Abstract
We have shown previously that immunization with herpes simplex virus type 1 (HSV-1) glycoprotein K (gK) exacerbated corneal scarring (CS) in ocularly infected mice. In this study, we investigated whether higher levels of CS were correlated with higher levels of latency and T cell exhaustion in gK-immunized mice. BALB/c mice were vaccinated with baculovirus-expressed gK or gD or mock immunized. Twenty-one days after the third immunization, mice were ocularly infected with 2 × 104 PFU/eye of virulent HSV-1 strain McKrae. On day 5 postinfection, virus replication in the eye was measured, and on day 30 postinfection, infiltration of the trigeminal ganglia (TG) by CD4, CD8, programmed death 1 (PD-1), and T cell immunoglobulin and mucin domain-containing protein 3 (Tim-3) was monitored by immunohistochemistry and quantitative real-time PCR (qRT-PCR). This study demonstrated that higher levels of CS were correlated with higher levels of latency, and this was associated with the presence of significantly higher numbers of CD4+PD-1+ and CD8+PD-1+ cells in the TG of the gK-immunized group than in both the gD- and mock-immunized groups. Levels of exhaustion associated with Tim-3 were the same among gK- and mock-vaccinated groups but higher than levels in the gD-vaccinated group. In this study, we have shown for the first time that both PD-1 and Tim-3 contribute to T cell exhaustion and an increase of latency in the TG of latently infected mice.
We have previously demonstrated that immunization of mice with glycoprotein K (gK), but not with any other known herpes simplex virus type 1 (HSV-1) glycoprotein, significantly exacerbates corneal scarring (CS) and facial dermatitis following ocular HSV-1 infection in different strains of mice (17-20). The exacerbated CS is independent of mouse or virus strains (18). gK is an essential HSV-1 gene product (20, 25, 35) and is thought to be an important determinant of virus-induced cell fusion since single amino acid changes within gK caused extensive virus-induced cell fusion (4, 9, 32, 47). Thus, because of the essential nature of gK in HSV-1 infectivity, we have used recombinant viruses (rather than deleting the gK gene) having two extra copies of gK and have shown that, similarly to gK immunization, mice infected with this recombinant virus had elevated levels of CS (41). This exacerbation of disease is of particular interest, as it appears to mimic the clinical disease process as we have shown for the role of anti-gK antibody in individuals with a history of HSV-1 recurrences (41). Similarly to our studies where elevation of anti-gK antibody in individuals with herpes stromal keratitis (HSK) was correlated with severity of eye disease, we also have shown that transfer of whole serum or purified IgG from gK-immunized mice to naive mice resulted in the same severe exacerbation of CS following ocular HSV-1 infection as that seen in gK-immunized mice (18).
Our T cell depletion studies have shown that this CS enhancement was mediated by a CD8+CD25+ T cell response (2, 39). In addition, we have identified a highly conserved CD8+ T cell epitope (ITAYGLVL) within the signal sequence of gK (39). This peptide increased levels of viral neurovirulence and virus-induced CS in ocularly infected mice. Moreover, in HSV-infected “humanized” HLA-A*0201 transgenic mice, the gK peptide induced strong cytotoxic responses (38). Recently, we have shown that the level of HSV-1 latency correlates with severity of CS and exhaustion of CD8+ T cells in the trigeminal ganglia (TG) of latently infected mice (38). As described above, gK immunization (2, 17-20), gK recombinant viruses expressing two extra copies of gK (41), or a gK peptide (39) exacerbates eye disease in different strains of mice. However, very little is known regarding what role, if any, gK-induced exacerbation of CS may play in the levels of latency and T cell exhaustion. In this study, we sought to determine if the severity of CS in gK-immunized mice ocularly infected with HSV-1 is associated with (i) increased virus replication in the eye during primary infection, (ii) the load of latent virus, as determined by the amount of latency-associated transcript (LAT) and viral glycoprotein B (gB) DNA, and (iii) increased levels of various immune infiltrates in TG (as determined by TaqMan real-time PCR [RT-PCR] and immunostaining). To address these questions, in this study, mice were vaccinated using the following 3 different vaccine regimens: (i) gK vaccination, which causes severe eye disease, (ii) mock vaccination, which causes moderate eye disease, and (iii) gD immunization, which protects from eye disease. Our results suggested a strong correlation among the severity of eye disease, the load of latent virus in the TG, the number of T cells in the TG, and the levels of programmed death 1 (PD-1) and T cell immunoglobulin and mucin domain-containing protein 3 (Tim-3) in the TG. Both Tim-3 and PD-1 are associated with exhaustion (dysfunction and deletion) of T cells, suggesting the possibility that increased PD-1 and Tim-3 levels might result in a decreased number of functional CD4+ and CD8+ T cells at the site of latency, thus resulting in more latent virus and consequently higher levels of CS.
MATERIALS AND METHODS
Virus and cells.
Plaque-purified McKrae, a neurovirulent HSV-1 strain, was grown in rabbit skin (RS) cell monolayers in minimal essential medium (MEM) containing 5% fetal calf serum as described previously (20).
Mice.
BALB/cJ mice (female, 6 weeks old) were obtained from Jackson Laboratory (Bar Harbor, ME). Animals were handled in accordance with the Association for Research in Vision and Ophthalmology (ARVO) statement for the Use of Animals in Ophthalmic and Vision Research under an approved IACUC protocol.
Immunization.
Mice were immunized three times at 3-week intervals subcutaneously (s.c.) with baculovirus-expressed recombinant gK as described previously (20). As a positive control, additional BALB/c mice were immunized similarly with recombinant baculovirus expressing gD (21). Subcutaneous injections were done using complete Freund's adjuvant (CFA) on day 0 and incomplete Freund's adjuvant (IFA) on days 21 and 42. For s.c. injections, gK- or gD-expressed glycoproteins were emulsified 1:1 in CFA or IFA. Each vaccination consisted of extracts from 1 × 106 cells, which contained approximately 5 μg of gK or gD, based on the intensity of each expressed glycoprotein band on Coomassie brilliant blue-stained gels. Mock-immunized mice were similarly inoculated three times with wild-type baculovirus-infected Sf9 cells. Mock vaccine consisted of extract from the same number of cells infected with wild-type baculovirus.
Ocular infection.
To reduce the incidence of death in the gK- and mock-immunized groups, we ocularly infected mice with 2 × 104 PFU rather than 2 × 105 PFU of HSV-1 strain McKrae. Infection was done 3 weeks after the third immunization by using a 2-μl drop into an eye that had no prior corneal scarification.
