Abstract
Herpesviruses minimally require the envelope proteins gB and gH/gL for virus entry and cell-cell fusion; herpes simplex virus (HSV) additionally requires the receptor-binding protein gD. Although gB is a class III fusion protein, gH/gL does not resemble any documented viral fusion protein at a structural level. Based on those data, we proposed that gH/gL does not function as a cofusogen with gB but instead regulates the fusogenic activity of gB. Here, we present data to support that hypothesis. First, receptor-positive B78H1-C10 cells expressing gH/gL fused with receptor-negative B78H1 cells expressing gB and gD (fusion in trans). Second, fusion occurred when gH/gL-expressing C10 cells preexposed to soluble gD were subsequently cocultured with gB-expressing B78 cells. In contrast, prior exposure of gB-expressing C10 cells to soluble gD did not promote subsequent fusion with gH/gL-expressing B78 cells. These data suggest that fusion involves activation of gH/gL by receptor-bound gD. Most importantly, soluble gH/gL triggered a low level of fusion of C10 cells expressing gD and gB; a much higher level was achieved when gB-expressing C10 cells were exposed to a combination of soluble gH/gL and gD. These data clearly show that gB acts as the HSV fusogen following activation by gD and gH/gL. We suggest the following steps leading to fusion: (i) conformational changes to gD upon receptor binding, (ii) alteration of gH/gL by receptor-activated gD, and (iii) upregulation of the fusogenic potential of gB following its interaction with activated gH/gL. The third step may be common to other herpesviruses.
Herpesviruses enter cells by fusing their envelopes with host cell membranes either by direct fusion at the plasma membrane or by pH-dependent or -independent endocytosis, depending on the target cell (27, 29, 39). Although the entry pathways of other enveloped viruses are similarly diverse (8), all systems for which molecular details have been obtained rely on a single fusion protein (43); herpesviruses are unique in their use of gB and the gH/gL heterodimer as their core fusion machinery (17, 37). Some herpesviruses employ additional receptor-binding glycoproteins, e.g., herpex simplex virus (HSV) gD, and others require gH/gL-associated proteins, e.g., UL128-131 of cytomegalovirus (CMV) (34) or gp42 of Epstein-Barr virus (EBV) (42). This complexity has made it difficult to unravel the mechanism of herpesvirus entry.
Ultrastructural and biochemical studies have shown that for HSV entry, binding of gD to one of its receptors, either HVEM or nectin-1 (36), activates the downstream events that drive gB- and gH/gL-dependent fusion (17). The structure of the gB ectodomain (18) bears striking structural homology to the postfusion form of the single fusion protein G of vesicular stomatitis virus (VSV) (33). However, unlike the other class III viral fusion proteins, VSV G and baculovirus gp64 (5), gB requires gH/gL to function in virus-cell and cell-cell fusion (17). A number of investigations support the concept that gH/gL might also be fusogenic (13, 41). Some have suggested that a multiprotein complex comprised of gD, gH/gL, and gB might be assembled to cause fusion (14). Using bimolecular complementation (BiMC), we and others showed that interactions can occur between half enhanced yellow fluorescent protein (EYFP)-tagged gB (e.g., gBn) and tagged gD (e.g., gDc) or between tagged gD and tagged gH (1, 3). However, because these occur in the absence of one of the other essential components, e.g., a receptor, we could not assess their functional significance. Importantly, gH/gL and gB interact with each other only in response to receptor binding by gD (1-3, 12). We subsequently showed that this interaction precedes fusion and is required for it to occur (2). Thus, we were able to conclude that gH/gL must interact with gB, whether transiently or stably, in order for fusion to occur. Whether gD was indeed involved in a multiprotein complex was not clear, nor was the role of gH/gL in promoting fusion initiated by gD-receptor binding. The lack of structural data for gH/gL left its potential role as a fusogen unresolved.
However, in 2010, the structure of gH/gL of HSV-2 was solved in collaboration with Chowdary et al. (12). Structurally, gH/gL does not resemble any known viral fusogen, thereby forcing a reconsideration of its function in promoting virus-cell and cell-cell fusion. We hypothesized that gH/gL does not likely act as a cofusogen with gB but rather regulates fusion by gB (12).
In this report, we argue that as a regulator of fusion, gH/gL might not have to be in the same membrane as gB in order to regulate its activity, i.e., gH/gL on one cell might promote fusion of gB expressed by another cell, as long as gD and a gD receptor are also present. In support of this, it was recently shown that gH/gL and gB of human cytomegalovirus (HCMV) can cause cell-cell fusion when expressed by distinct cells (in trans) (41). We present evidence that HSV gB and gH/gL can cause cell-cell fusion when they are expressed in trans, a process that requires both gD and a gD receptor. Although the efficiency of fusion in trans is low compared with that of fusion when gB and gH/gL are in cis (as they would be when in the virus), separation of these proteins onto two different cells enabled us to dissect the order in which each protein acts along the pathway to fusion. Moreover, we found that a combination of soluble gD (not membrane bound) and soluble gH/gL (also not membrane bound) could trigger fusion of receptor-bearing cells that had been transfected with the gene for gB. Our data show that gD, gH/gL, and gB act in a series of steps whereby gD is first activated by binding its cell receptor. Previous studies showed that receptor binding causes gD to undergo conformational changes (17). Based on the data in this paper, we propose that these changes then enable gD to activate gH/gL into a form that in turn binds to and activates the fusogenic activity of gB. Although we do not know whether any of these reactions result in the formation of a stable complex, our data suggest that gB is the sole HSV fusogen and that gD and gH/gL act to upregulate cell-cell fusion and most likely virus-cell fusion, leading to HSV entry.
MATERIALS AND METHODS
Cells and media.
Mouse melanoma cells (B78H1) expressing nectin-1 (C10) were grown in 10% fetal bovine serum-Dulbecco modified Eagle medium containing 500 μg/ml G418 (26). The parental cell line B78H1 was propagated in the absence of G418.
Plasmids.
