Abstract
Immunodeficiency and lymphoid malignancy are hallmarks of the human disease Nijmegen breakage syndrome (NBS; OMIM 251260), which is caused by NBS1 mutations. Although NBS1 has been shown to bind to the T-cell receptor alpha (TCRα) locus, its role in TCRβ rearrangement is unclear. Hypomorphic mutations of Nbs1 in mice and patients result in relatively mild T-cell deficiencies, raising the question of whether the truncated Nbs1 protein might have clouded a certain function of NBS1 in T-cell development. Here we show that the deletion of the entire Nbs1 protein in T-cell precursors (Nbs1T-del) results in severe lymphopenia and a hindrance to the double-negative 3 (DN3)-to-DN4 transition in early T-cell development, due to abnormal TCRβ coding and signal joints as well as the functions of Nbs1 in T-cell expansion. Chromatin immunoprecipitation (ChIP) analysis of the TCR loci reveals that Nbs1 depletion compromises the loading of Mre11/Rad50 to V(D)J-generated DNA double-strand breaks (DSBs) and thereby affects resection of DNA termini and chromatin conformation of the postcleavage complex. Although a p53 deficiency relieves the DN3→DN4 transition block, neither a p53 deficiency nor ectopic expression of TCRαβ rescues the major T-cell loss in Nbs1T-del mice. All together, these results demonstrate that Nbs1's functions in both repair of V(D)J-generated DSBs and proliferation are essential for T-cell development.
DNA double-strand breaks (DSBs) are generated by exogenous DNA damage stimuli (e.g., ionizing radiation) and endogenous metabolic intermediates, such as collapsed replication forks. Upon DSB generation, NBS1 together with MRE11 and RAD50 form the MRN complex (26). MRE11, which contains two DNA binding domains, mediates the MRN complex binding to the exposed DNA ends. The adjacent MRN-associated DNA ends could be further tethered together through the coiled-coil domain (zinc hook) of RAD50 (19, 43). The chromatin loading of MRN stimulates the ATM kinase that subsequently phosphorylates the downstream effectors, such as p53 and CHK2, for cell cycle regulation, apoptosis, and repair initiation (9, 27, 40). In addition to transducing the repair signal, the NBS1/MRN complex participates in modulating DNA damage response (DDR) pathways by promoting error-free homology-directed repair (HDR) while repressing nonhomologous end joining (NHEJ) to minimize the generation of errant, therefore potentially “dangerous,” DNA joints in S- and G2/M-phase cells (47).
Mutations in any of the MRN complex proteins increase genomic instability and cause human genomic instability disorders. NBS1 hypomorphism causes the autosomal recessive disorder Nijmegen breakage syndrome (NBS; OMIM 251260). NBS patients show multisystemic defects, among which immunodeficiency and a predisposition to lymphoid malignancies originating from B- or T-cell lineages are the major hallmarks (11, 22). NBS patients are prone to opportunistic infections due to agammaglobulinemia, primarily IgA and IgG. Other immune defects include a reduced total number of CD3+ and CD4+ but not CD8+ single-positive (SP) T cells (11, 22, 30). The MRN complex is therefore proposed to play an important role in the sensing (as a DDR component) and repairing (as a component of NHEJ) of V(D)J-generated DSBs and thereby to have its regulatory function in lymphoid development (2, 13). However, NBS patient cells carrying hypomorphic mutations of the NBS1 gene fail to demonstrate a direct role of NBS1 in V(D)J recombination (15, 20, 48).
During lymphocyte development, G1-phase-specific endonucleases RAG1/2 generate the DSBs at recombination signal sequences (RSS) flanking functional V, D, and J gene segments, which are in turn joined mainly by the NHEJ machinery (13, 28). Recent studies using intrachromosomal recombination substrates in the MRN hypomorphic mouse B cells or a small interfering RNA (siRNA) knockdown of MRN in cell lines demonstrate that the MRN complex stabilizes the DSB ends generated by RAG1/2 to promote canonical NHEJ (C-NHEJ; LigIV-Xrcc4 dependent) and also alternative NHEJ (A-NHEJ; LigIV-Xrcc4 independent) if C-NHEJ is not available (8, 10, 12, 17, 32, 34, 44, 46).
NBS1 consists of multiple domains and interacts with several key DDR molecules at both the early or late stages of the DDR cascade (49). While hypomorphic mutation supports cell and mouse survival, the complete loss of any of the MRN components causes embryonic lethality, suggesting a vital role of Nbs1 in viability and proliferation. Hypomorphic Nbs1 mice show a T-lymphoid defect, characterized by a specific reduction of CD4+ CD8+ double-positive (DP) and CD4+ single-positive (SP) T-cell populations but a rather normal amount of CD8+ SP and CD4− CD8− double-negative (DN) T cells (17, 23). During T-cell development, Nbs1 localizes to the T-cell receptor alpha (TCRα) locus (7), and unjoined TCRα coding ends are accumulated in the hypomorphic Nbs1 murine T cells (17). Collectively, these findings indicate that Nbs1/the MRN complex may function in V(D)J recombination during the late stage of T-cell development by specifically affecting the TCRα locus rearrangement (17). Given the essential function of NBS1, or MRN, in sensing and repairing DSBs, cell cycle control, and cell viability, full-length NBS1 may be expected to have a strong impact on lymphoid development. Current working models depicting that Nbs1 is involved in the repair of DSBs during V(D)J recombination have been developed from cells containing truncated Nbs1 isoforms (for example, p72), which may mask additional functions of NBS1. In the present study, we engineer mice with an Nbs1-specific deletion in early developing thymocytes and explore the additional function of the entire NBS1 protein in T-cell development, which might have not been seen previously in an Nbs1 hypomorphic background.