Evaluation of corneal scarring.
Clinical eye disease patterns were determined by examining the eyes of the mice on day 30 postinfection (p.i.). HSV-induced corneal scarring (epithelial keratitis) was evaluated by slit lamp biomicroscopy by using 1% fluorescein stain. The magnitude of stromal disease was scored as 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 3.5, or 4, with 0, 1, 2, 3, and 4 representing no disease and disease involving 25, 50, 75, and 100% of the corneal surface, respectively.
Analysis of replication and clearance of HSV-1 from the eye.
Eyes were swabbed with a Dacron swab (spectrum type 1) once daily on days 1, 3, and 5 after ocular infection. The swab was transferred to a 12- by 75-mm culture tube containing 1 ml of medium and then frozen and thawed; virus titers were determined using standard plaque assays on RS cells.
Immunostaining of TG.
. TG from individual mice that survived ocular infection were collected on day 30 postinfection. The TG were embedded in optimal cutting temperature compound (OCT; Tissue-Tek, Sakura) for cryosectioning and stored at −80°C. Cryostat sections, 8 to 10 μm thick, were cut and air dried overnight and then fixed in 4% paraformaldehyde overnight at 4°C. Single, double, and triple immunostaining was performed using monoclonal antibodies against CD4 (clone GK1.5), CD8 (clone 53-6.7), PD-1 (clone RMP1-30) (eBioscience), and Tim-3 (GenBank accession number AAL65156) (R&D Systems) according to manufacturer protocol at 4°C overnight. Secondary antibodies were labeled with Alexa-488, -594 or -647 (Invitrogen) and incubated on tissue for 1 h at room temperature. Washed sections were dried and mounted with DAPI (4′,6-diamidino-2-phenylindole) ProLong gold (Invitrogen). Counts were made for at least 10 sections per group in a double-blind fashion.
DNA extraction and PCR analysis for HSV-1 genomic DNA.
DNA was isolated from homogenized individual TG by using a commercially available DNeasy blood and tissue kit (Qiagen, Stanford, CA; catalog no. 69506) according to the manufacturer's instructions. PCR analyses were done using gB-specific primers (forward, 5′-AACGCGACGCACATCAAG-3′; reverse, 5′-CTGGTACGCGATCAGAAAGC-3′; and probe, 5′-FAM-CAGCCGCAGTACTACC-3′). The amplicon length for this primer set is 72 bp. The relative copy numbers for gB DNA were calculated using standard curves generated from the plasmid pAc-gB1. In all experiments, GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used for the normalization of transcripts.
RNA extraction, cDNA synthesis, and TaqMan quantitative RT-PCR (qRT-PCR).
Individual TG from mice that survived ocular infection were collected 30 days postinfection and immersed in RNAlater RNA stabilization reagent and stored at −80°C until processing. Tissue processing, total RNA extraction, and RNA yield were carried out as we have described previously (40, 41). Following RNA extraction, 1,000 ng of total RNA was reverse transcribed using random hexamer primers and murine leukemia virus (MuLV) reverse transcriptase from a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA) in accordance with the manufacturer's recommendations. The levels of various RNAs were evaluated using commercially available TaqMan gene expression assays (Applied Biosystems, Foster City, CA) with optimized primer and probe concentrations. Primer-probe sets consisted of two unlabeled PCR primers and a 6-carboxyfluorescein (FAM) dye-labeled TaqMan MGB probe formulated into a single mixture. Additionally, all cellular amplicons included an intron-exon junction to eliminate signal from genomic DNA contamination. The assays used in this study were as follows: (i) CD4 (ABI assay identifier Mm00442754_m1; amplicon length, 72 bp), (ii) CD8 (α chain; ABI assay identifier Mn01182108_m1; amplicon length, 67 bp), (iii) PD-1 (programmed death 1 [also known as CD279]; ABI assay identifier Mm00435532_m1; amplicon length, 65 bp), (iv) Tim-3 (ABI assay identifier Mm00454540_m1; amplicon length, 98 bp), and (v) GAPDH (ABI assay identifier Mm999999.15_G1; amplicon length, 107 bp). The custom-made primers and probe set for LAT were as follows: forward primer, 5′-GGGTGGGCTCGTGTTACAG-3′; reverse primer, 5′-GGACGGGTAAGTAACAGAGTCTCTA-3′; and probe, 5′-FAM-ACACCAGCCCGTTCTTT-3′ (amplicon length, 81 bp; corresponding to LAT nucleotides 119553 to 119634). Relative copy numbers for LAT were calculated using standard curves generated from the plasmid pLAT5301.
Quantitative real-time PCR was performed using an ABI Prism 7900HT sequence detection system (Applied Biosystems, Foster City, CA) in 384-well plates as we described previously (40, 41). Real-time PCR was performed in triplicate for each tissue sample. The threshold cycle (CT) values, which represents the PCR cycle at which there is a noticeable increase in the reporter fluorescence above baseline, were determined using SDS 2.2 software. GAPDH RNA was used for the normalization of transcripts.
Statistical analysis.
Student's t test and analysis of variance (ANOVA) were performed using the computer program InStat (GraphPad, San Diego, CA) to analyze protective parameters. Results were considered statistically significant when P values were <0.05.
RESULTS
gK immunization increases the level of latency in the TG of latently infected mice.
