Abstract
Bone marrow stromal cell antigen 2 (BST-2, also known as tetherin/CD317/HM1.24) inhibits the release of human immunodeficiency virus type 1 (HIV-1) and other enveloped viruses by tethering virus particles to the cell surface. In this study, we provide evidence not only that the yield of cell-free HIV-1 particles is significantly reduced by BST-2 but also that the infectivity of these progeny virions is severely impaired. The lowered virion infectivity is due to the accumulation of pr55 Gag precursor and the p40Gag intermediates as well as to the loss of a mature core in the majority of HIV-1 particles. These data suggest that, in addition to impeding the release of HIV-1 particles from host cells, BST-2 may also interfere with the activation of viral protease and, as a result, impairs viral Gag processing as well as maturation of HIV-1 particles.
Cellular restriction factors constitute an important defense system that hosts have evolved to combat pathogen infection (39). In recent years, a number of cellular restriction factors have been discovered that inhibit replication of human immunodeficiency virus type 1 (HIV-1) by targeting distinct steps of the viral replication cycle. Examples include APOBEC3G (apolipoprotein B mRNA editing enzyme 3G) that causes hypermutation of HIV-1 cDNA during viral RNA reverse transcription (34), Trim5α (tripartite motif 5α) from Old World monkeys that targets the incoming HIV-1 core and destroys the viral reverse transcription complex (36), and BST-2 (bone marrow stromal cell antigen 2, also known as tetherin/CD317/HM1.24) which inhibits HIV-1 production by impeding the release of progeny virions from the cell surface (25, 37).
Since the identification of BST-2 as a restriction factor to HIV-1, it has also been shown to restrict the production of other enveloped viruses including HIV-2, simian immunodeficiency virus (SIV), Kaposi's sarcoma herpes virus (KSHV), Lassa virus, Marburg virus, and Ebola virus (13, 14, 22, 30). In order to evade the restriction imposed by BST-2, different viruses have evolved various countermeasures. In the case of HIV-1, the viral protein Vpu causes downregulation of BST-2 from the cell surface and, as a result, removes BST-2 from virus budding sites (37). Vpu may exert this effect either by sequestering BST-2 at the trans-Golgi network or by diverting BST-2 trafficking to proteasomes or lysosomes for degradation through recruitment of the β-TrCP (beta-transducin repeats-containing protein) E3 ubiquitin ligase (2, 3, 21, 23). In contrast to HIV-1, most primate lentiviruses overcome the antiviral action of BST-2 using the viral Nef protein; these include SIVsmm (SIV in sooty mangabey), SIVmac (macaque), SIVsyk (Syke's monkey), SIVagm (African green monkey), SIVcpz (chimpanzee) and SIVgor (gorilla) (12, 32, 40). HIV-2 uses its envelope protein to combat BST-2 (18), whereas SIVtan (tantalus monkey) requires both Nef and Env to effectively overcome BST-2 restriction (8, 40). In a similar fashion, the Ebola virus counteracts BST-2 with its viral envelope protein (14, 19). In the case of KSHV, viral protein K5/MIR2 causes ubiquitination and subsequent degradation of BST-2 (22, 26).
The antiviral function of BST-2 is attributed to its unique structure. BST-2 bears two domains that anchor its two ends at the plasma membrane: a transmembrane domain proximal to the N terminus and a C-terminal glycosylphosphatidylinositol (GPI) moiety (16). In particular, the GPI structure mediates association of BST-2 with lipid raft microdomains on which HIV-1 Gag proteins multimerize and form virus particles (16). As a result, BST-2 is incorporated into the envelope of virus particles (4, 27). It is supposed that some BST-2 molecules may have one terminus already inserted in the viral envelope whereas the other terminus is still in the plasma membrane, thus holding the newly formed virus particles to the cell surface (27). In addition, the extracellular coiled-coil domain mediates disulfide-linked dimerization and is thought to be necessary for the tethering activity of BST-2 (4, 10, 27). These structural features, rather than the primary sequences, endow BST-2 with its tethering activity. This notion is supported by the potent virion retention activity of an “artificial” tetherin that contains the transmembrane domain, the coiled-coil domain, and the GPI anchor from heterogeneous proteins (27). It is generally accepted that a reduction in the number of cell-free virus particles is the primary result of the tethering action of BST-2. Interestingly, several studies have shown that BST-2 diminishes the amounts of infectious HIV-1 particles to a higher degree than HIV-1 p24/CA antigen or reverse transcriptase that has been released into the culture supernatant (6, 25, 32, 37). This suggests a potential impact of BST-2 on the infectivity of HIV-1 particles. We now provide evidence that, in addition to physically tethering virus particles to the cell surface and thus diminishing the production of cell-free virions, BST-2 also impairs Gag processing and virus maturation and thus decreases virus infectivity.
MATERIALS AND METHODS
Plasmid DNA and antibodies.
Human BST-2 (hBST-2) and African green monkey BST-2 (agmBST-2) cDNA sequences were cloned into the expression vector pcDNA3.1 (Invitrogen), as previously described (29). Both hBST-2 and agmBST-2 proteins have a Flag tag attached to their N termini. The infectious HIV-1 viral clone BH10 and a Vpu cDNA clone (pcDNA-Vphu) were obtained from the NIH AIDS Research and Reference Reagent Program. BH10 with a deletion of Vpu [BH10(Vpu−)] has the initiation codon ATG of Vpu changed to ACG (29). Flag antibody and tubulin antibody were purchased from Sigma, HIV-1 p24 antibody was from ID Labs Inc., transferrin receptor antibody was from Novus Biologicals, and antiserum against human BST-2 and HIV-1 Vpu was obtained from the NIH AIDS Research and Reference Reagent Program (20, 24).
Cell culture and transfection.