MATERIALS AND METHODS
Generation of Nbs1T-del, Nbs1T-del p53−/−, and Nbs1F/F Lck-Cre+ Rag2−/− AND+ mice.
Nbs1F6/F6 mice (14) were crossed with Lck-Cre mice (kindly provided by Christopher Wilson, University of Washington, Seattle, WA), giving rise to Nbs1F6/F6 Lck-Cre+ mice, i.e., Nbs1T-del mice (mice with deletion of the entire Nbs1 protein in the T cells). Additionally, Nbs1T-del mice were crossed with p53 knockout mice to generate Nbs1T-del p53−/− mice. In addition, Nbs1T-del mice were crossed with Rag2+/− AND+ mice (kindly provided by André Nussenzweig, NIH) to generate mice with the genotype Nbs1F6/F6 Lck-Cre+ Rag2−/− AND+ (Nbs1T-del Rag2−/− AND+) (24, 36). All animals were maintained under specific-pathogen-free conditions at the animal facility of the Leibniz Institute for Age Research-Fritz Lipmann Institute (FLI), Jena, Germany. Animal care and experiments were performed in accordance with the ethic committee guidelines. For PCR genotyping, the following two primers were used to identify the Nbs1F6 allele (1.2 kb) and the Nbs1-deleted (Δ) allele (0.6 kb): loxPtestR, AATACAGTGACTCCTGGAGG, and Intron5F, ATAAGACAGTCACCACTGCG. For a specific genotyping of the different stages of DN cells, the additional primer, EX6, CAGGGCGACATGAAAGAAAAC, together with the above-mentioned primers, was used to identify the Nbs1F6 allele (0.3 kb) and the Nbs1-deleted (Δ) allele (0.6 kb). The p53 knockout allele can be detected by PCR using the following primers: X7, TATACTCAGAGCCGGCCT; X6.5, ACAGCGTGGTGGTACCTTAT; and Neo, CATTCAGGACATAGCGTTGG. To genotype the Rag2 knockout allele, we used 3f, GCAAGGACGCTCTAGGAATG, and 3r, TAGTCCCGTTTCCCATGTTG. To detect the TCRαβ transgene, the following primers were used: TCR-f, GACTTGGAGATTGCCAACCCATATCTAAGT, and TCR-r, TGAGCCGAAGGTGTAGTCGGAGTTTGCATT.
Flow cytometry analysis.
The thymi and spleens were mashed through a 40-μm nylon membrane and then treated with the ACK buffer (0.15 mM NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA [pH 7.2]). The cells were resuspended in cold phosphate-buffered saline (PBS) containing 2% fetal calf serum (FCS), subjected to staining with phycoerythrin (PE)-, fluorescein isothiocyanate (FITC)-, allophycocyanin (APC)-, and PE-Cy5-conjugated monoclonal antibodies, and analyzed with a FACSCanto flow cytometer equipped with FACSDiva software (Becton Dickinson, Mountain View, CA). The panel of FITC-labeled monoclonal antibodies included CD19 and CD44 (Pharmingen, San Diego, CA). The PE-conjugated antibodies used were CD25, CD3, and CD4 (Pharmingen). The APC and PE-Cy5-conjugated antibodies used were CD8 and CD4 (Pharmingen). Apoptosis was analyzed by staining cells with FITC-conjugated annexin V antibody (Pharmingen) in the annexin V staining solution (10 mM HEPES-NaOH at pH 7.4, 140 mM NaCl, 2.5 mM CaCl2).
Lymphocyte sorting and stimulation.
After the T-cell isolation and antibody staining, the DN, DP, and SP thymocyte subpopulations were purified from thymi to >95% purity using a FACSAria cell sorter (Becton Dickinson). For in vitro T-cell stimulation, sorted thymocytes were treated simultaneously with anti-CD3 and anti-CD28 antibodies (10 μg/ml each) or 5 μg/ml phytohemagglutinin (PHA; Sigma-Aldrich, Munich, Germany) together with 10 ng/ml phorbol myristate acetate (PMA; Sigma-Aldrich). Prior to stimulation, lymphocytes were stained with 2.5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) (29). The cells were analyzed by flow cytometry at the indicated time.
V(D)J recombination profiling.
Genomic DNA was isolated from the sorted DP, SP, and DN T cells and amplified by PCR using the following primers: for the β locus coding joint, Vβ13, 5′-GAGGAAAGGTGACATTGAGC-3′, and Jβ2.7, 5′-TGAGAGCTGTCTCCTACTATCGATT-3′; for the β locus signal joint (5), Dβ1, 5′-AGAGGAGCAGCTTATCTGGTGG-3′, and Vβ14, 5′-CTTTGGTGACTTCTGACTTGA-3′; and for the α locus signal joint (38), SJα56, 5′-CAGTAGGGGATGGATGCTAACATGA-3′, and ADV8-RS, 5′-CCTGCACCCTTGGTTCATGTG-3′.
The PCR products were gel purified and subsequently cloned into the PCR-Topo TA vector (Invitrogen, Karlsruhe, Germany). The sequences were compared with those in the UCSC database (http://genome.ucsc.edu/) for a further characterization of the resolved V(D)J recombination. The raw sequences of the TCR status are provided in Tables S1 to S4 in the supplemental material.
ChIP assay.