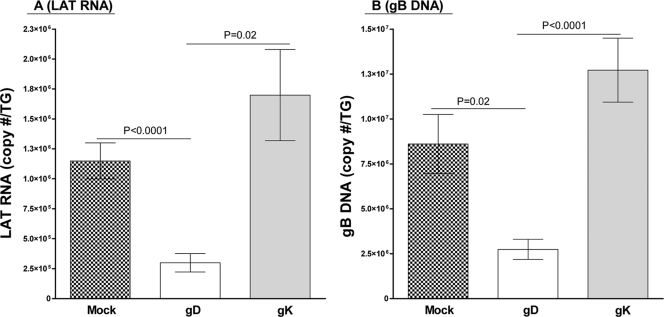
We have previously shown that immunization with gK caused exacerbation of CS and facial dermatitis following HSV-1 infection in mice (17-20). To determine if this higher level of CS is correlated with higher levels of latency, groups of 35 to 50 BALB/c mice from two separate experiments were immunized three times with gK or gD or mock immunized as described in Materials and Methods. Three weeks after the final vaccination, mice were infected ocularly with 2 × 104 PFU/eye of HSV-1 strain McKrae. On day 30 p.i., 28 of 50 (56%), 38 of 50 (76%), and 35 of 35 (100%) gK-, mock-, and gD-infected mice had survived ocular infection, respectively. After euthanization on day 30, the TG from the surviving mice were harvested, and the levels of LAT RNA and gB DNA were determined as described in Materials and Methods. The amount of LAT RNA detected was significantly higher in the TG of gK- and mock-immunized mice than in the TG of gD-immunized mice (Fig. 1A, P < 0.0001 for mock and P = 0.02 for gK [Student's t test]). Similar results were obtained when we looked at the copy number of gB DNA per TG (Fig. 1B, P = 0.02 for mock and P < 0.0001 for gK [Student's t test]). In addition, there was more LAT RNA (1.5-fold) and gB DNA (1.5-fold) in the TG of gK-immunized mice than in the TG of mock-immunized mice (Fig. 1). Taken together, the results suggest a trend where gK-immunized mice had a higher level of latency than the mock-immunized group, while both mock and gK groups had a higher latent viral load in the TG than the gD-immunized group.
FIG. 1.
HSV-1 latency is increased in gK-immunized mice. BALB/c mice were immunized three times at 3-week intervals s.c. with baculovirus-expressed recombinant gK or gD or mock vaccinated with empty vector. Mice were infected with 2 × 104 PFU of strain McKrae 3 weeks after final immunization. Thirty days p.i., mice were sacrificed, TG were removed, and total RNA and DNA were harvested. Quantitative RT-PCR and PCR were performed for the presence of LAT RNA and gB DNA, respectively. In each experiment, an estimated relative copy number of LAT and gB was calculated using standard curves generated from pGem-5317 and pAC-gB, respectively. Briefly, the DNA template was serially diluted 10-fold such that 5 μl contained 103 to 1011 copies of LAT or gB and then subjected to TaqMan PCR with the same set of primers. By comparing the normalized threshold cycle of each sample to the threshold cycle of the standard, the copy number for each reaction was determined. GAPDH expression was used to normalize the relative expression of LAT and gB in the TG. (A) LAT; (B) gB. Bars, means ± standard errors of the means (SEM) from 20 TG per group.
Lack of correlation of virus replication in mouse tears with the level of latency.
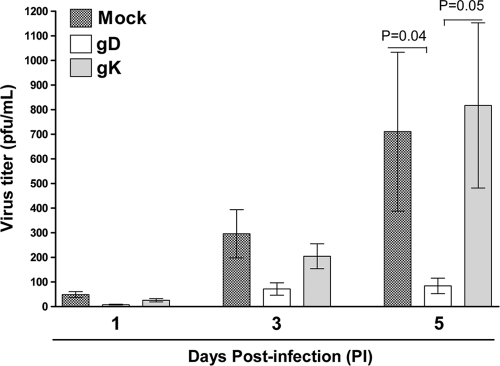
The virus titers in the tear films that had been collected on days 1, 3, and 5 p.i. from the mice as described above were determined using plaque assays on RS cells. On days 1 and 3 p.i., there was no significant difference in virus replication among the gK, gD, and mock groups (Fig. 2). However, by day 5 p.i., as expected, both mock- and gK-immunized mice had significantly higher levels of virus titers than the gD group (Fig. 2). Thus, it appears that there was no direct correlation between gK immunization and the increase of virus replication in the eyes of infected mice.
FIG. 2.
gD immunization reduces HSV-1 viral titer by day 5 p.i. BALB/c mice were immunized and infected as described in the legend to Fig. 1. Tear swabs were taken on days 1, 3, and 5 postinfection, and standard plaque assay was performed on RS cells to determine virus titer (PFU/milliliter). Bars, means ± SEM from three independent titration experiments for 20 mice per group (n = 40 eyes).
Upregulation of immune infiltrates and exhaustion markers in the TG of gK-immunized mice.
Recent studies have shown that T cell exhaustion occurred as a result of chronic infection with several different viruses (3, 8, 30) and that the presence of low-level viral antigen during chronic/latent infection leads to T cell exhaustion (42). HSV-1 latency was originally thought to be a completely dormant situation with no viral gene expression, but recent studies have shown that this is not the case (11, 31, 34). Thus, to investigate potential differences in the TG from gK-, gD-, and mock-immunized mice, we performed qRT-PCR analysis on the same TG used above to determine the expression levels of mRNAs characteristic of T cells (CD4, CD8) and exhaustion markers (PD-1, Tim-3).
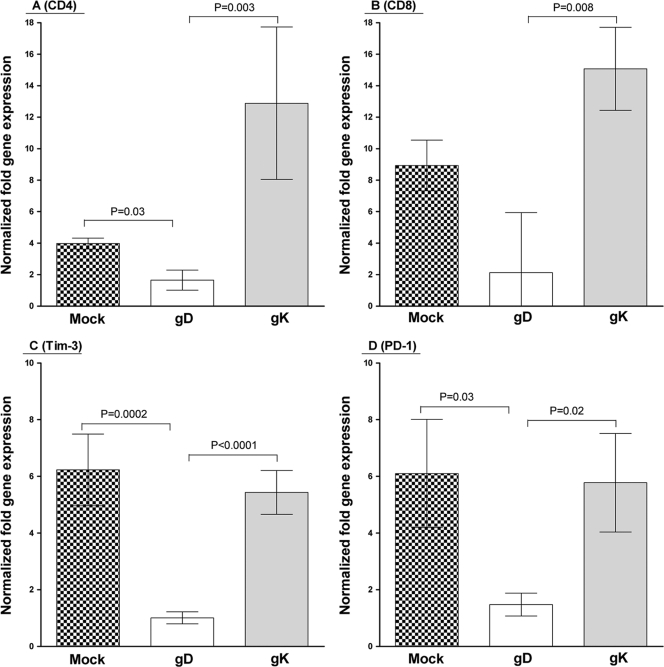
We observed a significant increase in CD4 (Fig. 3A, P = 0.03) and CD8 (Fig. 3B, P = 0.008) expression in the gK-immunized group compared with that in the gD-immunized group. We also observed a significant increase in the expression of the exhaustion marker Tim-3 in both mock- and gK-immunized groups compared to that in the gD group (Fig. 3C, P = 0.0002 for mock and P < 0.0001 for gK). In addition, we detected a significant increase in the expression of exhaustion marker PD-1 in mock- and gK-immunized groups compared to that in the gD group (Fig. 3D, P = 0.03 for mock and P = 0.02 for gK). However, there was no significant difference between gK- and mock-immunized groups for either PD-1 or Tim-3 expression (Fig. 3C and D). Thus, our results suggest that immunization using different antigens can alter the patterns of immune infiltrates and exhaustion markers in the TG of latently infected mice.