Human embryonic kidney cells (293T), HeLa cells, and TZM-bl cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (Invitrogen). TZM-bl cells are used as indicator cells to measure the infectivity of HIV-1 particles (38). SupT1 cell lines that express hBST-2 with induction by doxycycline were cultured in RPMI 1640 medium supplemented with 10% tetracycline-free fetal bovine serum (Clontech), 1 mg/ml G418, and 2 μg/ml puromycin (29). Twenty-four hours prior to transfection, 293T cells were seeded in 12-well plates at 2 × 105 cells per well or in 10-cm dishes at 2.5 × 106 cells per dish. Eighteen hours prior to transfection with small interfering RNA (siRNA), HeLa cells were seeded in 10-cm plates at 0.6 × 106 cells per dish. A BST-2 siRNA oligonucleotide (5′-AGAAAGTGGAGGAGCTTGA-3′) designed to target human BST-2 mRNA at nucleotide positions 332 to 350 was purchased from Ambion together with a control siRNA (catalog number 4611). Transfection was performed with Lipofectamine 2000 (Invitrogen) in accordance with the manufacturer's instructions.
Western blotting.
Cell lysates were mixed with loading buffer containing 1% SDS, boiled for 5 min before loading, and separated in sodium dodecyl sulfate-polyacrylamide gels (10%). Western blots were probed with primary antibodies against a Flag tag (1:2,000 dilution), BST-2 (1:2,500 dilution), HIV-1 p24 (1:5,000 dilution), HIV-1 Vpu (1:2,000 dilution), transferrin receptor ([TfR] 1:1,000 dilution), or tubulin (1:5,000 dilution), followed by a second incubation with the horseradish peroxidase-conjugated secondary antibodies (1:5,000).
Purifying HIV-1 particles.
Supernatants from transfected 293T cells or HeLa cells were first clarified by centrifugation at 3,000 rpm at 4°C for 30 min in a benchtop Beckman centrifuge. Viral particles were pelleted by ultracentrifugation through a 15% sucrose cushion at 100,000 × g for 1 h at 4°C. The pelleted materials were loaded on the top of a 15% to 50% continuous sucrose gradient and centrifuged in an SW41 rotor (Beckman) at 100,000 × g for 16 h at 4°C. Twelve 1-ml fractions were collected from the top of the gradient. Presence of HIV-1 particles in each fraction was detected by Western blotting using anti-HIV-1 p24 antibody.
Measuring viral reverse transcriptase activity.
Viral reverse transcriptase activity was measured to determine the amounts of virus in culture supernatants. Briefly, 10 μl of culture supernatant was mixed with 40 μl of reaction buffer containing 0.5 unit/ml poly(rA)-oligo(dT) (Midland Certified Reagent Co.) and 0.1 mCi/ml [3H]dTTP (Perkin-Elmer). After a 3-h incubation at 37°C, reactions were terminated by the addition of 10% trichloroacetic acid (TCA). The precipitated oligonucleotides were collected by filtering the reaction mixtures through Millipore MultiScreen Glass Fiber FC plates (Millipore). After two washes with 10% TCA and one wash with ethanol, levels of 3H that were retained on the filters were scored in a liquid scintillation counter (Perkin-Elmer).
Measuring virus infectivity.
Virus infectivity was determined by infecting the TZM-bl indicator cells (38). Briefly, TZM-bl indicator cells were seeded in a 24-well plate 1 day prior to infection with 50 μl of culture supernatant. At 40 h postinfection, cells were lysed in 100 μl of 1× passive lysis buffer (Promega), and luciferase activity in 10 μl of cell lysate was measured using a luciferase assay kit (Promega). The luciferase activity indicates the relative infectivity of viruses.
Membrane flotation assay.
Transfected 293T cells were harvested and Dounce homogenized on ice in a buffer containing 10% sucrose, 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 2 mM EDTA, 0.1% 2-mercaptoethanol, and protease inhibitor cocktails (Roche Diagnostics, Laval, Quebec City, Canada). After clarification at 3,000 rpm for 30 min at 4°C to remove nuclei and cell debris, the clear fraction was mixed with 85% sucrose to a final concentration of 73% and loaded at the bottom of a 5-ml ultracentrifuge tube (Beckman). Two more layers of sucrose solutions (1 ml of 10% and 2.5 ml of 65%) were added before ultracentrifugation at 100,000 × g for 16 h at 4°C. All sucrose solutions were prepared in cell lysis buffer. Eight fractions were collected from the top of the sucrose gradient, and the membrane-associated materials at the 10%-65% interface were harvested as fraction 2. Western blotting was performed to detect HIV-1 Gag protein and the membrane marker transferrin receptor in each fraction.
Electron microscopy.
293T cells were transfected with DNA of Vpu-deleted HIV-1 alone or together with human BST-2 cDNA. Following clarification at 3,000 rpm at 4°C for 30 min, culture supernatants were passed through a 0.45-μm-pore-size filter and subjected to ultracentrifugation through a 15% sucrose cushion to pellet HIV-1 particles. After the supernatants were carefully discarded, 500 μl of 1% low-melting-point agarose was added to collect virus particles at the bottom of the centrifuge tube. Virus samples were then fixed in 2.5% glutaraldehyde overnight at 4°C and underwent routine processing and embedding procedures for electron microscopy. The thin-sectioned samples were stained with lead citrate and uranyl acetate and examined with a JEOL JEM-2000 FX transmission electron microscope equipped with a Gatan 792 Bioscan 1,024- by 1,024-byte wide-angle Multiscan charge-coupled device camera.
RESULTS
Expression of human BST-2 leads to production of poorly infectious HIV-1 particles.