Plasmids pEP98 (gB), pEP99 (gD), pEP100 (gH), and pEP101 (gL), encoding full-length type I glycoproteins, were gifts of P. G. Spear (30). pTC510 (gH2) and pTC579 (gL2), encoding full-length type II glycoproteins, have been described previously (10, 11). The construction of EYFP-tagged gB (Bc) and gH (Hn) has been described elsewhere (1).
Antibodies.
The following antibodies were used for immunofluorescence: A22 and SS55 anti-gB monoclonal antibodies (MAbs) (6), MC5 and MC23 anti-gD MAbs (1), and R137 anti-gH1 and R176 anti-gH2 polyclonal antibodies (10, 31). For blocking experiments, the following antibodies were used: DL11 gD MAb and C226 and A22 gB MAbs (6). H1781 MAb was purchased from Virusys Corp.
Soluble proteins.
Soluble gD306t, gB730, and gH2t/gL2 were purified form baculovirus-infected cells (Sf9) as described previously (7, 35, 44).
Transfection and cell cocultures.
B78H1 or C10 cells were seeded on glass coverslips and cultured overnight at 37°C to the desired density. Cells were transfected with GenePorter reagents (Gene Therapy Systems) according to the manufacturer's instructions with various plasmids (as indicated in each experiment). For the coculture experiments, B78H1 and C10 cells were transfected with the indicated plasmids for 8 h at 37°C. C10 cells were detached with trypsin or EDTA and overlaid on top of the B78H1 cells. The two cell monolayers were cocultured for 40 h at 37°C. Samples were then processed for immunofluorescence.
Triggering of fusion with soluble proteins and antibody blocking.
To synchronize fusion, C10 cells were transfected with the plasmid for gD, gB, gH2, or gL2 for 8 h as described above. At that time, 250 μg/ml of soluble gB730, gD306, or gH2/gL2 was added and cells were incubated with the protein for an additional 40 h. For blocking of fusion, C10 cells were transfected with plasmids (Table 1 ) for 8 h, then overlaid onto B78 cells in the presence of 100 μg/ml of MAb C226, A22, or H1781 (shown in Fig. 2) (6), and incubated for an additional 40 h.
TABLE 1.
Number of syncytia per coverslip when two transfected cell populations were cocultured
| Protein(s) in cell population 1 (B78) | Protein(s) in cell population 2 (B78 or C10a) | No. of synctia at 40 h | Avg no. of nuclei/synctiumc | Total no. of fusion events | Fusion indexd (% of ideal index) |
|---|---|---|---|---|---|
| gB, gD, gH/gL | 0 | 0 | 0 | 0 | |
| gB, gD, gH/gL | N | 600 | 15 | 9,000 | 100 |
| gB, gH/gL, N | gD | 310 | 12 | 3,720 | 41 |
| gB, gD | gH/gL, N | 80 | 8 | 640 | 7.1 |
| gB | gD, gH/gL, N | 95 | 9 | 855 | 9.5 |
| gD | gH/gL, N | 0 | 0 | 0 | 0 |
| gB, gD | N | 0 | 0 | 0 | 0 |
| gB | gH/gL, gD306b | 0 | 0 | 0 | 0 |
| gB | gH/gL, N, gD306b | 140 | 9 | 1,260 | 14 |
| gB | gH/gL, N, gD306 | 130 | 6 | 780 | 8.7 |
| gB, N, gD306 | gH/gL | 8 | 3 | 24 | 0.3 |
N, nectin.
Soluble gD306 was added at the time of coculture.
A syncytium was counted as such when 3 or more nuclei were surrounded by one fluorescent membrane.
The fusion index was determined by considering the combination of gB, gD, and gH/gL in cell population 1 and nectin in cell population 2 to be 100%. For all other variations, the total number of fusion events was divided by the ideal of 9,000 and multiplied by 100.
Immunofluorescence.
The procedure was essentially as described in detail elsewhere (1, 2). Briefly, transfected cells were fixed with paraformaldehyde and then incubated with the indicated glycoprotein-specific primary antibodies, followed by fluoroconjugated secondary antibodies. Nuclei were stained with To-Pro-3 iodide (Invitrogen). Coverslips were mounted in ProLong Gold antifade reagent (Invitrogen) and examined by confocal microscopy with a Nikon TE-300 inverted microscope coupled to a Bio-Rad confocal imaging system. In the merged images in the figures, the far-red nuclear stain was artificially colored in white. All images were taken at ×60 magnification.
Syncytium counting.
After the cells were stained with the indicated antibodies, syncytia were counted on the entire surface of each coverslip (Fig. 1 to 4) for the presence of syncytia at ×60 magnification. In some cases, particularly when counts were low, we averaged the number seen on at least two coverslips. A syncytium was defined as such when one fluorescent membrane enclosed 3 or more nuclei. For the coculture experiments, B78H1 and C10 cells were transfected with the indicated plasmids for 8 h at 37°C. C10 cells were detached with trypsin or EDTA and overlaid on top of the B78H1 cells. The two cell monolayers were cocultured for 40 h at 37°C. Samples were then processed for immunofluorescence. To block fusion, 100 μg/ml MAb was added at the time of coculture.
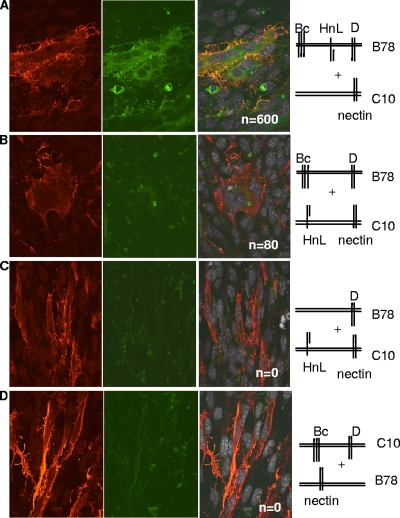
FIG. 1.