FIG. 3.
Effect of the higher levels of latency and CS on T cells and exhaustion markers in gK-immunized mice. BALB/c mice immunized and infected as described in the legend to Fig. 1 were allowed to progress to day 30 p.i. At this time, mice were sacrificed, TG were removed, total RNA was harvested, and gene expression was measured using TaqMan qRT-PCR. Expression is represented as a fold increase or decrease normalized to naive BALB/c expression. GAPDH expression was used to normalize the relative expression of each transcript. Bars, means ± SEM from 20 TG.
Exhaustion markers are increased in T cells from the TG of gK-immunized mice.
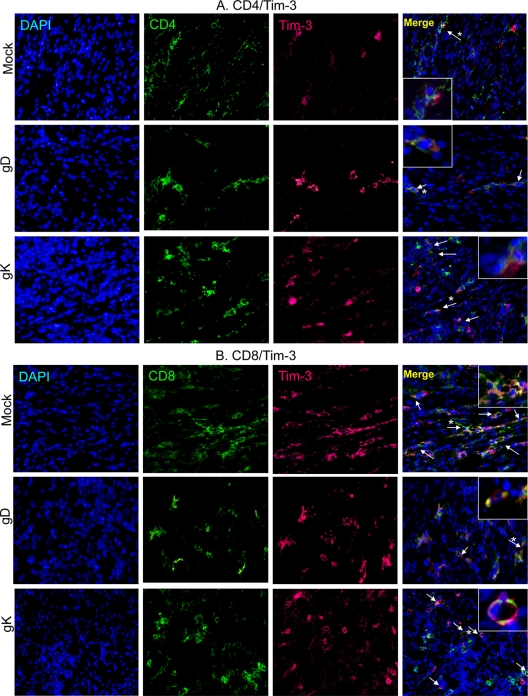
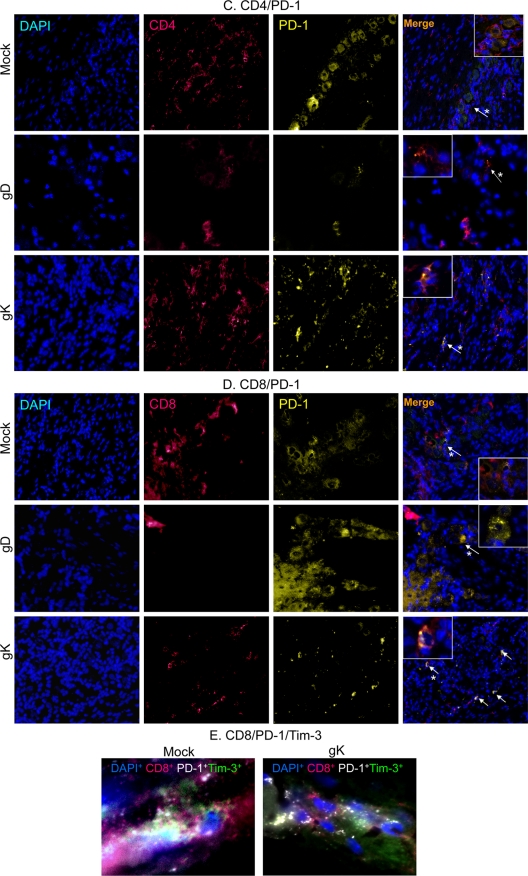
Our qRT-PCR results described for Fig. 3 above suggest that the levels of PD-1 and Tim-3 were increased in the gK-immunized group compared to those in the gD-immunized group. To demonstrate that the increase in exhaustion markers is from exhausted T cells, as these exhaustion markers can also be expressed on other immune cell types, such as macrophages (13), as well as murine retinal neurons (6, 7), we performed double fluorescent immunohistochemistry for CD4+Tim-3+, CD4+PD-1+, CD8+Tim-3+, and CD8+PD-1+ cells in the TG of latently infected mice as described in Materials and Methods. We observed the highest colocalization of CD4+ and Tim3+ cells in mock- and gK-immunized TG (Fig. 4A). Similarly, most of the CD8+ T cells in mock- and gK-immunized TG stained positive for Tim-3 expression (Fig. 4B). PD-1 expression levels were similar between mock- and gD-immunized TG for both CD4+ T cells (Fig. 4C) and CD8+ T cells (Fig. 4D). In contrast, more CD4+ and CD8+ T cells were positive for PD-1 expression in the TG of the gK-immunized group (Fig. 4C and D, respectively). Finally, we observed CD8+ T cells positive for both Tim-3 and PD-1 expression in both mock- and gK-immunized groups (Fig. 4E), representing a third category of exhausted T cells which express both PD-1 and Tim-3 markers. In contrast, no CD8+PD-1+Tim-3+ T cells were detected in the TG of the gD group (not shown).
FIG. 4.
T cell exhaustion markers, PD-1 and Tim-3, colocalize to T cells in the TG of immunized mice during latency. BALB/c mice were immunized and infected as described in the legend to Fig. 1. On day 30 p.i., mice were sacrificed, TG were removed, and 15-μm sections were processed for the presence of CD4+Tim-3+, CD8+Tim-3+, CD4+PD-1+, and CD8+PD-1+ cells from 10 TG per group. (A) Tim-3-Alexa-647 (red), CD4-fluorescein isothiocyanate (FITC) (green); (B) Tim-3-Alexa-647 (red), CD8-FITC (green); (C) PD-1-tetramethyl rhodamine isothiocyanate (TriTc) (yellow), CD4-Alexa-647 (red); and (D) PD-1-TriTc (yellow), CD8-Alexa-647 (red) (20× direct magnification); (E) CD8-Alexa-647 (red), Tim-3-Alexa-488 (green), PD-1-Biotin-594 (white) (63× direct magnification). The nuclear stain DAPI (blue) is included in all panels. Arrows indicate positive stain and asterisks indicate the cell selected for inlay (63× magnification).