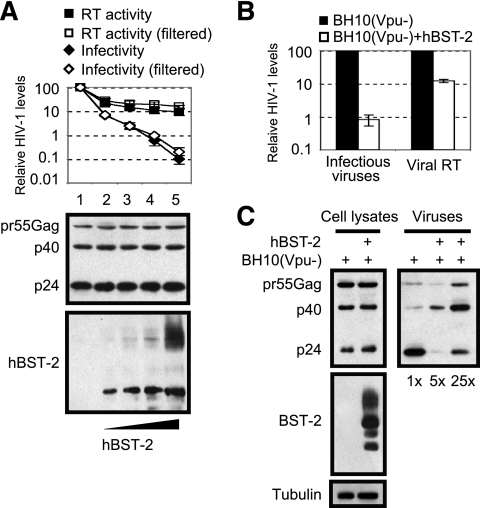
We first asked whether BST-2, in addition to hindering the release of HIV-1 particles from the cell surface, also exerts any effect on the infectivity of cell-free virions. To answer this question, we transfected 293T cells with Vpu-deleted HIV-1 clone BH10(Vpu−) DNA and various amounts of human BST-2 cDNA. The amounts of progeny virions in the culture supernatants were determined by measuring viral reverse transcriptase activity; the levels of infectious HIV-1 particles in the supernatants were determined by infecting the TZM-bl indicator cells. The results shown in Fig. 1A indicate a maximum 10-fold decrease in viral reverse transcriptase activity, whereas the levels of infectious cell-free virus particles diminished by as much as 1,000-fold. A similar observation was made when the culture supernatants were first clarified using the 0.22-μm-pore-size filters before viral reverse transcriptase activity and levels of infectious virus particles were determined (Fig. 1A). Therefore, in addition to physically inhibiting the release of HIV-1 particles from the cell surface and thus reducing the production of cell-free viruses, BST-2 also has a deleterious effect on the infectivity of HIV-1 particles, which leads to a greater decrease in the numbers of infectious cell-free virions.
FIG. 1.
Human BST-2 reduces HIV-1 infectivity. (A) Vpu-deleted HIV-1 clone BH10(Vpu−) DNA (100 ng) was transfected into 293T cells (2 × 105) together with various amounts of human BST-2 cDNA (0, 10, 20, 50, and 100 ng). Expression of viral Gag protein and BST-2 in cells was assessed by Western blotting using antibodies against HIV-1 p24 and Flag tag. Amounts of HIV-1 particles in supernatants were determined by measuring viral reverse transcriptase (RT) activity. Levels of infectious HIV-1 were assessed by infecting the TZM-bl indicator cells. In order to remove aggregated virus particles, supernatants were also clarified using 0.22-μm-pore-size filters before they were subjected to reverse transcriptase assays and used to infect TZM-bl cells (labeled as open diamonds and open squares in the graph). Values that were obtained from experiments with BH10(Vpu−) alone are set as 100. Results shown represent arithmetic means ± standard deviations of three independent experiments. (B) BH10(Vpu−) DNA (2 μg) was cotransfected with hBST-2 DNA (1 μg) into 293T cells (2.5 × 106). Levels of HIV-1 in supernatants were determined either by measuring viral reverse transcriptase activity or by infecting TZM-bl cells. (C) Viral Gag expression and processing in cells and in virus particles. In the case of BST-2 expression, viruses were harvested from a 5- or 25-times-greater volume of supernatant than in the absence of BST-2 so as to better visualize Gag signals in Western blots. Western blotting was performed with antibodies against HIV-1 p24, Flag tag, or tubulin.
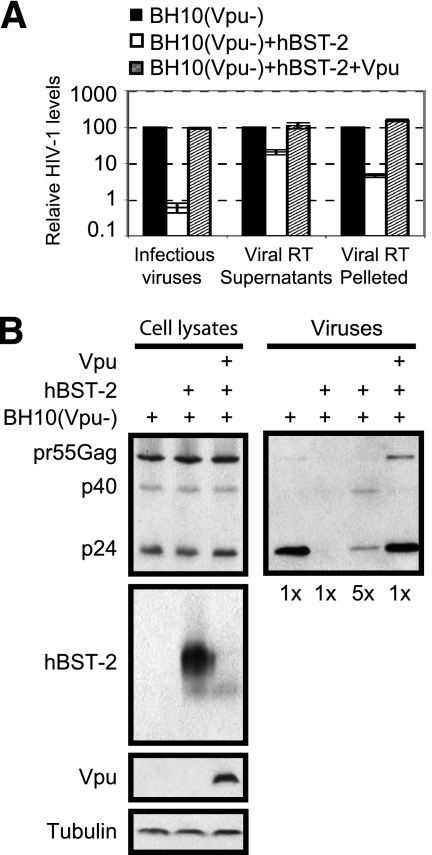
In order to investigate the defects in HIV-1 particles that were caused by BST-2, we purified cell-free HIV-1 particles in the supernatants by ultracentrifugation and examined Gag proteins and their derivatives by Western blotting. Given the significantly small amounts of viruses that were produced in the presence of BST-2 (Fig. 1B), a 5- or 25-times-greater volume of supernatant from BST-2 transfection than from transfection without BST-2 was used to concentrate viruses by ultracentrifugation. The results of Western blotting showed a significant accumulation of the pr55Gag precursor and the p40Gag intermediate products in virus samples that were collected from BST-2 transfection (Fig. 1C). Cotransfection of Vpu cDNA increased the production of infectious HIV-1 virions to close to control levels and also restored normal Gag processing in virus particles (Fig. 2). We noted that reverse transcriptase activity associated with pelleted virions decreased more dramatically than that measured for the culture supernatants as a result of hBST-2 expression (Fig. 2A), which is consistent with a previous report (4). Nevertheless, Vpu restored reverse transcriptase activities of the culture supernatants and of pelleted virions to control levels (Fig. 2A). Together, these data indicate that BST-2 impedes processing of Gag proteins and, as a result, diminishes the infectivity of HIV-1 particles.
FIG. 2.
Vpu rescues production of infectious HIV-1 particles in the presence of human BST-2. (A) 293T cells were transfected with either BH10(Vpu−) DNA alone or together with human BST-2 cDNA or with human BST-2 and Vpu cDNA constructs. Levels of HIV-1 in supernatants were determined by measuring viral reverse transcriptase (RT) activity or by infecting the TZM-bl indicator cells. Virus particles in the culture supernatants were pelleted through a 15% sucrose cushion by ultracentrifugation (100,000 × g), and the samples were measured for viral reverse transcriptase activity. (B) Viral Gag processing in cells and in virus particles was assessed by Western blotting using anti-HIV-1 p24 antibody.
African green monkey BST-2 reduces the infectivity of HIV-1 particles.
