambor, J. C. Kappes, M. S. Saag, M. S. Cohen, J. Mulenga, C. A. Derdeyn, S. Allen, E. Hunter, M. Markowitz, P. Hraber, A. S. Perelson, T. Bhattacharya, B. F. Haynes, B. T. Korber, B. H. Hahn, and G. M. Shaw. 2009. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J. Exp. Med. 206:1273-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneidewind, A., Y. Tang, M. A. Brockman, E. G. Ryland, J. Dunkley-Thompson, J. C. Steel-Duncan, M. A. St. John, J. A. Conrad, S. A. Kalams, F. Noel, T. M. Allen, C. D. Christie, and M. E. Feeney. 2009. Maternal transmission of HIV escape mutations subverts HLA-B57 immunodominance but facilitates viral control in the haploidentical infant. J. Virol. 83:8616-8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang, O. O. 2009. Candidate vaccine sequences to represent intra- and inter-clade HIV-1 variation. PLoS One 4:e7388. [DOI] [PMC free article] [PubMed] [Google Scholar]