Fusion occurs when gB and gH/gL are in trans. (A to D) Nectin-1-expressing B78H1-C10 cells and/or B78 cells were transfected for 8 h with plasmids for the glycoproteins as shown in the stick diagrams. The C10 cells were trypsinized and overlaid onto coverslips containing B78 cells, and the bilayer was cocultured for 40 h. Cells were fixed and stained with anti-gH/gL polyclonal antibody (red). Propidium iodide was added to visualize nuclei (gray). Cells were analyzed by immunofluorescent assay for protein (red channel), EYFP (green channel), and nuclei (far-red channel). Images in the far-red channel were artificially colored white, as seen in the merged images (third set of images). Confocal images are at ×60 magnification and were captured using the same camera setting. n, number of syncytia per coverslip.
RESULTS
Fusion occurs when gB and gH/gL are in trans.
We previously used bimolecular complementation (BiMC) to show that fusion occurs when nectin-1-expressing C10 cells are transfected with split EYFP-tagged versions of gB (Bc) and gH/gL (HnL) along with untagged gD (all in cis) (1-3, 12). Importantly, EYFP fluorescence, indicative of a gB-gH interaction, was detected in each syncytium (1, 2). The split EYFP tags were at the C termini of gB and gH/gL (cytoplasmic side), and the observed BiMC indicated that the C termini of gB and gH interact during fusion. Additionally, certain virus-neutralizing MAbs to the gB or gH/gL ectodomain blocked both BiMC and fusion (2, 12), showing that the ectodomains of gB and gH/gL also interact in a functional sense. Importantly, no nonneutralizing MAbs to the ectodomain of either protein blocked this interaction. We therefore concluded that specific portions of the ectodomains of gB and gH/gL must interact as a critical step for HSV glycoprotein-mediated cell fusion. Although these studies were carried out when gB and gH/gL were in cis, our structural data suggested that gH/gL is unlikely to serve as a “cofusogen” but may instead regulate gB-mediated fusion (12). Thus, it was theoretically possible that a similar interaction could occur when gB and gH/gL were in trans with each other and that this could also drive fusion.
To test this hypothesis, we used a coculture assay (38, 39) whereby B78 (no receptor) and nectin-1-bearing C10 cells were first transfected for 8 h with various combinations of Bc, Hn, L, and/or gD and then cocultured for 40 h prior to fixation and microscopic analysis. First, to validate this variation of our original assay (1), C10 cells were overlaid onto B78 cells that had been transfected with Bc, Hn, L, and gD (Fig. 1A and Table 1). In this case, only the receptor was in trans. This mimics what occurs between the virus and cell. Multiple syncytia formed, and all syncytia exhibited EYFP fluorescence, which is indicative of BiMC. Thus, the coculture system recapitulated the results obtained using the monolayer system where all of the glycoproteins were transfected into receptor-bearing C10 cells; i.e., the HSV proteins and the receptor were in cis. The negative control was to transfect the glycoproteins into B78 cells, and no syncytia formed as expected (Table 1). When Bc and gD were in B78 cells and Hn and L were in C10 cells, there was no BiMC, but fusion still occurred at 7% of the ideal level (Fig. 1B and Table 1). A similar level of fusion was seen when gB was expressed in B78 cells and gD and gH/gL were expressed in C10 cells (Table 1). Although fusion with gB and gH/gL in trans was less efficient than fusion with these proteins in cis, the syncytia were large and easy to recognize. Other combinations with gB and gH/gL were done, and the fusion indexes were similar (not shown). As expected, when either gB (Fig. 1C) or gH/gL was omitted from the cocultures (Fig. 1D), there was no BiMC or cell fusion. Thus, fusion in trans depends on the presence of gH/gL, gB, and gD and a gD receptor. Finally, the absence of BiMC when gH/gL and gB were in trans (Fig. 1B) shows that the carboxy termini of gB and gH do not have to interact with each other for fusion to occur; i.e., they do not have to be in cis. However, because there was no BiMC, we could not formally conclude that fusion in trans was the result of a direct interaction of the ectodomains of Bc and HnL, as occurs when the two proteins are in cis (2).
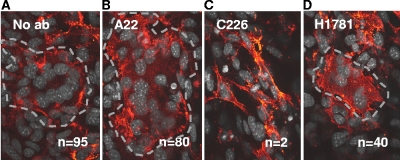
To address this issue, we tested whether fusion with gB and gH/gL in trans could be blocked with virus-neutralizing anti-gB monoclonal antibodies that would be expected to block the interaction between gB and gH/gL (2). We previously mapped a panel of gB-specific neutralizing MAbs to three functional regions (FR) (6). Antibodies to FR1 and FR2 blocked both BiMC and fusion (2), while MAbs to FR3 blocked only fusion. Blocking of fusion by MAbs to FR1 prevented insertion of the fusion loops into the target membrane (15). In contrast, MAbs to FR2 were proposed to interfere with the interaction between gB and gH/gL, so these were the ones tested here. Of the available MAbs that map to FR2, MAb C226 was the most potent at blocking fusion and BiMC (2). This MAb also has very high titers of virus-neutralizing activity, indicating that it neutralizes by blocking this critical interaction during virus entry. Additionally, MAb 1781, which partially blocked both BiMC and fusion with gB and gH/gL in cis (2), also partially blocked fusion in trans (Fig. 2D).
FIG. 2.
Cell-cell fusion in trans is blocked by the anti-gB neutralizing MAb C226. B78H1 cells transfected with gB and gD were cocultured with C10 cells transfected with gH/gL in the absence (A) or presence (B) of the nonneutralizing MAb A22 or in the presence of the neutralizing MAb C226 (C) or H1781 (D). Conditions for coculture, fixation, and staining are the same as for Fig. 1. For easier identification, the syncytia were outlined with a dotted gray line. n, number of syncytia per coverslip.