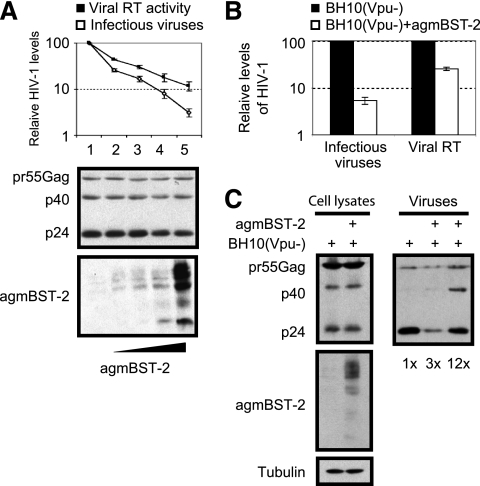
We next tested whether BST-2 from other species can also decrease HIV-1 infectivity. To this end, African green monkey BST-2 was cotransfected with Vpu-deleted HIV-1 DNA. The results showed that the decrease in infectious HIV-1 particles was always greater than the reduction in the total amount of virus as measured by viral reverse transcriptase assay (Fig. 3A and B). We then pelleted HIV-1 particles from supernatants and assessed virion-associated viral Gag protein and its derivatives by Western blotting. The results showed that African green monkey BST-2 also caused significant accumulation of pr55Gag precursor and the p40Gag intermediate products (Fig. 3C). Therefore, both human and African green monkey BST-2 proteins impede Gag processing and diminish HIV-1 infectivity.
FIG. 3.
African green monkey BST-2 diminishes the infectivity of HIV-1 particles. (A) BH10(Vpu−) DNA (100 ng) was transfected into 293T cells (2 × 105) together with various amounts of agmBST-2 cDNA (0, 10, 20, 50, and 100 ng). Levels of HIV-1 in supernatants were determined by measuring viral reverse transcriptase activity or by infecting the TZM-bl indicator cells. Expression of viral Gag protein and agmBST-2 in cells was examined by Western blotting. (B) 293T cells (2.5 × 106) were transfected with 2 μg of BH10(Vpu−) DNA and 1 μg of agmBST-2 cDNA constructs. Levels of viruses in supernatants were determined as described above. Gag processing in cells and in virus particles was examined by Western blotting with HIV-1 p24 antibody.
Knockdown of endogenous BST-2 in HeLa cells increases the production of infectious HIV-1 particles.
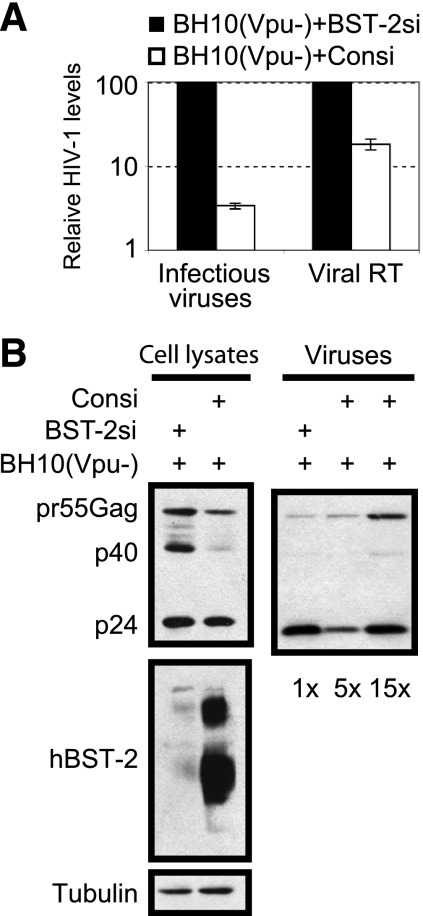
We next asked whether the endogenous BST-2 is able to diminish HIV-1 infectivity in addition to inhibiting virus release. To answer this question, we used siRNA oligonucleotides to deplete endogenous BST-2 protein in HeLa cells and then transfected the cells with Vpu-deleted HIV-1 DNA [BH10 (Vpu−)]. The results showed that knockdown of endogenous BST-2 led to a 5-fold increase in the total amount of HIV-1 particles and a 30-fold increase in the number of infectious HIV-1 virions (Fig. 4A). A further examination of virion-associated Gag protein and its derivatives showed a marked accumulation of pr55Gag precursor in virus samples that were harvested from transfections with control siRNA compared with those from transfections with BST-2 siRNA (Fig. 4B). Therefore, endogenous BST-2 delays processing of virion-associated Gag protein and diminishes HIV-1 infectivity.
FIG. 4.
Knockdown of endogenous BST-2 in HeLa cells increases production of infectious HIV-1 particles. (A) Levels of HIV-1 particles in supernatants were quantified by measuring viral reverse transcriptase (RT) activity or by infecting the TZM-bl indicator cells. (B) Western blotting was used to assess expression of BST-2 and viral Gag protein. BST-2si, BST-2 siRNA; Consi, control siRNA.
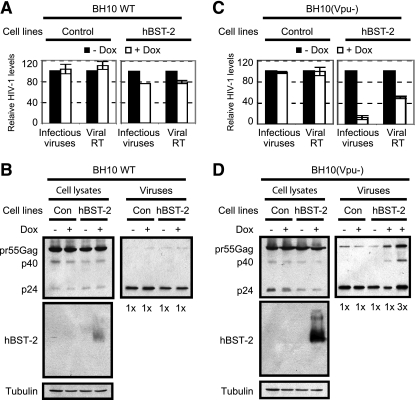
BST-2 diminishes HIV-1 infectivity in SupT1 cells.
We next tested whether BST-2 exerts an adverse effect on HIV-1 infectivity in its natural target, CD4+ T cells. To this end, we used a SupT1 cell line that we created expressing human BST-2 with induction by doxycycline (29). A SupT1 cell line that was stably transduced with the empty retroviral vector served as the control. Prior to infection with wild-type or Vpu-deleted HIV-1, SupT1 cell lines were exposed to doxycycline (500 μg/ml) for 16 h to induce BST-2 expression. Amounts of cell-free virus in the culture supernatants were measured 40 h after infection. The results showed that production of infectious wild-type HIV-1 as well as its Gag processing was not evidently affected by BST-2 (Fig. 5A and B), whereas the infectivity of Vpu-deleted HIV-1 particles decreased by ∼8-fold and viral reverse transcriptase activity associated with cell-free virions decreased ∼2-fold (Fig. 5C). The more significant reduction in virus infectivity caused by BST-2 was further shown to result from a marked accumulation of pr55Gag and p40Gag products in Vpu-deleted HIV-1 particles (Fig. 5D). Therefore, BST-2 decreases both the quantity and the infectivity of HIV-1 particles that are produced by CD4+ T cells in the absence of Vpu.