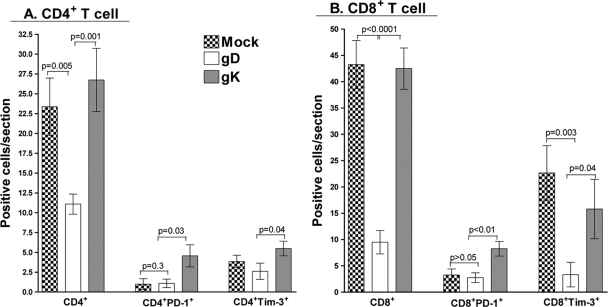
We quantified the above-described staining and observed higher numbers of PD-1+ and Tim-3+ T cells (CD4+ and CD8+) in the gK-immunized group than in the gD-immunized group (Fig. 5A and B). We also saw an increase in CD8+Tim-3+, CD8+PD-1+, CD4+Tim-3+, and CD8+PD-1+ T cells in mock-immunized TG compared to those in the gD group (Fig. 5A and B). The level of Tim-3+ T cells in the mock-immunized group was not statistically different from that of the gK-immunized group. The increases in the gK-immunized group were an approximate 3-fold increase in the number of CD8+PD-1+ T cells, a 5-fold increase in the number of CD4+PD-1+ T cells, and a 2-fold increase in the number of CD4+Tim-3+ T cells compared to the numbers in both mock- and gD-immunized groups. In addition, there was a 12-fold (gK) and 19-fold (mock) difference in the number of CD8+Tim-3+ T cells in gK- and mock-immunized tissues compared to the number of cells in the gD group. These results suggested that the level of T cell infiltrates present in the TG correlated with more latent virus and higher levels of PD-1 and Tim-3. Conversely, they also demonstrated that lower levels of latency in gD-immunized mice lead to a reduction in the number of exhausted T cells. Overall, our results demonstrated a significant increase in the number of PD-1+ T cells in the gK group compared to that in both the mock and gD groups. The number of Tim-3+ T cells was decreased in the gD group compared to that in both the mock and gK groups (there was no difference between mock and gK in regard to Tim-3 expression). Finally, we observed the presence of CD8+PD-1+Tim-3+ T cells in the TG of both mock and gK groups but not in the TG of the gD group.
FIG. 5.
Upregulation of exhaustion markers on T cells in the TG of immunized mice. TG from BALB/c mice were processed as described in the legend to Fig. 4. More than 30 sections from 10 TG were stained and counted in a double-blind fashion for the presence of T cell markers (CD4 or CD8) and for the number of T cells positive for the exhaustion markers (PD-1 or Tim-3). (A) Quantification of CD4+, CD4+PD-1+, and CD4+Tim-3+ T cells; (B) quantification of CD8+, CD8+PD-1+, and CD8+Tim-3+ T cells. Bars, means ± SEM from 30 independent, double-blind sections from 10 TG per group.
DISCUSSION
The main goal of the present study was to examine the expression of specific exhaustion markers in response to different glycoprotein vaccines in HSV-1-infected mice and to correlate these patterns of exhaustion with changes in viral latency in the TG and viral replication in the eye. In a previous study, we correlated higher levels of CS with increased expression of CD8 and PD-1 in the TG of HSV-1-infected mice (38). We have also previously observed a higher level of virus replication and a reduction in viral clearance in the TG of gK-immunized mice (2, 20). We sought to further this observation to include the glycoprotein vaccines to determine if the higher levels of CS observed in gK-immunized and infected animals were correlated with higher levels of virus latency and T cell exhaustion. As controls, we used mice vaccinated with HSV-1 gD (protects from CS) or mock vaccinated (moderate CS relative to that with gK). In this report, we found a correlation between gK immunization and the expression level of many of the transcripts we examined in the TG of latently infected mice. These included the amount of LAT RNA and gB DNA (which are indicative of the relative amount of HSV-1 latency) and the mRNA levels of CD4, CD8, PD-1, and Tim-3. However, we did not detect any correlation between the level of latency in the TG of latently infected mice and virus titer in the eyes of infected mice during acute infection. In addition, by immunohistochemistry, we observed increased numbers of CD4+ and CD8+ T cells in the TG in both mock- and gK-immunized groups compared with the number of cells in the TG of the gD group. As a consequence of higher gB and LAT copy numbers, we anticipate that gK-immunized animals would be able to reactivate from latency more efficiently, and this could be a cause of the increase in immune infiltrates in the gK group. The observed increase in T cells combined with the presence of latent virus, higher immunopathology (CS), and expression of T cell exhaustion markers led us to question if these T cells were functional or exhausted.
Previously, we and others have shown that reduced functionality of CD8+ T cells is associated with the expression of the exhaustion marker PD-1 (15, 38). CD8+ T cell exhaustion has been observed in a multitude of chronic infections, such as cytomegalovirus (CMV), HSV-1, Friend virus, simian immunodeficiency virus (SIV), hepatitis C virus (HCV), hepatitis B virus (HBV), and Mycobacterium tuberculosis (27, 28, 33, 42, 44, 46, 53). These PD-1-expressing, exhausted T cells display reduced numbers of cytotoxic T lymphocytes (CTL), proliferation, and production of interleukin 2 (IL-2), followed by a loss in tumor necrosis factor alpha (TNF-α) production and finally impairment in gamma interferon (IFN-γ) production (42, 55). Furthermore, virus-specific T cells can even be deleted in severe chronic exhaustion (16, 37, 55, 56). This suggests that in the gK-immunized and mock-immunized groups, the increased latency leads to chronic antigen presentation and exhaustion of T cells, while the gD-immunized group is able to effectively clear the virus, leading to a reduction in latent virus, antigen exposure, and T cell exhaustion.
The discovery of the PD-1 exhaustion marker has led investigators to exploit it in therapeutic strategies to reverse T cell exhaustion. Similarly to this study, in which gD vaccination reduced T cell exhaustion, it was previously shown that CD8+PD-1+ T cells were present only during SIV chronic infection, and these cells were not observed after vaccination (54). Blocking the PD-1:PD-L1 produced therapeutic effects in reversing CD8+ T cell exhaustion in chronic lymphocytic choriomeningitis virus (LCMV) infection (24). Similar results were observed in reversing T cell dysfunction when PD-1 was blocked in conjunction with CTLA-4 (another inhibitory exhaustion marker) for both HCV (44) and human immunodeficiency virus (HIV) (29) infections. Furthermore, it has been shown that the ligand for PD-1, PD-L1, negatively regulates CD4+CD25+FoxP3+ T regulatory (Treg) cells in persons with chronic HCV (14) and HBV (45) infections. Finally, these PD-1-expressing Tregs were also seen in association with remission in lupus patients who had undergone hemopoietic stem cell transplantation (57), suggesting a potentially positive role in humans.