In this and the coculture experiments that followed, we used untagged versions of gB and gH/gL since we could no longer visualize BiMC when these proteins were in trans. B78 cells transfected with gB were cocultured with C10 cells transfected with gD and gH/gL in the absence or presence of anti-gB MAbs. When we used the nonneutralizing MAb A22 (previously shown to have no effect on BiMC) (Fig. 2A and B) (2, 12), there were nearly as many syncytia (80) as when no MAb was added (95) (Fig. 2A and Table 1). In contrast, in three experiments where MAb C226 was included in the overlay (Fig. 2C), only 0 to 2 syncytia were detected on the coverslip. Another MAb to FR2, H1781, was able to reduce the number of syncytia though not as dramatically as MAb C226 (Fig. 2D), consistent with previous results for this MAb for fusion in cis (2). We noted similar inhibition by MAb C226 when gD was expressed in the B78 cells rather than in the C10 cells (data not shown). We conclude that when gB and gH/gL are in trans, fusion involves an interaction between their ectodomains. However, this experiment does not exclude the possibility that when these proteins are in cis, other regions contribute to the interaction, e.g., the transmembrane regions or cytoplasmic tail.
Receptor-activated gD triggers gH/gL into a form that promotes the fusogenic activity of gB.
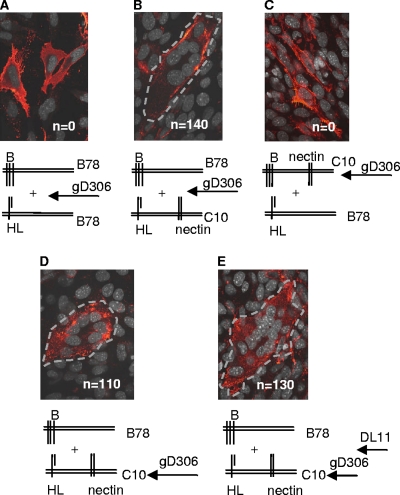
We previously showed that soluble gD triggers fusion of receptor-bearing C10 cells transfected with both gB and gH/gL (1, 2, 12). We presumed that upon receptor binding, gD interacted with either gB or gH/gL (or possibly with both) as a necessary prelude to the gB-gH/gL interaction. However, the EYFP-tagged version of gD interacted with EYFP-tagged gB or with EYFP-tagged gH in the absence of fusion; thus, we were unable to clarify whether these represented functional interactions, i.e., ones that are necessary for fusion to occur. Similar interactions were noted by others using BiMC (3, 4). The fact that we could detect fusion when these proteins were in trans meant that we could use the trans assay to resolve this question. To be certain we could do this, we first transfected B78 cells with gB and overlaid them onto a second monolayer of B78 cells transfected with gH/gL. Soluble gD306 was added at the time of coculture and left on the cells for 16 h. As expected, there was no fusion since there was no receptor on either monolayer (Fig. 3A and Table 1). As a positive control, we transfected nectin-1-bearing C10 cells with gB and cocultured them with B78 cells bearing gH/gL, adding gD306 at the time of coculture to trigger fusion (Fig. 3B and Table 1). As expected, since all of the essential proteins were present, fusion occurred. To determine whether gD acted upon gB or gH/gL or both, we then transfected nectin-1-bearing C10 cells with gB and at 8 h posttransfection added soluble gD306 for 16 h. Unbound gD was then removed by washing the cells with culture medium. The cells were detached and overlaid onto B78 cells that had been transfected with gH/gL. After 24 h of coculture, there was no evidence of cell-cell fusion (Fig. 3C and Table 1). In contrast, when C10 cells expressing gH/gL were preexposed to soluble gD306 for 16 h, washed, and then cocultured with B78 cells transfected with gB (Fig. 3C), cell-cell fusion was readily observed (130 syncytia) (Table 1). When we included the gD-neutralizing MAb DL11 in the overlay to eliminate any residual gD-receptor interactions (28), we again saw fusion and the number of syncytia was marginally affected by the presence of this MAb (Fig. 3E and Table 1). DL11 is a gD antibody that neutralizes the virus by blocking virion gD as well as purified gD from binding to both receptors (23, 28), and it is unlikely to interfere with downstream events. Therefore, no residual unbound gD remained that might have triggered gB. We conclude that receptor-activated gD interacts with gH/gL, and this activated form of gH/gL triggers gB into a fusogenic state.
FIG. 3.
Fusion in trans is triggered by preexposure of C10 cells expressing gH/gL to soluble gD306. (A) B78 cells expressing gB were cocultured with B78 cells expressing gH/gL. Soluble gD306 was added at the time of coculture (negative control). (B) B78 cells expressing gB were cocultured with C10 cells expressing gH/gL. Soluble gD306 was added at the time of coculture (positive control). (C) C10 cells expressing gB were incubated with gD306 (soluble gD) overnight. The medium was removed, and the cells were washed once with medium, overlaid with fresh medium and serum, and cocultured with B78 cells expressing gH/gL. (D) C10 cells expressing gH/gL were incubated with gD306 (soluble gD) overnight. The medium was removed, and the cells were washed once with medium, overlaid with fresh medium and serum, and cocultured with B78 cells expressing gB. (E) Same as in panel D, except that MAb DL11 was added at the time of coculture between the C10 and B78 cells. Dotted gray lines in panels B, D, and E outline the identified syncytia. n, number of syncytia per coverslip.
Soluble forms of both gD and gH/gL can trigger cell-cell fusion.