Freshly isolated thymocytes (1 × 107) were cross-linked with 1% formaldehyde, and chromatin immunoprecipitations (ChIPs) were performed as described previously (18) using 4 μg of a polyclonal antibody specific for Rad50 (Upstate, Lake Placid, NY), γ-H2AX (Upstate), or acetyl-histone H4 (Ac-H4; Upstate). Input DNA and immunoprecipitated DNA were analyzed by PCR for the presence of Vβ1, Vβ30, Vα5, and Vα21 recombination signal sequences (RSSs). The primers used to amplify the sequences of interest included the following: Vβ1, 5′CTGGGGACAAAGAGGTCAAA3′ and 5′GGGAAGTCTGGGTAGGAAGG3′; Vβ30, 5′TCTGGGGCTACAGCTGATTT3′ and 5′GCATTAGGCATGAGGGAAAA3′; Vα5, 5′CTGGGAAGCGTCTTCAGTTC3′ and 5′AAAAGTGTGCCACTCCATCC3′; and Vα21, 5′TTGGTACCGACAGGTTCCTC3′ and 5′TGCTGAGCTCATTGCTCACT3′. PCR products were resolved on a 2% agarose gel and stained with ethidium bromide.
Chromosome metaphase preparation and cytogenetic analysis.
The thymocytes from 8-week-old control and Nbs1T-del mice were isolated as described above. Thymocytes (1 × 107 to 5 × 107) were cultured in complete RPMI 1640 medium supplemented with 10% FCS and stimulated with 10 units/ml interleukin-2 (IL-2; Chiron, Ratingen, Germany), 10 ng/ml PMA (Sigma-Aldrich), and 750 ng/ml ionomycin (Sigma-Aldrich) (33). After 48 h of incubation at 37°C, 20 ng/ml colcemid (Sigma-Aldrich) was added, and the metaphases were prepared according to the standard protocol. For telomere fluorescence in situ hybridization (T-FISH), hybridization of FITC-labeled peptide nucleic acid (PNA) telomeric probes (Dako, Hamburg, Germany) onto metaphases was conducted by following the company's manual. The pictures of metaphase were captured and analyzed by BandView and FISHView softwares (Applied Spectral Imaging system) installed on a Zeiss Imager M1 microscope.
Immunoblotting.
Proteins extracted from cells in a RIPA buffer (20 mM HEPES at pH 7.6, 20% glycerol, 0.5 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA at pH 8.0, 0.5% NP-40, 1 mM dithiothreitol [DTT], 1 mM phenylmethylsulfonyl fluoride [PMSF], 5 mg/ml leupeptin, 2 mg/ml aprotinin, 1 mM β-glycerophosphate, 1 mM Na3VO4, and 10 mM NaF) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), blotted with antibodies in 1× Tris-buffered saline-Tween 20 (TBS-T) containing 5% nonfat dried milk, followed by an incubation with horseradish peroxidase-conjugated secondary antibodies, and detected by ECL reagents (Amersham Biosciences, Buckinghamshire, United Kingdom). The following antibodies were used: rabbit anti-Nbs1 antibody (1:1,000; Cell Signaling, Danvers, MA), mouse anti-PARP-1 (1:2,000; R&D Systems, Wiesbaden-Nordenstadt, Germany), and mouse anti-Chk2 (1:1,000; Upstate).
Histopathology and immunohistological staining.
The tissues used for histological examination were fixed in 4% buffered formalin and embedded in paraffin. The sections (5 μm) were stained with hematoxylin and eosin (H&E) and mounted for microscopy. The pictures are processed with AxioVision software.
RESULTS
T-cell-specific deletion of Nbs1 in mice results in central and peripheral T-cell lymphopenia.
To investigate the essential function of Nbs1 in lymphoid development and V(D)J recombination, we crossed Nbs1F6/F6 mice (14) with Lck-Cre transgenic mice, which express Cre recombinase in T-cell precursors (DN cells). PCR and Western blot analyses confirmed that Nbs1F6/F6 Lck-Cre+ mice had a deletion of Nbs1 specifically in the thymi and all T-cell populations but not CD19+ B cells (Fig. 1A to D). Hence, we designated Nbs1F6/F6 Lck-Cre+ as Nbs1T-del. The control group of mice (Nbs1T-ctr) includes Nbs1+/+ Lck-Cre−, Nbs1+/+ Lck-Cre+, Nbs1+/F6 Lck-Cre−, Nbs1+/F6 Lck-Cre+, and Nbs1F6/F6 Lck-Cre− mice, all of which carried at least one functional Nbs1 allele.
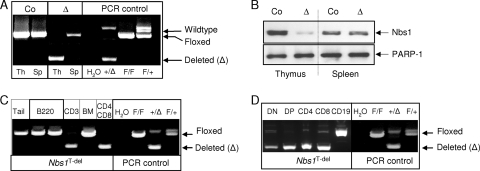
FIG. 1.
The specific deletion of Nbs1 in the T-cell lineage. (A) PCR analysis of the Nbs1 deletion in the thymus (Th) and spleen (Sp) of the indicated genotype. “Co” and “Δ” represent samples obtained from Nbs1T-ctr and Nbs1T-del mice, respectively. PCR controls used Nbs1F6 (F) and Nbs1-deleted (Δ) alleles. (B) Western blot analysis of the Nbs1 deletion in thymi and spleens of 4-week-old Nbs1T-ctr and Nbs1T-del mice. PARP-1 is used as a loading control. (C) PCR analysis of the Nbs1 deletion in genomic DNA from sorted CD3+, B220+, and CD4+ CD8+ populations from Nbs1T-del thymi and spleens as well as bone marrow (BM) cells. Tail DNA (tail) is used to control the floxed allele. (D) PCR analysis of the Nbs1 deletion in sorted CD19+, CD4+, CD8+, and CD4+ CD8+ populations from Nbs1T-del thymi and spleens.