FIG. 5.
Effect of human BST-2 on HIV-1 infectivity in SupT1 cells. The control or the hBST-2 SupT1 cells were treated with doxycycline (Dox; 500 μg/ml) for 16 h before being infected with wild-type or Vpu-deleted HIV-1, as indicated. Forty hours after infection, levels of viruses in the culture supernatants were determined either by measuring viral reverse transcriptase (RT) activity or by infecting the TZM-bl indicator cells (A and C). Cell- and virion-associated viral Gag proteins were assessed by Western blotting using the anti-HIV-1 p24 antibody (B and D). Expression of BST-2 was detected by Western blotting using the anti-Flag antibody. Con, control.
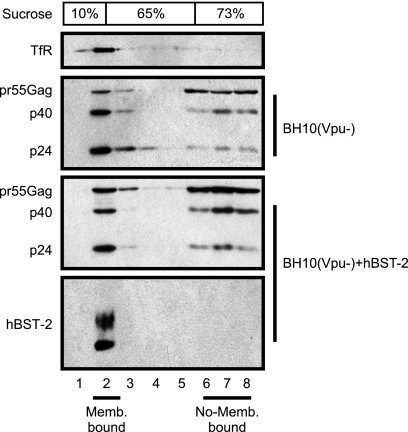
BST-2 delays processing of cellular membrane-associated Gag proteins.
HIV-1 protease is activated during or shortly after virus budding at the plasma membrane. The activated viral protease cleaves Gag protein into mature proteins, which results in transformation of an immature virus particle into an infectious mature particle that is characterized by the formation of a conical core (5). Given that BST-2 causes accumulation of pr55Gag precursor and Gag intermediate products in HIV-1 particles, we asked whether BST-2 starts to exert this deleterious effect on Gag processing before HIV-1 leaves the cell surface. To this end, we transfected 293T cells with Vpu-deleted HIV-1 DNA and human BST-2 cDNA. After being washed extensively with cold 1× phosphate-buffered saline to remove cell-free virus particles, cells were collected and lysed in a buffer without detergent. Membrane-associated Gag molecules were isolated by ultracentrifugation through a sucrose step gradient. Results of Western blotting showed that BST-2 and a membrane marker, TfR, were detected in fraction 2 where membrane-bound Gag molecules were enriched (Fig. 6). Notably, an evident accumulation of membrane-associated pr55Gag precursor was observed in the presence of BST-2 expression (Fig. 6). This result suggests that BST-2 delays Gag processing before viral particles leave the cell surface.
FIG. 6.
Effect of BST-2 on processing of membrane-associated Gag protein. 293T cells were transfected with BH10(Vpu−) DNA alone or together with BST-2 cDNA construct. Membrane-associated Gag protein was isolated in a membrane flotation assay. Processing of Gag protein was examined by Western blotting using anti-HIV-1 p24 antibody. BST-2 and the membrane marker TfR in the sucrose step gradient were also detected by Western blotting.
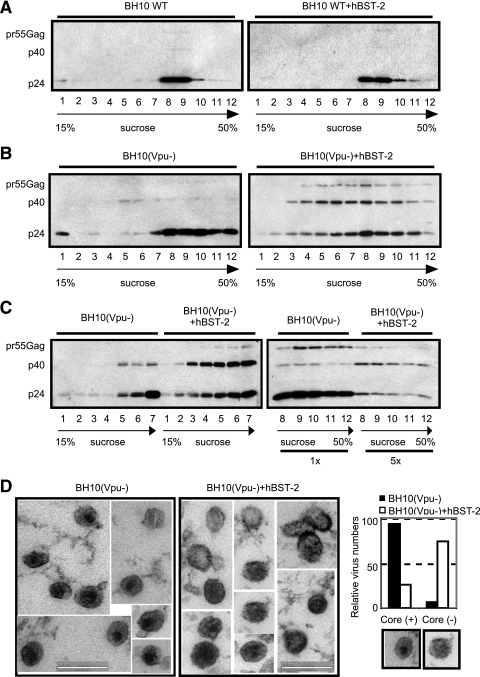
BST-2 adversely affects formation of the core structure in HIV-1 particles.
We next tried to identify the structural defects in HIV-1 particles that were caused by BST-2. First, HIV-1 particles were concentrated by ultracentrifugation, followed by a second round of ultracentrifugation through a 15% to 50% continuous sucrose gradient. Distribution of virus particles in the gradient was examined by Western blotting using anti-HIV-1 p24 antibody. BST-2 did not exert any effect on the distribution of wild-type HIV-1 particles in the sucrose gradient after ultracentrifugation (Fig. 7A). In the absence of BST-2, Vpu-deleted HIV-1 particles were located mainly in fractions 8 to 10. BST-2 expression caused not only a dramatic decrease in the number of virus particles in fractions 8 to 10 but also a marked increase in HIV-1 particles in the lighter fractions (2 to 6) as well as an accumulation of pr55Gag precursor and the p40Gag intermediate product (Fig. 7B). We then processed fraction samples 1 to 7 with or without BST-2 expression in the same Western blot to directly assess the impact of BST-2 on the production of low-density HIV-1 particles. Again, markedly stronger Gag signals were seen for samples that were collected with BST-2 expression (Fig. 7C). Therefore, concurrent with diminishing production of HIV-1 particles of normal density (fractions 8 to 10), BST-2 enhances production of HIV-1 particles of lower density (fractions 2 to 6).
FIG. 7.