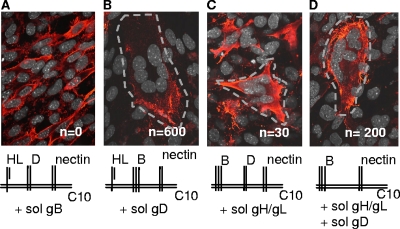
Thus far, we have shown that gH/gL can function both in cis and in trans with gB to cause fusion. We wondered whether gH/gL could carry out its function even when not membrane bound, i.e., as a soluble protein. In contrast, since gB functions as the fusogen, we predicted that it could function only when membrane anchored. To test this, we first transfected C10 cells with gH/gL and gD and added soluble gB, and as expected, no fusion was detected (Fig. 4 and Table 2). Next, we transfected C10 cells with gH/gL and gB and added soluble gD. In this case, as seen before (1, 2, 12), many syncytia formed and we termed this the “ideal” situation for a monolayer system to which a soluble glycoprotein was added. When gB-plus-gD-transfected C10 cells were incubated in the absence of any added protein, no syncytia formed (1, 2, 12; also data not shown). A small number (28) of syncytia were detected when we added soluble gH/gL to C10 cells transfected with gD and gB (Fig. 4C and Table 2). Of particular interest, however, was the fact that significant fusion occurred when we added a combination of soluble gD and gH/gL to C10 cells transfected with gB. In this case, 200 syncytia formed in one experiment (Table 2) and 140 in another, although in that experiment the number of syncytia seen with soluble gD as the trigger was also lower (data not shown). The fusion index for the experiment whose results are shown in Fig. 4 was 38% of that in the ideal situation. Taken together, these experiments show that while gB must be membrane anchored, neither gD nor gH/gL needs be membrane anchored to function (see Fig. 5 for models). One caveat to be noted is that gB730 may be a postfusion form of gB and in that case would not be expected to trigger fusion. However, that does not take away from the key observation that membrane-bound (prefusion) gB can be triggered to carry out fusion by the combination of gD and gH/gL when neither protein is membrane anchored. On the basis of these results, we hypothesize that both gD (once activated by receptor) and gH/gL (once activated by the active form of gD) serve a regulatory role and that gB is the fusogen that is acted upon to ultimately cause fusion (modeled in Fig. 5). Moreover, our data suggest that gB is the sole protein that acts as the HSV fusogen, although it must be activated to do so by both receptor-bound gD and gD-activated gH/gL.
FIG. 4.
Neither gD nor gH/gL needs to be membrane anchored to trigger cell-cell fusion. (A to D) C10 cells were transfected with plasmids for the proteins indicated in the diagrams for 8 h, followed by addition of soluble gB (A), gD (B), or gH/gL (C) or a combination of gD and gH/gL (D). Panels A and B were stained for gH/gL; panels C and D were stained for gB. Syncytia are outlined for easier identification. n, number of syncytia per coverslip.
TABLE 2.
Number of syncytia per coverslip when a monolayer of transfected cells was exposed to a soluble protein
| Protein(s) in cell population 1 (C10) | Soluble protein(s) added | No. of synctia at 40 h | Avg no. of nuclei/synctiuma | Total no. of fusion events | Fusion indexb (% of ideal index) |
|---|---|---|---|---|---|
| gD, gH/gL | gB730 | 0 | 0 | 0 | 0 |
| gB, gH/gL | gD306 | 600 | 7 | 4,200 | 100 |
| gB, gD | gH2L2 | 30 | 3 | 90 | 2.1 ± 0.2c |
| gB | gH2gL2 | 10 | 3 | 30 | 0.7 ± 0.1c |
| gB | gH2gL2, gD306 | 200 | 8 | 1,600 | 38 |
| gB | gD306 | 0 | 0 | 0 | 0 |
A syncytium was counted as such when 3 or more nuclei were surrounded by one fluorescent membrane.
The fusion index was determined by considering the fusion of gB and gH/gL in C10 cells with soluble gD306 to be 100%. For all other variations, the total number of fusion events was divided by the ideal of 4,200 and multiplied by 100.
Results are averages ± standard errors from 3 independent experiments.
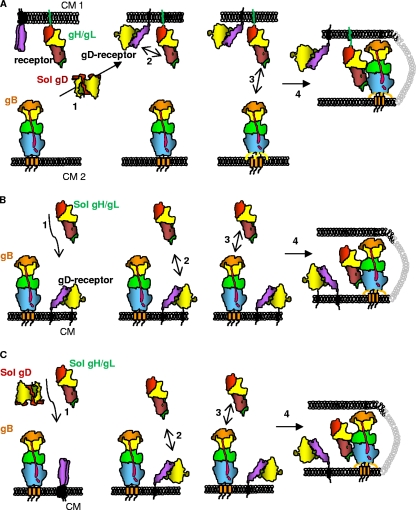
FIG. 5.
Schemes for the steps in fusion when gB is triggered by receptor-bound soluble gD and/or gH/gL. (A to C) Each protein is depicted in cartoon form, shaped like the actual structure. Glycoproteins gB and gH/gL are colored according to the color scheme used to depict their domains (12, 18). gD is colored according to the color scheme used to depict its dimeric structure (24). (A) In step 1, soluble gD binds to the cell-bound receptor (purple) and undergoes conformational changes that enable it to interact with cell-bound gH/gL (step 2). Then, cell-bound gH/gL interacts with cell-bound gB (step 3) to cause fusion (step 4). (B) Soluble gH/gL is added to cells containing gD bound to the receptor (steps 1 and 2). Then, steps 3 and 4 happen as in panel A. (C) A combination of soluble gD and gH/gL is added to receptor-bearing cells expressing gB. gD binds the receptor (step 1), undergoes conformational changes, and then acts upon gH/gL (step 2), which is then able to upregulate the fusogenic activity of gB (steps 3 and 4). CM, cell membrane.
DISCUSSION
We previously transfected receptor-bearing B78H1-C10 cells with half EYFP-tagged forms of gB (Bc) and gH (Hn) as well as untagged gL and then triggered both BiMC and cell fusion by addition of soluble gD306 (1, 2, 12). We also showed that this interaction could be blocked by a subset of anti-gB neutralizing MAbs but not by any nonneutralizing MAbs (2). Based on its lack of resemblance to fusion proteins (12), we predicted that HSV gH/gL should still function when presented in the membrane of a cell that did not express gB; precedence for this was reported by Vanarsdall and colleagues for HCMV gH/gL and gB (41). Indeed, we found that HSV gH/gL and gB do function in trans, although this process is much more efficient when these proteins are in cis. This is to be expected, as the proteins in the virion are optimally arranged to accomplish fusion.
Interestingly, we no longer observed BiMC between the YFP-tagged forms of gB and gH, likely because the tags are on the C termini and the interaction occurs between the ectodomains before membrane mixing. Perhaps this interaction is not all that stable when the tags are in separate membranes prior to fusion and the two proteins are separate from each other after fusion. This observation is important in visualizing the topology of the trans interactions and how they can lead to fusion (Fig. 5). To verify that gH/gL and gB do still interact during fusion in trans, we blocked fusion with the neutralizing anti-gB MAbs C226 and H1781. Both recognize epitopes in FR2 of the ectodomain (6), which is likely part of the interface with gH/gL when formed in cis. We propose that the same part of gB interacts with gH/gL when the two proteins are in trans.