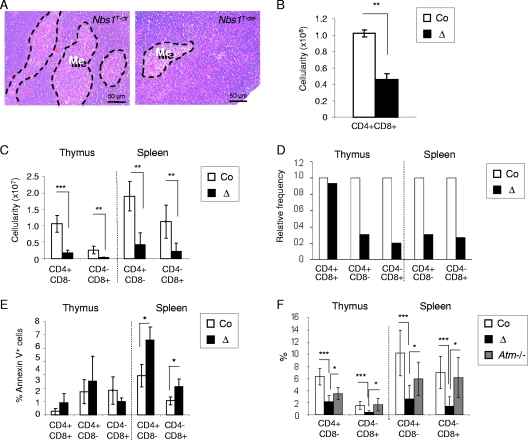
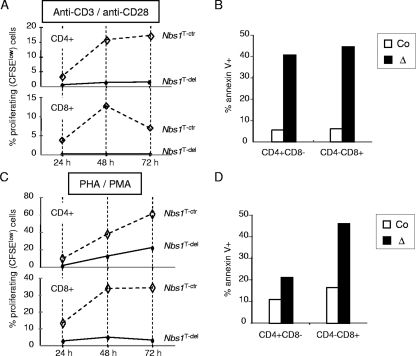
Young Nbs1T-del mice exhibited a marked atrophy of the medullar compartment in the thymi (Fig. 2A). Flow cytometry analysis of the thymi and spleens from 4-week-old mice revealed a greatly reduced cellularity and proportion of all distinct T-cell populations. Whereas the average cellularity of DP cells was 10 × 107 in Nbs1T-ctr mice, it reached only 4.6 × 107 in Nbs1T-del mice (∼2-fold reduction) (Fig. 2B). The reduction of CD4+ or CD8+ SP thymocytes was even more pronounced, from 1.1 × 107 CD4+ SP cells in Nbs1T-ctr mice to 0.17 × 107 CD4+ SP cells in Nbs1T-del mice (∼6.5-fold reduction) and from 0.26 × 107 CD8+ SP cells in Nbs1T-ctr to 0.03 × 107 CD8+ SP cells in Nbs1T-del mice (∼8.7-fold reduction) (Fig. 2C). The relative frequency and cellularity of CD4+ and CD8+ SP cells were also reduced in the Nbs1T-del spleen and blood samples (Fig. 2C and D; also data not shown). Among the general loss of SP cells, TCRα2- and TCRα8.3-expressing mature T cells were greatly reduced (data not shown). In addition, a considerably high percentage of apoptotic T cells was present in the thymi and spleens from mutants (Fig. 2E). After in vitro stimulation with PHA/PMA or TCR cross-linking antibodies (against CD3/CD28), Nbs1T-del T cells failed to proliferate (Fig. 3A and C) and showed prominent apoptosis (Fig. 3B and D), which is similar to the response of NBS patient T cells (42). All these data demonstrate dramatic defects of T-cell development and function when Nbs1 is completely deleted in an early stage.
FIG. 2.
Nbs1T-del mice develop central and peripheral T-cell lymphopenia. (A) H&E staining of thymus sections from 4-week-old Nbs1T-ctr and Nbs1T-del mice. Me, medulla. (B) Numbers of CD4+ CD8+ DP T cells in the thymi of Nbs1T-ctr and Nbs1T-del mice (n > 6; 3 to 5 weeks of age). (C) Numbers of CD4+ and CD8+ SP T cells in the thymi and spleens of Nbs1T-del mice are compared to those in the thymi and spleens of Nbs1T-ctr mice (n > 6; 3 to 5 weeks of age). (D) Frequency of CD4+ CD8+ DP, CD4+ SP, and CD8+ SP T cells in the thymi and spleens of Nbs1T-del mice relative to the controls (n > 6; 3 to 5 weeks of age). (E) Proportion of annexin V-positive T cells in the thymi and spleens of Nbs1T-ctr and Nbs1T-del mice. (F) Comparison of lymphopenia in Nbs1T-del and Atm−/− mice. The histogram shows the comparison of the average percentages of CD4+ and CD8+ SP T cells in the thymi and spleens of Nbs1T-ctr, Nbs1T-del, and Atm−/− mice (n > 4; 3 to 5 weeks of age). Co, Nbs1T-ctr; Δ, Nbs1T-del; *, P < 0.05; **, P < 0.01; ***, P < 0.001. P values were obtained using Student's unpaired t test.
FIG. 3.
Cellular response to T-cell activation in Nbs1T-del mice. (A) Thymocytes were stimulated with anti-CD3/anti-CD28 antibodies and labeled with CFSE. The proliferation of Nbs1T-ctr and Nbs1T-del CD4+ and CD8+ SP T cells was analyzed at the indicated time points by flow cytometry, according to the CFSE dilution. (B) Apoptotic cells after stimulation with anti-CD3/anti-CD28 antibodies were determined by annexin V staining. (C) Thymocytes were stimulated with PMA/PHA and labeled with CFSE. The proliferation of Nbs1T-ctr and Nbs1T-del CD4+ and CD8+ SP T cells was analyzed at the indicated time points by flow cytometry, according to the CFSE dilution. (D) Apoptosis after PMA/PHA treatment was determined by annexin V staining. Co, Nbs1T-ctr; Δ, Nbs1T-del.
Since ATM and NBS1 act in the same pathway upon DSB generation (27), we further compared the T-cell profile of Atm−/− mice with that of our Nbs1T-del mice (1). Atm−/− mice showed only a mild percentage of CD4+ SP reduction but no reduction of CD8+ SP cells compared to those of wild-type controls (Fig. 2F), which is consistent with previous reports (1, 41). Compared to Atm−/− mice, Nbs1T-del mice have significantly fewer CD4+ and CD8+ SP cells in the thymi and also in the spleens (Fig. 2F). These data seem to indicate that the functions of ATM and NBS1 in T-cell development are not identical, despite their biochemical functions in the same DSB response.