Effect of BST-2 on the morphology of HIV-1 particles. Wild-type (WT) and Vpu-deleted [BH10(Vpu−)] HIV-1 particles were produced in the absence or the presence of BST-2, followed by ultracentrifugation through a continuous sucrose gradient. The presence of virus particles in each fraction was detected by Western blotting using anti-HIV-1 p24 antibody. (C) In order to directly compare the levels of HIV-1 particles in the low-density fractions (1 to 7) between transfections with and without BST-2, fraction samples of two sucrose gradients under both conditions were assessed in the same Western blot using anti-HIV-1 p24 antibody. (D) HIV-1 particles in the culture supernatants were harvested and processed for electron microscopy. Images shown are thin-sectioned HIV-1 particles. For each virus sample, more than 100 virus particles were examined for the presence or absence of the core structure. The results are summarized in the bar graph. Bar, 200 nm.
Next, we examined the morphology of HIV-1 particles by electron microscopy. Instead of checking cell-associated virus particles, we harvested cell-free progeny virions that had been released into culture supernatants. In order to observe the interior structure of HIV-1 particles by thin-section microscopy, viruses were pelleted by ultracentrifugation and collected with solidified low-melting-point agarose that served as a matrix to permit thin-sectioning and sample processing for electron microscopy. The results shown in Fig. 7D indicate that in the absence of BST-2, the majority of Vpu-deleted HIV-1 particles exhibited a mature morphology characterized by an electron-dense core that was not distinguishable from that of Vpu-positive wild-type HIV-1 particles. In contrast, such a core was rarely seen in Vpu-deleted HIV-1 particles that were produced from cells expressing BST-2 (Fig. 7D). These particles did not show an electron-dense ring directly underneath the viral envelope, which is characteristic of immature HIV-1 particles. This is consistent with the results of Western blotting shown in Fig. 1C indicating that Gag protein was indeed processed by viral protease, albeit in an incomplete fashion, which led to accumulation of pr55Gag precursor and the p40Gag intermediate product that interfered with construction of the core structure.
DISCUSSION
BST-2 reduces the production of cell-free HIV-1 particles from virus-producing cells by tethering progeny virions to the cell surface (25, 33, 37). These tethered viral particles are often internalized through endocytosis and are likely destroyed within the cell. The potential outcomes of BST-2 restriction in the context of natural virus infection may include decreased viral load and reduced virus transmission. In addition to physically tethering HIV-1 particles to the cell surface and diminishing the yield of cell-free virus particles, our data further show that BST-2 also diminishes the infectivity of progeny virions, which is consistent with the data reported by other groups (6, 25, 32, 37). It is noted that Vpu promotes HIV-1 release from A3.01 cells, but Vpu is dispensable for HIV-1 infectivity in this cell line (15). The cause for the discrepancy between the latter finding and our data is unclear. We speculate that this may partially arise from the use of different cell lines in different studies.
HIV-1 spreads more efficiently by cell-cell transmission than by cell-free virus infection (31, 35). Since BST-2 tethers HIV-1 particles to the cell surface, it is speculated that the increased virus presence at the cell surface may promote formation of a viral synapse and consequently increase HIV-1 spread through the cell-cell transmission mode. In contrast to this speculation, recent data show that BST-2 diminishes productive cell-cell transmission of HIV-1 by causing virion aggregation (1). Results of our study further suggest that this reduced virus transmission also partially results from the ability of BST-2 to decrease the infectivity of HIV-1 particles.
Our study shows that the adverse effect of BST-2 on the infectivity of HIV-1 particles is associated with abnormal accumulation of virion-associated pr55Gag precursor and the intermediate p40Gag products. We observed this Gag processing defect in virus samples that were produced by 293T, HeLa, and SupT1 cells. A few groups of investigators have also performed Western blotting to examine the protein profiles of Vpu-deleted HIV-1 particles that were produced in the presence of BST-2 (8, 13, 25). Their data highlighted the levels of mature CA (p24) but did not explicitly show pr55Gag and p40Gag, likely due to the rather small amounts of Vpu-deleted HIV-1 samples examined in their Western blotting.
It is currently unknown how BST-2 causes accumulation of pr55Gag and p40Gag in the released HIV-1 particles. Since Gag processing and subsequent virus maturation rely on activation of viral protease that is originally produced as a domain of the Gag-Pol precursor protein, we speculate that BST-2 may interrupt viral protease activation by a direct or an indirect mechanism. The viral protease is dormant when it is part of the viral Pol protein. Activation of the viral protease during virus assembly is tightly regulated to ensure that Gag and Gag-Pol proteins are not cleaved before virus morphogenesis is completed at the plasma membrane. Although the timing and the cues of viral protease activation have not been fully defined, it is believed that Gag processing starts during or shortly after virus budding and that virus budding and viral protease activation are linked with each other (5). In this connection, any factor that slows down virus budding may disturb viral protease activation and, as a result, affect virus maturation. One example of this kind are the late domain mutants that are budding defective and exhibit an immature morphology due to incomplete Gag cleavage by viral protease (7, 11). Although BST-2 does not block virus budding, it may still affect the kinetics of viral protease activation by a certain means and lead to incomplete Gag processing.
Incomplete processing of Gag prevents formation of the core structure in virus particles. In theory, the p40 intermediate Gag products that were seen to associate with virus particles in Fig. 1C can represent either matrix (MA)/CA/SP1 (or p17/p24/p2) or CA/SP1/NC/SP2/p6 (or p24/p2/p7/p1/p6) on the basis of their molecular weights. It is known that viral protease cleaves Gag at different rates at distinct cleavage sites. The first cleavage takes place at the SP1/CA junction, the second cleavage occurs at the SP2/p6 junction with a 9-fold-lower efficiency, and the third cleavage occurs at the MA/CA junction with a 14-fold-lower efficiency (28). Therefore, the vast majority of the p40Gag intermediate molecules should be MA/CA/SP1. Results of a recent study showed that even 10% of uncleaved MA/CA protein relative to the amount of wild-type Gag protein reduced virus infectivity by more than 10-fold (17). This small portion of uncleaved MA/CA is believed to poison the mature CA (p24) population and disrupt construction of a conical core in virus particles (17). On the basis of these results, we propose that BST-2 causes incomplete processing of Gag protein in virus particles, which leads to accumulation of p40Gag intermediate products that interfere with mature CA (p24) to construct a conical core.