A second important finding was that exposure of gH/gL-expressing C10 cells to soluble gD led to fusion with cells expressing gB that had not been exposed to gD. In sharp contrast, exposure of gB-transfected C10 cells to soluble gD did not lead to fusion with cells expressing gH/gL that had not seen gD. We interpret this to mean that receptor-bound gD alters gH/gL in such a way as to allow it to activate membrane-bound gB. Although our bias is that gD and gH/gL interact directly, this activation could involve a cell protein as well.
Finally, gH/gL, like gD, did not have to be membrane anchored to induce fusion of nectin-1-bearing C10 cells expressing gB and gD (modeled in Fig. 5A and B). In fact, fusion of membrane-anchored gB in C10 cells could be triggered when exposed to a combination of soluble gD and gH/gL (modeled in Fig. 5C). Taken together, our data support the idea that gD binds the receptor and this form of gD activates gH/gL to upregulate gB into a fusogenic state. This process involves an interaction, whether direct or indirect, between the ectodomains of gD and gH/gL as well a direct interaction between the ectodomains of gB and gH/gL. We believe the interaction between gD and gH/gL is likely transient or weak; when we stabilized the interaction of the C termini of the two proteins with EYFP tags, no fusion occurred when gB and the receptor were also present. The interaction between gD and gB (in the context of fusion), although not required for fusion itself (1), could trigger the insertion of gB fusion loops into the target membrane but not into a fusogenic state. This is powerful evidence that gB is the sole fusogen of HSV. It should be noted, however, that portions of gB, gD, and gH other than their ectodomains may contribute substantially to the normal fusion process (e.g., in the context of the virus). Such contributions might account, at least in part, for why fusion in cis is so much more efficient than fusion in trans. Indeed, several reports have shown that residues in the carboxy termini of both gB and gH influence the efficiency of cell fusion and/or virus entry (20-22).
The fact that gH/gL does not have to be membrane anchored to promote fusion appears to contradict the previous idea that this protein can promote hemifusion (40) while gB is needed for full fusion. While we did not examine hemifusion in our studies, it is difficult to imagine how a non-membrane-anchored version of gH/gL could carry out lipid mixing of two membranes. The structure of the gH/gL ectodomain appears to rule out that possibility; i.e., there is no classical fusion peptide or loop (12). However, we cannot exclude the possibility that gH/gL, in addition to a regulatory role, does have some ability to interact with lipids when anchored to cells via its transmembrane region.
How does HSV-induced fusion compare to fusion by other enveloped viruses?
In most enveloped viruses, fusion requires the conversion of a surface glycoprotein from a prefusion metastable state to a more stable postfusion form (5, 16). This involves a rearrangement of domains (fold-back) so that fusion peptides (class I) or fusion loops (classes II and III) can be inserted into a target membrane while the transmembrane anchor holds another portion of the protein in the virion envelope. Unlike VSV G, gB mediates entry through either the plasma membrane or an endosome (17). Furthermore, gB does not function on its own but instead requires gH/gL for all fusion, regardless of the site of entry. As such, we have hypothesized that gH/gL upregulates the fusogenic capabilities of gB (12). Based on the literature on viral fusion proteins, it is likely that gH/gL helps to trigger conversion of the prefusion form of gB into a postfusion conformation, though pH may also play a key role during endosomal entry of the virus.
Why do we see fusion in trans in contradiction to the literature on HSV?
In two previous reports (9, 32), fusion by HSV glycoproteins was induced only when gD, gB, and gH/gL and a gD receptor were in cis. This discrepancy with our data is a bit puzzling but may be partially explained by the sensitivity of the assays used or by issues that were raised above. In one case (9), the authors did not count fused cells with fewer than 11 nuclei, a number close to the maximum that we saw occurring in trans (Table 1). In the second case, a luciferase assay was used to measure fusion (32). In our study, fusion with gB and gH/gL in trans occurred with less than 10% of the efficiency of fusion when all of the glycoproteins were in cis; this level of fusion could well have been below the level of detection by the luciferase assay (32).
Why is the efficiency of fusion in trans low compared to that of fusion in cis?
Several factors may be involved in addition to those mentioned above. First, if transfection occurred in only 40 to 50% of each cell population, the chances that gH/gL and gB would “find each other” would be no greater than 20 to 25% of the total population. To some extent, this artifact was overcome by comparing fusion when gB and gH/gL were in different cells with fusion when all of the glycoproteins were in the same cell (cis) and only the receptor nectin-1 was in a different cell. We called this the ideal situation for fusion between two cell populations (Table 1). When gB, gH/gL, and nectin are in one cell and gD is in the other, fusion occurs with less than half the efficiency of that in the ideal situation, evidence that it is easier for gD to find gH/gL when both are in the same cell. Moreover, these two proteins as well as gB are in cis when in the virus, so it is possible that the affinity of gD for gH/gL or of gB for gH/gL is greater when they are adjacent to each other (cis) rather than across from each other (trans). Finally, of course, all of these proteins are always in cis when in the virion envelope, as noted above. However, some trans interactions might occur during cell-cell spread of the virus.
How does binding of gH/gL to gB trigger gB into a fusogenic state?
One way would be to enhance the rate by which the prefusion form of gB is converted into a fusion-competent state. Thus, gH/gL might alter the equilibrium between prefusion and postfusion states of gB. Indeed, a mutant form of EBV gB has the ability to fuse cells, and several rate-of-entry and syn mutants with changes in gB and gH/gL have been reported (19, 21, 25). Future studies using soluble gH/gL to trigger fusion by these mutants might offer additional clues about the mechanism by which gH/gL upregulates gB.
Acknowledgments
We thank Tina M. Cairns, J. Charles Whitbeck, and other members of our lab for helpful discussions of the work reported here. We also thank Huan Lou for providing purified soluble glycoproteins. Special thanks to Leslie B. King of the School of Veterinary Medicine at the University of Pennsylvania for careful editing of the manuscript.