The absence of Nbs1 specifically hampers the DN3-to-DN4 transition in T-cell development.
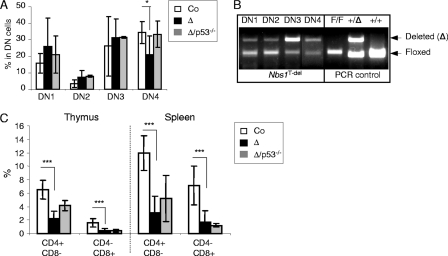
To determine the stage at which T-lymphocyte development is affected, we investigated early T-cell development from T-cell precursors. Double staining with CD25 and CD44 antibodies, which mark the developmental stages before TCRα rearrangement, revealed that the Nbs1T-del thymus contained normal or even slightly higher double-negative 1 (DN1; CD44+ CD25−), DN2 (CD44+ CD25+), and DN3 (CD44− CD25+) populations (Fig. 4A). However, the frequency of Nbs1T-del DN4 (CD44− CD25−) cells was significantly decreased (21.2%) compared to that of controls (34.5%; P = 0.029) (Fig. 4A). PCR analysis revealed that the Nbs1 deletion was readily detectable at the DN1 stage, and the majority (>80%) was deleted at the DN3 stage (Fig. 4B). It seems that the percentage of DN4 cells (Fig. 4A) in our Nbs1T-del mice was overrepresented because a high fraction of DN4 cells (∼50%) still contained the intact Nbs1 allele (Fig. 4B), which might be derived from DN3 cells escaping the deletion of the Nbs1 locus.
FIG. 4.
The Nbs1-null mutation affects the transition from the DN3 to DN4 stages in early T-cell development. (A) The histogram shows the different DN subpopulations in the total DN population, according to their surface markers CD44 and CD25. These data are the mean percentages for at least 6 mice from each genotype. (B) PCR analysis of the Nbs1 deletion in sorted DN subpopulations, according to their CD44 and CD25 expression profiles. F, Nbs1 floxed allele; Δ, Nbs1-deleted allele. (C) Frequencies of CD4+ and CD8+ SP T cells obtained from 4-week-old littermates of Nbs1T-ctr, Nbs1T-del, and Nbs1T-del p53−/− mice. These data are the mean percentages for at least three mice from each genotype. Co, Nbs1T-ctr; Δ, Nbs1T-del; Δ/p53, Nbs1T-del p53−/−; *, P < 0.05; ***, P < 0.001. P values were obtained using Student's unpaired t test.
Loss of Nbs1 impairs the processing of V(D)J-associated DSBs in TCRβ coding joints.
The transition from DN3 to DN4 corresponds to the stage of TCRβ gene rearrangement (16). To further investigate how Nbs1 contributes to the repair of DSBs in V(D)J recombination, we analyzed the V(D)J coding joints of the TCRβ locus in Nbs1T-del T cells. To this end, we chose and amplified Vβ13-Dβ1/2-Jβ2.7 recombination products by PCR and sequenced these joints (Fig. 5A; see also Tables S1 and S2 in the supplemental material). We found that Nbs1T-del T cells had a significantly higher portion of V(D)J joints with longer Dβ segments (93% in mutants compared to 73% in controls; here, we used 4 nucleotides [nt] as the threshold to define long or short Dβ1/2 segments) (Fig. 5B and C; see also Tables S1 and S2 in the supplemental material). Meanwhile, the nucleotide insertion rate in the Vβ13-Dβ1/2 junction region was also significantly increased (63% in controls versus 84% in mutants; P < 0.05) (Fig. 5B and C) and similarly increased in the Dβ1/2-Jβ2.7 joint, where the nucleotide insertion rate was consistently elevated albeit not statistically significant (66% in controls versus 77% in mutants; P > 0.05). In addition, for the Vβ13-Dβ1/2-Jβ2.7 coding joint deletion, we detected less frequently degraded coding ends during V(D)J recombination, although they cannot reach a statistically significant level (P > 0.05) (Fig. 5B and C). Nevertheless, as combined consequences of the above-mentioned phenotypes, the Nbs1T-del T cells had formed longer coding joint regions than those of controls (6.2 nt in Nbs1T-del versus 3.5 nt in controls; P < 0.05) (Fig. 5D). These findings suggest a defective DNA end processing or a resection of V(D)J termini when Nbs1 is completely deleted.
FIG. 5.
Nbs1 deletion results in aberrant processing of V(D)J coding ends and signal ends. (A) Scheme for V(D)J recombination in TCR Vβ 13, Dβ1 or Dβ2, and Jβ 2.7 gene loci. Arrowheads indicate primer positions. (B) TCRβ gene rearrangement analyzed by sequencing the PCR products of indicated segments in genomic DNA isolated from 4-week-old Nbs1T-ctr and Nbs1T-del thymocytes. n, number of PCR products analyzed. The original data are shown in Tables S1 and S2 in the supplemental material. (C) PCR sequencing analysis of TCRβ gene rearrangements in thymocyte DNA isolated from 4-week-old Nbs1T-ctr and Nbs1T-del mice. The frequency of the V(D)J joints is plotted. (D) Size of resolved Vβ13-Dβ1/2-Jβ2.7 regions in Nbs1T-ctr and Nbs1T-del mice. (E) Nucleotide usages in Vβ14-Dβ1 and Jα56-ADV8 signal joints of control and Nbs1T-del mice (n, number of sequences used for the GC/AT ratio calculation). The P value is calculated using the percentage of GC in imperfect joints (see Tables S3 and S4 in the supplemental material). Co, Nbs1T-ctr; Δ, Nbs1T-del; *, P < 0.05; **, P < 0.01; n.s., not significant. The chi-square test was used for calculations in panels B and C, and the unpaired Student's t test was used for calculations in panels D and E.