As does the major exhaustion marker PD-1 (3), upregulation of T cell immunoglobulin and mucin domain-containing protein 3 (22, 26) also contributes to T cell exhaustion. In this study, we looked at the possible involvement of Tim-3 in T cell exhaustion and the increase of HSV-1 latency. For both gK- and mock-immunized groups, but not the gD-immunized group, we observed an increase in the expression of both PD-1 and Tim-3 on CD8+ T cells in the TG. For the first time in the context of latent HSV-1 infection, we demonstrated significant elevation in the number of CD8+Tim-3+ T cells in the TG of mock- and gK-immunized groups. While there is little difference at the current dose of HSV-1 between mock- and gK-immunized groups in regard to Tim-3 expression, the increased expression in these groups over that in the gD group is highly significant. Thus, our results suggest that, similarly to our PD-1 findings, upregulation of Tim-3 also is correlated with the increase of HSV-1 latency. Previously, it was shown that Tim-3 is an inhibitory transmembrane receptor expressed on the surface of fully differentiated TH1 T cells (36) which functioned to terminate TH1 immunity (48). Tim-3 also is important in regulating both peripheral tolerance and the expansion of TH1 populations (49, 50). Blocking Tim-3 and its interaction with its ligand (Galectin-9pu) has been shown to reverse T cell exhaustion and boost immunity (22, 43). Thus, the Tim-3 pathway may represent an early mechanistic approach to enhancing HSV-1 vaccines by blocking Tim-3 inhibition and thus boosting immunity against HSV-1 in infected individuals, whereas PD-1 is observed more strongly in the gK-immunized group and thus may represent a more potent blockage to HSK in therapeutic vaccines.
In this study, we also have looked at CD4+ T cell exhaustion in immunized mice by using PD-1 and Tim-3 markers. CD4+ T cell exhaustion, while currently less widely studied, appears to be no less prevalent, as it has already been implicated in chronic Trypanosoma cruzi, CMV, and HIV infections (1, 10, 12). More recently, CD4+Tim-3+ T cells were implicated in primary HSV-1 infection, and by altering the interaction of Tim-3 with its ligand, the authors could produce therapeutic effects on ocular lesions (51, 52).
In summary, our results suggest that the increased severity of CS observed in gK-immunized mice is correlated with higher latency and that, in turn, this chronic exposure to viral antigen leads to a higher level of T cell exhaustion, shown by higher levels of expression of PD-1 and Tim-3 exhaustion markers. Our results also suggest that there are at least three populations of exhausted T cells: one that expresses PD-1, one that expresses Tim-3, and one that expresses both PD-1 and Tim-3. Since T cell exhaustion is a commonality observed across many chronic viral infections and as a function of T cell exhaustion, targeting and reversing T cell exhaustion in combination with current vaccination strategies represent a global yet highly specific approach to create more efficacious vaccines that could positively boost an individual's response to antigen as well as their response to therapeutics aimed at boosting immunity.
Acknowledgments
This work was supported by Public Health Service grant EY13615 from the National Eye Institute to H.G.
Footnotes
Published ahead of print on 22 September 2010.
REFERENCES
- 1.Albareda, M. C., G. C. Olivera, S. A. Laucella, M. G. Alvarez, E. R. Fernandez, B. Lococo, R. Viotti, R. L. Tarleton, and M. Postan. 2009. Chronic human infection with Trypanosoma cruzi drives CD4+ T cells to immune senescence. J. Immunol. 183:4103-4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, S. J., K. R. Mott, A. V. Ljubimov, and H. Ghiasi. 2010. Exacerbation of corneal scarring in HSV-1 gK-immunized mice correlates with elevation of CD8+CD25+ T cells in corneas of ocularly infected mice. Virology 399:11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barber, D. L., E. J. Wherry, D. Masopust, B. Zhu, J. P. Allison, A. H. Sharpe, G. J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682-687. [DOI] [PubMed] [Google Scholar]
- 4.Bond, V. C., and S. Person. 1984. Fine structure physical map locations of alterations that affect cell fusion in herpes simplex virus type 1. Virology 132:368-376. [DOI] [PubMed] [Google Scholar]
- 5.Reference deleted.
- 6.Chen, L., V. Pai, R. Levinson, A. H. Sharpe, G. J. Freeman, J. Braun, and L. K. Gordon. 2009. Constitutive neuronal expression of the immune regulator, programmed death 1 (PD-1), identified during experimental autoimmune uveitis. Ocul. Immunol. Inflamm. 17:47-55. [DOI] [PubMed] [Google Scholar]
- 7.Chen, L., C. W. Sham, A. M. Chan, L. M. Francisco, Y. Wu, S. Mareninov, A. H. Sharpe, G. J. Freeman, X. J. Yang, J. Braun, and L. K. Gordon. 2009. Role of the immune modulator programmed cell death-1 during development and apoptosis of mouse retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 50:4941-4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Day, C. L., D. E. Kaufmann, P. Kiepiela, J. A. Brown, E. S. Moodley, S. Reddy, E. W. Mackey, J. D. Miller, A. J. Leslie, C. DePierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E. J. Wherry, H. M. Coovadia, P. J. Goulder, P. Klenerman, R. Ahmed, G. J. Freeman, and B. D. Walker. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350-354. [DOI] [PubMed] [Google Scholar]
- 9.Debroy, C., N. Pederson, and S. Person. 1985. Nucleotide sequence of a herpes simplex virus type 1 gene that causes cell fusion. Virology 145:36-48. [DOI] [PubMed] [Google Scholar]
- 10.D'Souza, M., A. P. Fontenot, D. G. Mack, C. Lozupone, S. Dillon, A. Meditz, C. C. Wilson, E. Connick, and B. E. Palmer. 2007. Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J. Immunol. 179:1979-1987. [DOI] [PubMed]