This work was supported by NIH grants AI-056045 and AI-076231 to R.J.E and by NIH grant AI-18289 to G.H.C.
Experiments were carried out by D.A. and W.T.S. Intellectual input and manuscript preparation were done by R.J.E., D.A., and G.H.C.
Footnotes
Published ahead of print on 22 September 2010.
REFERENCES
- 1.Atanasiu, D., J. C. Whitbeck, T. M. Cairns, B. Reilly, G. H. Cohen, and R. J. Eisenberg. 2007. Bimolecular complementation reveals that glycoproteins gB and gH/gL of herpes simplex virus interact with each other during cell fusion. Proc. Natl. Acad. Sci. U. S. A. 104:18718-18723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atanasiu, D., J. C. Whitbeck, M. P. de Leon, H. Lou, B. P. Hannah, G. H. Cohen, and R. J. Eisenberg. 2010. Bimolecular complementation defines functional regions of herpes simplex virus gB that are involved with gH/gL as a necessary step leading to cell fusion. J. Virol. 84:3825-3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avitabile, E., C. Forghieri, and G. Campadelli-Fiume. 2007. Complexes between herpes simplex virus glycoproteins gD, gB, and gH detected in cells by complementation of split enhanced green fluorescent protein. J. Virol. 81:11532-11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avitabile, E., C. Forghieri, and G. Campadelli-Fiume. 2009. Cross talk among the glycoproteins involved in herpes simplex virus entry and fusion: the interaction between gB and gH/gL does not necessarily require gD. J. Virol. 83:10752-10760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Backovic, M., and T. S. Jardetzky. 2009. Class III viral membrane fusion proteins. Curr. Opin. Struct. Biol. 19:189-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bender, F. C., M. Samanta, E. E. Heldwein, M. P. de Leon, E. Bilman, H. Lou, J. C. Whitbeck, R. J. Eisenberg, and G. H. Cohen. 2007. Antigenic and mutational analyses of herpes simplex virus glycoprotein B reveal four functional regions. J. Virol. 81:3827-3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bender, F. C., J. C. Whitbeck, H. Lou, G. H. Cohen, and R. J. Eisenberg. 2005. Herpes simplex virus glycoprotein B binds to cell surfaces independently of heparan sulfate and blocks virus entry. J. Virol. 79:11588-11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bissonnette, M. L., S. A. Connolly, D. F. Young, R. E. Randall, R. G. Paterson, and R. A. Lamb. 2006. Analysis of the pH requirement for membrane fusion of different isolates of the paramyxovirus parainfluenza virus 5. J. Virol. 80:3071-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Browne, H., B. Bruun, and T. Minson. 2001. Plasma membrane requirements for cell fusion induced by herpes simplex virus type 1 glycoproteins gB, gD, gH and gL. J. Gen. Virol. 82:1419-1422. [DOI] [PubMed] [Google Scholar]
- 10.Cairns, T., R. S. B. Milne, M. Ponce-de-Leon, D. K. Tobin, G. H. Cohen, and R. J. Eisenberg. 2003. Structure-function analysis of herpes simplex virus type 1 gD and gH-gL: clues from gDgH chimeras. J. Virol. 77:6731-6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cairns, T. M., L. S. Friedman, H. Lou, J. C. Whitbeck, M. S. Shaner, G. H. Cohen, and R. J. Eisenberg. 2007. N-terminal mutants of herpes simplex virus type 2 gH are transported without gL but require gL for function. J. Virol. 81:5102-5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chowdary, T. K., T. M. Cairns, D. Atanasiu, G. H. Cohen, R. J. Eisenberg, and E. E. Heldwein. 2010. Crystal structure of the conserved herpesvirus fusion regulator complex gH-gL. Nat. Struct. Mol. Biol. 17:882-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galdiero, S., A. Falanga, G. Vitiello, M. Vitiello, C. Pedone, G. D'Errico, and M. Galdiero. 2010. Role of membranotropic sequences from herpes simplex virus type I glycoproteins B and H in the fusion process. Biochim. Biophys. Acta 1798:579-591. [DOI] [PubMed] [Google Scholar]
- 14.Gianni, T., M. Amasio, and G. Campadelli-Fiume. 2009. Herpes simplex virus gD forms distinct complexes with fusion executors gB and gH/gL in part through the C-terminal profusion domain. J. Biol. Chem. 284:17370-17382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hannah, B. P., T. M. Cairns, F. C. Bender, J. C. Whitbeck, H. Lou, R. J. Eisenberg, and G. H. Cohen. 2009. Herpes simplex virus glycoprotein B associates with target membranes via its fusion loops. J. Virol. 83:6825-6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison, S. C. 2008. Viral membrane fusion. Nat. Struct. Mol. Biol. 15:690-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heldwein, E. E., and C. Krummenacher. 2008. Entry of herpesviruses into mammalian cells. Cell. Mol. Life Sci. 65:1653-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heldwein, E. E., H. Lou, F. C. Bender, G. H. Cohen, R. J. Eisenberg, and S. C. Harrison. 2006. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 313:217-220. [DOI] [PubMed] [Google Scholar]
- 19.Highlander, S. L., W. H. Cai, S. Person, M. Levine, and J. C. Glorioso. 1988. Monoclonal antibodies define a domain on herpes simplex virus glycoprotein B involved in virus penetration. J. Virol. 62:1881-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Highlander, S. L., D. J. Dorney, P. J. Gage, T. C. Holland, W. Cai, S. Person, M. Levine, and J. C. Glorioso. 1989. Identification of mar mutations in herpes simplex virus type 1 glycoprotein B which alter antigenic structure and function in virus penetration. J. Virol. 63:730-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutchinson, L., F. L. Graham, W. Cai, C. Debroy, S. Person, and D. C. Johnson. 1993. Herpes simplex virus (HSV) glycoproteins B and K inhibit cell fusion induced by HSV syncytial mutants. Virology 196:514-531. [DOI] [PubMed] [Google Scholar]