Nbs1 deletion affects the joining of signal joints.
To test if a complete Nbs1 loss also affects signal joint formation, we examined signal joints for Vβ14-Dβ1 and Jα56-ADV8 (5, 38). A sequence analysis of cloned PCR products showed that more than 55% of the signal regions in both control and Nbs1T-del T cells had perfect joining (see Tables S3 and S4 in the supplemental material), indicating that Nbs1T-del mice have robust signal joint formation. This is consistent with the previous report which found that c-Abl-transformed MRN hypomorphic B cells have normal signal joint formation (10, 17). However, in those signal joints with imperfect joining, Nbs1-null T cells showed a high preference for the usages of the GC nucleotide rather than those of the AT nucleotide. For the β locus, the GC/AT ratio is 1.03 in Nbs1T-ctr T cells and 3.86 in Nbs1T-del T cells (P < 0.01); for the α locus, the GC/AT ratio is 1.5 in Nbs1T-ctr T cells and 2.1 in Nbs1T-del T cells (P > 0.05) (Fig. 5E; see also Tables S3 and S4 in the supplemental material). These interesting results suggest altered terminal deoxynucleotidyltransferase (TdT) activity in the TCRαβ loci in the absence of Nbs1.
Rag-induced DSB repair and chromatin conformation around V(D)J segments in Nbs1T-del T cells.
MRN helps stabilize the coding joints in the “postcleavage complex,” and the enzymatic activity of Mre11 can process DSB ends (10, 44). The abnormalities in V(D)J joints, especially in TCRβ coding joints of Nbs1T-del T cells, may reflect the Nbs1-null-mediated DSB repair defect to process coding ends prior to joining. To gain insight into the impact of the Nbs1-null mutation in V(D)J end processing and stability, we analyzed the DSB repair status around Rag-induced DSB sites by chromatin immunoprecipitation (ChIP) (Fig. 6A). We first examined the status of γ-H2AX, a DSB marker, in the V(D)J joints in both distal and proximal V regions of TCRβ and -α loci (Vβ1, Vβ30, Vα5, and Vα21). We recovered more γ-H2AX products from these regions in the Nbs1-null T cells than in controls, suggesting that Rag1/2-induced DSBs were persistent, reflecting inefficient repair in Nbs1-null T cells (Fig. 6A). Consistent with this notion, using the RAD50 antibody, we precipitated fewer products from Nbs1T-del T-cell extracts than from control cell extracts (Fig. 6A). This indicates that Rad50 loading to DSBs was compromised because of the Nbs1 deletion, which in turn might impair the stability and thereby the processing of the DSB ends. Since the H2AX phosphorylation-mediated chromatin response to DSBs facilitates MRN loading and further DNA end processing (37), we next monitored the chromatin status around V(D)J ends using an antibody against acetyl-histone H4 (Ac-H4), a marker of a relaxed chromatin conformation. Consistent with the γ-H2AX status, we found more products from V segments in Nbs1T-del thymocytes than from those in controls, indicating an open chromatin conformation around these gene segments (Fig. 6A). All together, the initiation of the DSB response in Nbs1T-del T cells seems normal, as indicated by the presence of γ-H2AX, but the processing of V(D)J termini is compromised.
FIG. 6.
Defects in V(D)J-initiated DNA repair, chromosome instability, and impaired DDR in Nbs1-deleted T cells. (A) ChIP analysis using antibodies against Rad50, γ-H2AX, and Ac-H4. These antibodies recovered distal and proximal Vβ (left) and Vα (right) regions of Nbs1T-ctr and Nbs1T-del thymocytes. Co, Nbs1T-ctr; Δ, Nbs1T-del; γ-H2AX, phospho-histone H2AX; Ac-H4, acetyl-histone H4. (B) Summary of the chromosomal stability in Nbs1T-ctr and Nbs1T-del T cells. The metaphases were prepared from 8-week-old Nbs1T-ctr and Nbs1T-del T cells 48 h after IL-2/PMA/ionomycin stimulation. n, number of metaphases analyzed. **, P < 0.01. Student's unpaired t test was used, except that the chi-square test was applied for calculation of the percentage of metaphases containing aberrations. (C) Western blot analysis of Nbs1 and Chk2 in whole-cell extracts from Nbs1T-ctr and Nbs1T-del thymocytes after 2 h of treatment with 0.2 μg/ml of adriamycin (Adr). PARP-1 is used as a loading control. The ratio of the phosphorylated form of Chk2 (upper band) was corrected to that of the nonphosphorylated form (lower band) by ImageJ software and indicated under the corresponding lanes. NA, not applicable.
The persistence of the γ-H2AX and relaxed chromatin in Nbs1T-del T cells indicates the defects in the repair process without Nbs1. We further confirmed the DNA repair defect in the Nbs1-null T cells by cytogenetic analysis of thymocytes upon treatment with PMA/IL-2/ionomycin. While the chromosome numbers were unchanged in Nbs1T-del T cells, we found significantly higher frequencies of chromosomal breaks and fusions in Nbs1T-del T cells than in Nbs1T-ctr T cells (Fig. 6B). In total, 73% of Nbs1T-del metaphases contained at least one aberration, in contrast to 53% in the control group (P < 0.01) (Fig. 6B). The defect is most likely mediated by the ATM-CHK2 pathway rather than the ATR-CHK1 pathway, since Nbs1T-del T cells show an attenuated ATM-CHK2 pathway (Fig. 6C) but not an attenuated ATR-CHK1 pathway (data not shown) after an in vitro challenge of Nbs1T-del T cells with adriamycin and UV, which is consistent with previous observations (35).