Several groups of investigators have conducted electron microscopy to examine HIV-1 particles that are tethered to the cell surface (4, 9, 25). These particles exhibit a mature morphology that is different from that of the cell-free Vpu-deleted HIV-1 particles, which are mostly devoid of the capsid core structure, as shown in this study. Although the fate of the tethered virus particles is largely unknown, the fact that protease digestion (such as by subtilisin) is required to release them argues that the vast majority of them might be destroyed by the cells rather than that they escape from the BST-2 leash and become cell-free single particles. In this sense, the single cell-free Vpu-deleted HIV-1 particles shown in Fig. 7D might have been generated via a pathway that mainly gives rise to abnormal virus particles. Interestingly, one study did report abnormal HIV-1 (Vpu-deleted) particles associated with A3.01 cells (15), suggesting that Vpu is required for normal virus morphogenesis, at least in certain cell types.
In summary, results of this study demonstrate that BST-2 not only inhibits release of Vpu-deleted HIV-1 particles by tethering them at the cell surface but also impairs the infectivity of progeny virions. The latter defect is a result of incomplete viral Gag processing and a loss of the core structure in virus particles. This observation suggests a novel antiviral action of BST-2.
Acknowledgments
We thank Qinghua Pan for technical support and Vicky Cheng and Bjorn Kuhl for critical reading of the manuscript.
This work was supported by funding from Canadian Institutes of Health Research and Canadian Foundation for AIDS Research. Chen Liang is a senior researcher of Fonds de la Recherché en Santé du Québec.
Footnotes
Published ahead of print on 22 September 2010.
REFERENCES
- 1.Casartelli, N., M. Sourisseau, J. Feldmann, F. Guivel-Benhassine, A. Mallet, A. G. Marcelin, J. Guatelli, and O. Schwartz. 2010. Tetherin restricts productive HIV-1 cell-to-cell transmission. PLoS Pathog. 6:e1000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Douglas, J. L., K. Viswanathan, M. N. McCarroll, J. K. Gustin, K. Fruh, and A. V. Moses. 2009. Vpu directs the degradation of the human immunodeficiency virus restriction factor BST-2/Tetherin via a βTrCP-dependent mechanism. J. Virol. 83:7931-7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dube, M., B. B. Roy, P. Guiot-Guillain, J. Binette, J. Mercier, A. Chiasson, and E. A. Cohen. 2010. Antagonism of tetherin restriction of HIV-1 release by Vpu involves binding and sequestration of the restriction factor in a perinuclear compartment. PLoS Pathog. 6:e1000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzpatrick, K., M. Skasko, T. J. Deerinck, J. Crum, M. H. Ellisman, and J. Guatelli. 2010. Direct restriction of virus release and incorporation of the interferon-induced protein BST-2 into HIV-1 particles. PLoS Pathog. 6:e1000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freed, E. O. 1998. HIV-1 Gag proteins: diverse functions in the virus life cycle. Virology 251:1-15. [DOI] [PubMed] [Google Scholar]
- 6.Goffinet, C., I. Allespach, S. Homann, H. M. Tervo, A. Habermann, D. Rupp, L. Oberbremer, C. Kern, N. Tibroni, S. Welsch, J. Krijnse-Locker, G. Banting, H. G. Krausslich, O. T. Fackler, and O. T. Keppler. 2009. HIV-1 Antagonism of CD317 is species specific and involves Vpu-mediated proteasomal degradation of the restriction factor. Cell Host Microbe 5:285-297. [DOI] [PubMed] [Google Scholar]
- 7.Gottlinger, H. G., T. Dorfman, J. G. Sodroski, and W. A. Haseltine. 1991. Effect of mutations affecting the p6 Gag protein on human immunodeficiency virus particle release. Proc. Natl. Acad. Sci. U. S. A. 88:3195-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta, R. K., S. Hue, T. Schaller, E. Verschoor, D. Pillay, and G. J. Towers. 2009. Mutation of a single residue renders human Tetherin resistant to HIV-1 Vpu-mediated depletion. PLoS Pathog. 5:e1000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Habermann, A., J. Krijnse-Locker, H. Oberwinkler, M. Eckhardt, S. Homann, A. Andrew, K. Strebel, and H. G. Krausslich. 2010. CD317/tetherin is enriched in the HIV-1 envelope and downregulated from the plasma membrane upon virus infection. J. Virol. 84:4646-4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinz, A., N. Miguet, G. Natrajan, Y. Usami, H. Yamanaka, P. Renesto, B. Hartlieb, A. A. McCarthy, J. P. Simorre, H. Gottlinger, and W. Weissenhorn. 2010. Structural basis of HIV-1 tethering to membranes by the BST-2/tetherin ectodomain. Cell Host Microbe 7:314-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang, M., J. M. Orenstein, M. A. Martin, and E. O. Freed. 1995. p6Gag is required for particle production from full-length human immunodeficiency virus type 1 molecular clones expressing protease. J. Virol. 69:6810-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia, B., R. Serra-Moreno, W. Neidermyer, A. Rahmberg, J. Mackey, I. B. Fofana, W. E. Johnson, S. Westmoreland, and D. T. Evans. 2009. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 5:e1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jouvenet, N., S. J. Neil, M. Zhadina, T. Zang, Z. Kratovac, Y. Lee, M. McNatt, T. Hatziioannou, and P. D. Bieniasz. 2009. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J. Virol. 83:1837-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaletsky, R. L., J. R. Francica, C. Agrawal-Gamse, and P. Bates. 2009. Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc. Natl. Acad. Sci. U. S. A. 106:2886-2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klimkait, T., K. Strebel, M. D. Hoggan, M. A. Martin, and J. M. Orenstein. 1990. The human immunodeficiency virus type 1-specific protein vpu is required for efficient virus maturation and release. J. Virol. 64:621-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kupzig, S., V. Korolchuk, R. Rollason, A. Sugden, A. Wilde, and G. Banting. 2003. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic 4:694-709. [DOI] [PubMed] [Google Scholar]