- 11.Feldman, L. T., A. R. Ellison, C. C. Voytek, L. Yang, P. Krause, and T. P. Margolis. 2002. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc. Natl. Acad. Sci. U. S. A. 99:978-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fletcher, J. M., M. Vukmanovic-Stejic, P. J. Dunne, K. E. Birch, J. E. Cook, S. E. Jackson, M. Salmon, M. H. Rustin, and A. N. Akbar. 2005. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J. Immunol. 175:8218-8225. [DOI] [PubMed] [Google Scholar]
- 13.Folkl, A., X. Wen, E. Kuczynski, M. E. Clark, and D. Bienzle. 2010. Feline programmed death and its ligand: characterization and changes with feline immunodeficiency virus infection. Vet. Immunol. Immunopathol. 134:107-114. [DOI] [PubMed] [Google Scholar]
- 14.Franceschini, D., M. Paroli, V. Francavilla, M. Videtta, S. Morrone, G. Labbadia, A. Cerino, M. U. Mondelli, and V. Barnaba. 2009. PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J. Clin. Invest. 119:551-564. doi: 10.1172/JCI36604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank, G. M., A. J. Lepisto, M. L. Freeman, B. S. Sheridan, T. L. Cherpes, and R. L. Hendricks. 2010. Early CD4(+) T cell help prevents partial CD8(+) T cell exhaustion and promotes maintenance of herpes simplex virus 1 latency. J. Immunol. 184:277-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuller, M. J., and A. J. Zajac. 2003. Ablation of CD8 and CD4 T cell responses by high viral loads. J. Immunol. 170:477-486. [DOI] [PubMed] [Google Scholar]
- 17.Ghiasi, H., S. Bahri, A. B. Nesburn, and S. L. Wechsler. 1995. Protection against herpes simplex virus-induced eye disease after vaccination with seven individually expressed herpes simplex virus 1 glycoproteins. Invest. Ophthalmol. Vis. Sci. 36:1352-1360. [PubMed] [Google Scholar]
- 18.Ghiasi, H., S. Cai, S. Slanina, A. B. Nesburn, and S. L. Wechsler. 1997. Nonneutralizing antibody against the glycoprotein K of herpes simplex virus type-1 exacerbates herpes simplex virus type-1-induced corneal scarring in various virus-mouse strain combinations. Invest. Ophthalmol. Vis. Sci. 38:1213-1221. [PubMed] [Google Scholar]
- 19.Ghiasi, H., R. Kaiwar, A. B. Nesburn, S. Slanina, and S. L. Wechsler. 1994. Expression of seven herpes simplex virus type 1 glycoproteins (gB, gC, gD, gE, gG, gH, and gI): comparative protection against lethal challenge in mice. J. Virol. 68:2118-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghiasi, H., S. Slanina, A. B. Nesburn, and S. L. Wechsler. 1994. Characterization of baculovirus-expressed herpes simplex virus type 1 glycoprotein K. J. Virol. 68:2347-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghiasi, H., S. L. Wechsler, S. Cai, A. B. Nesburn, and F. M. Hofman. 1998. The role of neutralizing antibody and T-helper subtypes in protection and pathogenesis of vaccinated mice following ocular HSV-1 challenge. Immunology 95:352-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golden-Mason, L., B. E. Palmer, N. Kassam, L. Townshend-Bulson, S. Livingston, B. J. McMahon, N. Castelblanco, V. Kuchroo, D. R. Gretch, and H. R. Rosen. 2009. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J. Virol. 83:9122-9130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reference deleted.
- 24.Ha, S. J., S. N. Mueller, E. J. Wherry, D. L. Barber, R. D. Aubert, A. H. Sharpe, G. J. Freeman, and R. Ahmed. 2008. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J. Exp. Med. 205:543-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutchinson, L., K. Goldsmith, D. Snoddy, H. Ghosh, F. L. Graham, and D. C. Johnson. 1992. Identification and characterization of a novel herpes simplex virus glycoprotein, gK, involved in cell fusion. J. Virol. 66:5603-5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones, R. B., L. C. Ndhlovu, J. D. Barbour, P. M. Sheth, A. R. Jha, B. R. Long, J. C. Wong, M. Satkunarajah, M. Schweneker, J. M. Chapman, G. Gyenes, B. Vali, M. D. Hyrcza, F. Y. Yue, C. Kovacs, A. Sassi, M. Loutfy, R. Halpenny, D. Persad, G. Spotts, F. M. Hecht, T. W. Chun, J. M. McCune, R. Kaul, J. M. Rini, D. F. Nixon, and M. A. Ostrowski. 2008. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J. Exp. Med. 205:2763-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jurado, J. O., I. B. Alvarez, V. Pasquinelli, G. J. Martinez, M. F. Quiroga, E. Abbate, R. M. Musella, H. E. Chuluyan, and V. E. Garcia. 2008. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J. Immunol. 181:116-125. [DOI] [PubMed] [Google Scholar]
- 28.Kasprowicz, V., J. Schulze Zur Wiesch, T. Kuntzen, B. E. Nolan, S. Longworth, A. Berical, J. Blum, C. McMahon, L. L. Reyor, N. Elias, W. W. Kwok, B. G. McGovern, G. Freeman, R. T. Chung, P. Klenerman, L. Lewis-Ximenez, B. D. Walker, T. M. Allen, A. Y. Kim, and G. M. Lauer. 2008. High level of PD-1 expression on hepatitis C virus (HCV)-specific CD8+ and CD4+ T cells during acute HCV infection, irrespective of clinical outcome. J. Virol. 82:3154-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaufmann, D. E., and B. D. Walker. 2009. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J. Immunol. 182:5891-5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keir, M. E., M. J. Butte, G. J. Freeman, and A. H. Sharpe. 2008. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26:677-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kramer, M. F., and D. M. Coen. 1995. Quantification of transcripts from the ICP4 and thymidine kinase genes in mouse ganglia latently infected with herpes simplex virus. J. Virol. 69:1389-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Little, S. P., and P. A. Schaffer. 1981. Expression of the syncytial (syn) phenotype in HSV-1, strain KOS: genetic and phenotypic studies of mutants in two syn loci. Virology 112:686-702. [DOI] [PubMed] [Google Scholar]
- 33.Maier, H., M. Isogawa, G. J. Freeman, and F. V. Chisari. 2007. PD-1:PD-L1 interactions contribute to the functional suppression of virus-specific CD8+ T lymphocytes in the liver. J. Immunol. 178:2714-2720. [DOI] [PubMed] [Google Scholar]