A p53 deficiency and the TCR transgene cannot fully correct Nbs1T-del lymphopenia.
T-cell lymphopenia in Nbs1T-del mice could be a combined defect of a Nbs1-mediated repair of DSBs and of proliferation. The transition from DN3 to DN4 corresponds to the stage of TCRβ gene rearrangement (16), and the reduction of DN4 cells in Nbs1T-del mice implies a defective Nbs1 function in repairing V(D)J-associated DSBs in the TCRβ locus, which could trigger the p53-dependent apoptotic pathway. In order to test this hypothesis, we crossed Nbs1T-del mice into a p53-deficient background. A p53 deletion in Nbs1T-del mice completely restored the DN4 cell population (Fig. 4A), suggesting that the affected DN3-to-DN4 transition was likely DNA breaks based and mediated by p53 activation. Although a p53 deficiency could rescue the DN4 cell loss in Nbs1T-del mice, the survival advantage given by the p53 loss did not significantly increase the total number of immature DP and mature SP T cells in the Nbs1T-del thymus and spleen (Fig. 4C). It is possible that the T-cell lymphopenia in Nbs1T-del mice is caused by the failure of resolving the V(D)J recombination termini and by the subsequent membrane-bound death receptor-mediated positive and negative selection or proliferation defects.
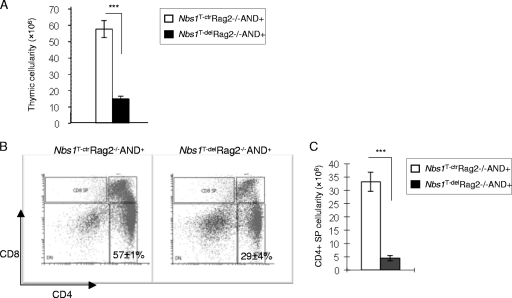
To further examine the contribution of DSBs and TCR function-mediated T-cell loss in the absence of Nbs1, we crossed Nbs1T-del mice into the Rag2−/− AND+ genetic background. Rag2−/− AND+ mice are devoid of endogenous V(D)J recombination because they lack Rag2-mediated DSBs in TCRs, but they ectopically express the transgenic TCRαβ receptor (AND+) (24, 36). Nbs1T-del Rag2−/− AND+ mice reached 26% of the thymic cellularity of Nbs1T-ctr Rag2−/− AND+ controls (Fig. 7A). In addition, the CD4+ SP fraction in Nbs1T-del Rag2−/− AND+ mice was about 51% of that of the Nbs1T-ctr Rag2−/− AND+ controls (29% in Nbs1T-del Rag2−/− AND+ mice and 57% in Nbs1T-ctr Rag2−/− AND+ mice) (Fig. 7B). Under the condition of Rag2 deficiency, the TCRαβ transgene partially restored the mature CD4+ T cells in the Nbs1T-del mice (51%, in comparison with ∼30% left in the Rag2+/+ background) (Fig. 2B and 7B). Despite this increased frequency, the total number of CD4+ SP cells was still significantly lower in Nbs1T-del Rag2−/− AND+ mice than in controls (Fig. 7C), arguing for a role of Nbs1 in T-cell proliferation and viability.
FIG. 7.
Rescue experiment of T-cell lymphopenia in Nbs1T-del mice with the TCRαβ transgene (AND) and a Rag2 deficiency. (A) The total thymus cellularity in Nbs1T-ctr Rag2−/− AND+ (n = 2) and Nbs1T-del Rag2−/− AND+ (n = 6) mice. (B) Representative T-cell profiles of the thymocytes obtained from two Nbs1T-ctr Rag2−/− AND+ mice and four age-matched Nbs1T-del Rag2−/− AND+ mice. (C) The total number of CD4+ SP cells obtained from thymi of Nbs1T-del mice (n = 2) relative to the controls (n = 4) in the Rag2−/− AND+ genetic backgrounds. ***, P < 0.001 using Student's unpaired t test.
DISCUSSION
A complete deletion of Nbs1 in the T-cell lineage resulted in a >6-fold reduction of CD4+ and CD8+ SP T cells and a 2-fold reduction of DP cells. These findings are in contrast with an approximately 2- to 3-fold reduction of CD4+ SP cells and yet a normal level of CD8+ SP cells in human NBS patients for whom NBS1 is hypomorphic (11, 22, 30). Moreover, hypomorphic mutations of Nbs1 in mice resulted in about a 30% reduction of SP T cells and DP T cells (17, 23). The mild T-cell phenotype of hypomorphic NBS1 in humans and mice is likely attributable to the presence of a truncated NBS1 protein, which supports survival and possibly proliferation (see below). Notably, although ATM and NBS1 act in a common DDR pathway in vitro, compared to our Nbs1T-del mice, Atm-null thymi show roughly a 25% reduction of the total cellularity but a normal frequency of CD4+ CD8+ DP cells and CD8+ SP cells (1, 41) (Fig. 2F).
V(D)J analysis of hypomorphic mutant Nbs1 and Mre11 mice and Atm-null mice reveals very similar V(D)J recombination defects, particularly in the TCRα locus, marked by unrepaired coding ends (17, 41). It seems that hypomorphic Nbs1 or Mre11 proteins and full-length Atm play largely overlapping roles in V(D)J processing and rather affect the later stage of T-cell development. In our study, in addition to recapitulating the later defect of TCRα locus recombination, as shown in Atm−/− mice and Nbs1/Mre11 hypomorphic mice (17, 21), the deletion of the complete Nbs1 protein in T cells unexpectedly affects the T-cell development as early as in the DN3-to-DN4 transition, which has not been reported in hypomophic MRN mutant mice or Atm-null mice. The similar but discrepant T-cell phenotypes of Atm-null and T-cell-specific Nbs1-null mice argue that ATM and NBS1 have overlapping, yet distinct, functions in T-cell lymphogenesis.