- 22.Jackson, J. O., E. Lin, P. G. Spear, and R. Longnecker. 2010. Insertion mutations in herpes simplex virus 1 glycoprotein H reduce cell surface expression, slow the rate of cell fusion, or abrogate functions in cell fusion and viral entry. J. Virol. 84:2038-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krummenacher, C., A. V. Nicola, J. C. Whitbeck, H. Lou, W. Hou, J. D. Lambris, R. J. Geraghty, P. G. Spear, G. H. Cohen, and R. J. Eisenberg. 1998. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J. Virol. 72:7064-7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krummenacher, C., V. M. Supekar, J. C. Whitbeck, E. Lazear, S. A. Connolly, R. J. Eisenberg, G. H. Cohen, D. C. Wiley, and A. Carfi. 2005. Structure of unliganded HSV gD reveals a mechanism for receptor-mediated activation of virus entry. EMBO J. 24:4144-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laquerre, S., R. Argnani, D. B. Anderson, S. Zucchini, R. Manservigi, and J. C. Glorioso. 1998. Heparan sulfate proteoglycan binding by herpes simplex virus type 1 glycoproteins B and C, which differ in their contributions to virus attachment, penetration, and cell-to-cell spread. J. Virol. 72:6119-6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller, C. G., C. Krummenacher, R. J. Eisenberg, G. H. Cohen, and N. W. Fraser. 2001. Development of a syngenic murine B16 cell line-derived melanoma susceptible to destruction by neuroattenuated HSV-1. Mol. Ther. 3:160-168. [DOI] [PubMed] [Google Scholar]
- 27.Milne, R. S., A. V. Nicola, J. C. Whitbeck, R. J. Eisenberg, and G. H. Cohen. 2005. Glycoprotein D receptor-dependent, low-pH-independent endocytic entry of herpes simplex virus type 1. J. Virol. 79:6655-6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicola, A. V., M. Ponce de Leon, R. Xu, W. Hou, J. C. Whitbeck, C. Krummenacher, R. I. Montgomery, P. G. Spear, R. J. Eisenberg, and G. H. Cohen. 1998. Monoclonal antibodies to distinct sites on herpes simplex virus (HSV) glycoprotein D block HSV binding to HVEM. J. Virol. 72:3595-3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicola, A. V., and S. E. Straus. 2004. Cellular and viral requirements for rapid endocytic entry of herpes simplex virus. J. Virol. 78:7508-7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okuma, K., M. Nakamura, S. Nakano, Y. Niho, and Y. Matsuura. 1999. Host range of human T-cell leukemia virus type I analyzed by a cell fusion-dependent reporter gene activation assay. Virology 254:235-244. [DOI] [PubMed] [Google Scholar]
- 31.Peng, T., M. Ponce-de-Leon, H. Jiang, G. Dubin, J. M. Lubinski, R. J. Eisenberg, and G. H. Cohen. 1998. The gH-gL complex of herpes simplex virus (HSV) stimulates neutralizing antibody and protects mice against HSV type 1 challenge. J. Virol. 72:65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pertel, P. E., A. Fridberg, M. L. Parish, and P. G. Spear. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279:313-324. [DOI] [PubMed] [Google Scholar]
- 33.Roche, S., S. Bressanelli, F. A. Rey, and Y. Gaudin. 2006. Crystal structure of the low-pH form of the vesicular stomatitis virus glycoprotein G. Science 313:187-191. [DOI] [PubMed] [Google Scholar]
- 34.Ryckman, B. J., B. L. Rainish, M. C. Chase, J. A. Borton, J. A. Nelson, M. A. Jarvis, and D. C. Johnson. 2008. Characterization of the human cytomegalovirus gH/gL/UL128-131 complex that mediates entry into epithelial and endothelial cells. J. Virol. 82:60-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sisk, W. P., J. D. Bradley, R. J. Leipold, A. M. Stoltzfus, M. Ponce de Leon, M. Hilf, C. Peng, G. H. Cohen, and R. J. Eisenberg. 1994. High-level expression and purification of secreted forms of herpes simplex virus type 1 glycoprotein gD synthesized by baculovirus-infected insect cells. J. Virol. 68:766-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spear, P. G., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 275:1-8. [DOI] [PubMed] [Google Scholar]
- 37.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. Methods 77:10179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stiles, K. M., and C. Krummenacher. 2010. Glycoprotein D actively induces rapid internalization of two nectin-1 isoforms during herpes simplex virus entry. Virology 399:109-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stiles, K. M., R. S. Milne, G. H. Cohen, R. J. Eisenberg, and C. Krummenacher. 2008. The herpes simplex virus receptor nectin-1 is down-regulated after trans-interaction with glycoprotein D. Virology 373:98-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subramanian, R. P., and R. J. Geraghty. 2007. Herpes simplex virus type 1 mediates fusion through a hemifusion intermediate by sequential activity of glycoproteins D, H, L, and B. Proc. Natl. Acad. Sci. U. S. A. 104:2903-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vanarsdall, A. L., B. J. Ryckman, M. C. Chase, and D. C. Johnson. 2008. Human cytomegalovirus glycoproteins gB and gH/gL mediate epithelial cell-cell fusion when expressed either in cis or in trans. J. Virol. 82:11837-11850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, X., W. J. Kenyon, Q. Li, J. Mullberg, and L. M. Hutt-Fletcher. 1998. Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J. Virol. 72:5552-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White, J. M., S. E. Delos, M. Brecher, and K. Schornberg. 2008. Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme. Crit. Rev. Biochem. Mol. Biol. 43:189-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willis, S. H., A. H. Rux, C. Peng, J. C. Whitbeck, A. V. Nicola, H. Lou, W. Hou, L. Salvador, R. J. Eisenberg, and G. H. Cohen. 1998. Examination of the kinetics of herpes simplex virus glycoprotein D binding to the herpesvirus entry mediator, using surface plasmon resonance. J. Virol. 72:5937-5947. [DOI] [PMC free article] [PubMed] [Google Scholar]