- 34.Margolis, T. P., F. L. Elfman, D. Leib, N. Pakpour, K. Apakupakul, Y. Imai, and C. Voytek. 2007. Spontaneous reactivation of herpes simplex virus type 1 in latently infected murine sensory ganglia. J. Virol. 81:11069-11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGeoch, D. J., M. A. Dalrymple, A. J. Davison, A. Dolan, M. C. Frame, D. McNab, L. J. Perry, J. E. Scott, and P. Taylor. 1988. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J. Gen. Virol. 69:1531-1574. [DOI] [PubMed] [Google Scholar]
- 36.Monney, L., C. A. Sabatos, J. L. Gaglia, A. Ryu, H. Waldner, T. Chernova, S. Manning, E. A. Greenfield, A. J. Coyle, R. A. Sobel, G. J. Freeman, and V. K. Kuchroo. 2002. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 415:536-541. [DOI] [PubMed] [Google Scholar]
- 37.Moskophidis, D., F. Lechner, H. Pircher, and R. M. Zinkernagel. 1993. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 362:758-761. (Erratum, 364:262.) [DOI] [PubMed] [Google Scholar]
- 38.Mott, K. R., C. J. Bresee, S. J. Allen, L. BenMohamed, S. L. Wechsler, and H. Ghiasi. 2009. Level of herpes simplex virus type 1 latency correlates with severity of corneal scarring and exhaustion of CD8+ T cells in trigeminal ganglia of latently infected mice. J. Virol. 83:2246-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mott, K. R., A. A. Chentoufi, D. Carpenter, L. Benmohamed, S. L. Wechsler, and H. Ghiasi. 2009. The role of a glycoprotein K (gK) CD8+ T-cell epitope of herpes simplex virus on virus replication and pathogenicity. Invest. Ophthalmol. Vis. Sci. 50:2903-2912. [DOI] [PubMed] [Google Scholar]
- 40.Mott, K. R., Y. Osorio, D. J. Brown, N. Morishige, A. Wahlert, J. V. Jester, and H. Ghiasi. 2007. The corneas of naive mice contain both CD4+ and CD8+ T cells. Mol. Vis. 13:1802-1812. [PubMed] [Google Scholar]
- 41.Mott, K. R., G. C. Perng, Y. Osorio, K. G. Kousoulas, and H. Ghiasi. 2007. A recombinant herpes simplex virus type 1 expressing two additional copies of gK is more pathogenic than wild-type virus in two different strains of mice. J. Virol. 81:12962-12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mueller, S. N., and R. Ahmed. 2009. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. U. S. A. 106:8623-8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakagawa, H., Y. Uchida, E. Takamura, Y. Nakagawa, H. Araki, and M. Watanabe. 1993. Diagnostic impression cytology for herpes simplex keratitis. Jpn. J. Ophthalmol. 37:505-513. [PubMed] [Google Scholar]
- 44.Nakamoto, N., H. Cho, A. Shaked, K. Olthoff, M. E. Valiga, M. Kaminski, E. Gostick, D. A. Price, G. J. Freeman, E. J. Wherry, and K. M. Chang. 2009. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 5:e1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nan, X. P., Y. Zhang, H. T. Yu, Y. Li, R. L. Sun, J. P. Wang, and X. F. Bai. 2010. Circulating CD4+CD25 high regulatory T cells and expression of PD-1 and BTLA on CD4+ T cells in patients with chronic hepatitis B virus infection. Viral Immunol. 23:63-70. [DOI] [PubMed] [Google Scholar]
- 46.Petrovas, C., D. A. Price, J. Mattapallil, D. R. Ambrozak, C. Geldmacher, V. Cecchinato, M. Vaccari, E. Tryniszewska, E. Gostick, M. Roederer, D. C. Douek, S. H. Morgan, S. J. Davis, G. Franchini, and R. A. Koup. 2007. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood 110:928-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pogue-Geile, K. L., and P. G. Spear. 1987. The single base pair substitution responsible for the Syn phenotype of herpes simplex virus type 1, strain MP. Virology 157:67-74. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez-Manzanet, R., R. DeKruyff, V. K. Kuchroo, and D. T. Umetsu. 2009. The costimulatory role of TIM molecules. Immunol. Rev. 229:259-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sabatos, C. A., S. Chakravarti, E. Cha, A. Schubart, A. Sanchez-Fueyo, X. X. Zheng, A. J. Coyle, T. B. Strom, G. J. Freeman, and V. K. Kuchroo. 2003. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat. Immunol. 4:1102-1110. [DOI] [PubMed] [Google Scholar]
- 50.Sanchez-Fueyo, A., J. Tian, D. Picarella, C. Domenig, X. X. Zheng, C. A. Sabatos, N. Manlongat, O. Bender, T. Kamradt, V. K. Kuchroo, J. C. Gutierrez-Ramos, A. J. Coyle, and T. B. Strom. 2003. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 4:1093-1101. [DOI] [PubMed] [Google Scholar]
- 51.Sehrawat, S., P. B. Reddy, N. Rajasagi, A. Suryawanshi, M. Hirashima, and B. T. Rouse. 2010. Galectin-9/TIM-3 interaction regulates virus-specific primary and memory CD8 T cell response. PLoS Pathog. 6:e1000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sehrawat, S., A. Suryawanshi, M. Hirashima, and B. T. Rouse. 2009. Role of Tim-3/galectin-9 inhibitory interaction in viral-induced immunopathology: shifting the balance toward regulators. J. Immunol. 182:3191-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takamura, S., S. Tsuji-Kawahara, H. Yagita, H. Akiba, M. Sakamoto, T. Chikaishi, M. Kato, and M. Miyazawa. 2010. Premature terminal exhaustion of Friend virus-specific effector CD8+ T cells by rapid induction of multiple inhibitory receptors. J. Immunol. 184:4696-4707. [DOI] [PubMed] [Google Scholar]
- 54.Velu, V., S. Kannanganat, C. Ibegbu, L. Chennareddi, F. Villinger, G. J. Freeman, R. Ahmed, and R. R. Amara. 2007. Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. J. Virol. 81:5819-5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wherry, E. J., J. N. Blattman, K. Murali-Krishna, R. van der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang, L., A. M. Bertucci, R. Ramsey-Goldman, R. K. Burt, and S. K. Datta. 2009. Regulatory T cell (Treg) subsets return in patients with refractory lupus following stem cell transplantation, and TGF-beta-producing CD8+ Treg cells are associated with immunological remission of lupus. J. Immunol. 183:6346-6358. [DOI] [PMC free article] [PubMed] [Google Scholar]