- 17.Lee, S. K., J. Harris, and R. Swanstrom. 2009. A strongly transdominant mutation in the human immunodeficiency virus type 1 gag gene defines an Achilles heel in the virus life cycle. J. Virol. 83:8536-8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Tortorec, A., and S. J. Neil. 2009. Antagonism to and intracellular sequestration of human tetherin by the human immunodeficiency virus type 2 envelope glycoprotein. J. Virol. 83:11966-11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez, L. A., S. J. Yang, H. Hauser, C. M. Exline, K. G. Haworth, J. Oldenburg, and P. M. Cannon. 2010. Ebola virus glycoprotein counteracts BST-2/tetherin restriction in a sequence-independent manner that does not require tetherin surface removal. J. Virol. 84:7243-7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maldarelli, F., M. Y. Chen, R. L. Willey, and K. Strebel. 1993. Human immunodeficiency virus type 1 Vpu protein is an oligomeric type I integral membrane protein. J. Virol. 67:5056-5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mangeat, B., G. Gers-Huber, M. Lehmann, M. Zufferey, J. Luban, and V. Piguet. 2009. HIV-1 Vpu neutralizes the antiviral factor Tetherin/BST-2 by binding it and directing its beta-TrCP2-dependent degradation. PLoS Pathog. 5:e1000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mansouri, M., K. Viswanathan, J. L. Douglas, J. Hines, J. Gustin, A. V. Moses, and K. Fruh. 2009. Molecular mechanism of BST2/tetherin downregulation by K5/MIR2 of Kaposi's sarcoma-associated herpesvirus. J. Virol. 83:9672-9681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell, R. S., C. Katsura, M. A. Skasko, K. Fitzpatrick, D. Lau, A. Ruiz, E. B. Stephens, F. Margottin-Goguet, R. Benarous, and J. C. Guatelli. 2009. Vpu antagonizes BST-2-mediated restriction of HIV-1 release via beta-TrCP and endo-lysosomal trafficking. PLoS Pathog. 5:e1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyagi, E., A. J. Andrew, S. Kao, and K. Strebel. 2009. Vpu enhances HIV-1 virus release in the absence of Bst-2 cell surface down-modulation and intracellular depletion. Proc. Natl. Acad. Sci. U. S. A. 106:2868-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neil, S. J., T. Zang, and P. D. Bieniasz. 2008. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451:425-430. [DOI] [PubMed] [Google Scholar]
- 26.Pardieu, C., R. Vigan, S. J. Wilson, A. Calvi, T. Zang, P. Bieniasz, P. Kellam, G. J. Towers, and S. J. Neil. 2010. The RING-CH ligase K5 antagonizes restriction of KSHV and HIV-1 particle release by mediating ubiquitin-dependent endosomal degradation of tetherin. PLoS Pathog. 6:e1000843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez-Caballero, D., T. Zang, A. Ebrahimi, M. W. McNatt, D. A. Gregory, M. C. Johnson, and P. D. Bieniasz. 2009. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell 139:499-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pettit, S. C., M. D. Moody, R. S. Wehbie, A. H. Kaplan, P. V. Nantermet, C. A. Klein, and R. Swanstrom. 1994. The p2 domain of human immunodeficiency virus type 1 Gag regulates sequential proteolytic processing and is required to produce fully infectious virions. J. Virol. 68:8017-8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rong, L., J. Zhang, J. Lu, Q. Pan, R. P. Lorgeoux, C. Aloysius, F. Guo, S. L. Liu, M. A. Wainberg, and C. Liang. 2009. The transmembrane domain of BST-2 determines its sensitivity to down-modulation by human immunodeficiency virus type 1 Vpu. J. Virol. 83:7536-7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakuma, T., T. Noda, S. Urata, Y. Kawaoka, and J. Yasuda. 2009. Inhibition of Lassa and Marburg virus production by tetherin. J. Virol. 83:2382-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sattentau, Q. 2008. Avoiding the void: cell-to-cell spread of human viruses. Nat. Rev. Microbiol. 6:815-826. [DOI] [PubMed] [Google Scholar]
- 32.Sauter, D., M. Schindler, A. Specht, W. N. Landford, J. Munch, K. A. Kim, J. Votteler, U. Schubert, F. Bibollet-Ruche, B. F. Keele, J. Takehisa, Y. Ogando, C. Ochsenbauer, J. C. Kappes, A. Ayouba, M. Peeters, G. H. Learn, G. Shaw, P. M. Sharp, P. Bieniasz, B. H. Hahn, T. Hatziioannou, and F. Kirchhoff. 2009. Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains. Cell Host Microbe 6:409-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sauter, D., A. Specht, and F. Kirchhoff. 2010. Tetherin: holding on and letting go. Cell 141:392-398. [DOI] [PubMed] [Google Scholar]
- 34.Sheehy, A. M., N. C. Gaddis, J. D. Choi, and M. H. Malim. 2002. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418:646-650. [DOI] [PubMed] [Google Scholar]
- 35.Sourisseau, M., N. Sol-Foulon, F. Porrot, F. Blanchet, and O. Schwartz. 2007. Inefficient human immunodeficiency virus replication in mobile lymphocytes. J. Virol. 81:1000-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stremlau, M., C. M. Owens, M. J. Perron, M. Kiessling, P. Autissier, and J. Sodroski. 2004. The cytoplasmic body component TRIM5α restricts HIV-1 infection in Old World monkeys. Nature 427:848-853. [DOI] [PubMed] [Google Scholar]
- 37.Van Damme, N., D. Goff, C. Katsura, R. L. Jorgenson, R. Mitchell, M. C. Johnson, E. B. Stephens, and J. Guatelli. 2008. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3:245-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei, X., J. M. Decker, H. Liu, Z. Zhang, R. B. Arani, J. M. Kilby, M. S. Saag, X. Wu, G. M. Shaw, and J. C. Kappes. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46:1896-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolf, D., and S. P. Goff. 2008. Host restriction factors blocking retroviral replication. Annu. Rev. Genet. 42:143-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang, F., S. J. Wilson, W. C. Landford, B. Virgen, D. Gregory, M. C. Johnson, J. Munch, F. Kirchhoff, P. D. Bieniasz, and T. Hatziioannou. 2009. Nef proteins from simian immunodeficiency viruses are tetherin antagonists. Cell Host Microbe 6:54-67. [DOI] [PMC free article] [PubMed] [Google Scholar]