Interestingly, the blocking of the DN3-DN4 transition is coincident with the TCRβ rearrangement, and the defective V(D)J recombination may be one of the reasons for the severe T-cell lymphopenia in Nbs1T-del mice. Nbs1 is required for a proper coding joint formation because a Nbs1 deletion in particular lengthened the V(D)J joints in the TCRβ locus of Nbs1T-del T cells. The less frequent deletion of the Vβ and Jβ joint regions and the less degraded Dβ1/2 segments might be due to inefficient processing of V(D)J joints in the Nbs1T-del TCRβ locus by Mre11 (the nuclease component of MRN). The MRN complex has the capacities of DNA bridging (by Rad50) and end processing (via the endonuclease and exonuclease activities of Mre11) (4, 43). The persistent DSBs or the defective processing of V(D)J coding ends in the Nbs1-null T cells could be due to a defective recruitment of MRN components for DNA end tethering and of DNA end resection factors, such as Mre11, for Rag-generated DSB end processing (44). Consistent with this notion, the binding of Rad50 to the DNA end coding joints in TCRβ as well as in the TCRα locus was attenuated in Nbs1-null T cells, thereby compromising the enzymatic activity of Mre11 to process the DNA ends. In addition, the retention of the unrepaired coding joints, suggested by the persistent γ-H2AX signals and open chromatin structure, leaves a broad time window for other terminal modifying enzymes, for example, template-independent TdT, to act on Rag1/2-generated DNA ends, which may account for longer coding joints in the TCRβ locus.
For signal joint formation, which is a direct and fast-joining process of two blunt DNA ends during V(D)J recombination, Nbs1T-del T cells efficiently joined signal ends, consistent with results from other reports (10), suggesting that Nbs1 is dispensable for the joining process. However, it is interesting to notice that the Nbs1T-del T cells signal joints showed a strong preference for the GC-nucleotide insertion, which has not been documented previously in Nbs1 hypomorphic mutant cells. The mechanism of such a biased use of nucleotides is unclear at the moment, but it is possible that delayed DNA end joining may affect the activity (albeit unintentionally) of the template-independent TdT. Interestingly, the analysis of T cells of Artemis−/− and SCID mice (DNA-PKcs mutated) also revealed an increased bias of GC/AT composition in the signal joints (39). The catalytic activity of TdT could be modified upon binding to DNA-PKcs (31). Previously, the failure of the hairpin opening caused by DNA-PKcs/Artemis in Atm−/− T cells indicates that ATM may be involved in the recruitment of DNA-PKcs/Artemis to V(D)J ends (3, 21). It is possible that the impaired MRN-ATM axis in Nbs1T-del T cells may compromise the loading of DNA-PKcs to the DNA ends of signal joints and thus affect the TdT activity and joint sequence diversity.
The severe T-cell lymphopenia in Nbs1T-del mice could also be attributed to increased apoptosis, which may be caused by chromosomal instability. A p53 deficiency relieved the block of DN3-to-DN4 transition and restored the DN4 cell population, indicating that the transition block is likely to be dependent on cell death triggered by persistent DNA coding ends generated during TCRβ V(D)J recombination (Fig. 6A and B). However, in contrast to the neural rescue effect of a p53 deficiency on Nbs1-central nervous system (CNS)-deleted mice (14), the p53 deficiency in Nbs1T-del mice did not restore the total thymic cellularity, suggesting that an Nbs1 deletion also has an impact on proliferative expansion in late T-cell development. Since TCRβ expression needs only one round of V(D)J recombination, while TCRα locus expression requires at least five rounds of V(D)J recombination, a p53 deficiency might be insufficient to rescue those DP cells with extensive unrepaired DNA breaks carrying on during TCRα rearrangements (21, 45).
Another reason for p53's failure to restore the total cellularity is perhaps due to proliferation defects of Nbs1T-del T cells. TCRαβ transgene expression in Atm−/− mice could restore up to 80% of the T-cell population (6), suggesting that the V(D)J defect and TCR selection are the major reasons for the T-cell loss in Atm−/− mice. Interestingly, when we inhibited the V(D)J-generated DNA breaks by inactivating Rag2 (36), the TCRαβ transgene partially restored the mature CD4+ T cells in Nbs1T-del mice, which show 51% of controls (1-fold reduction) (Fig. 7B) compared to ∼30% of controls (2-fold reduction) (Fig. 2B) in the Rag2+/+ background. The increased Nbs1T-del CD4+ SP population in the Rag2−/− AND+ background suggests a role for Nbs1 in the repair of the V(D)J-induced DSBs and perhaps in the positive and negative selection processes. However, even if DSBs are prevented by Rag2 deficiency and TCRαβ is provided to allow a normal positive and negative selection (in the Rag2−/− AND+ background), the total CD4+ SP numbers as well as thymic cellularity are still very low in Nbs1T-del Rag2−/− AND+ mice (Fig. 7A and C). These observations thus well indicate that the function of Nbs1 in cell proliferation is likely to be responsible for the major loss of the T cells in late stages, as suggested previously in Nbs1-deleted B cells (25, 35). Therefore, the loss of T cells after Nbs1 deletion is likely through its function in cell proliferation, viability, and not exclusively, cell death via β selection because of an aberrant V(D)J recombination.
Taken together, a complete deletion of Nbs1 in early T-cell precursors affects TCRβ V(D)J rearrangements and results in the persistence of Rag1/2-generated coding joint ends due to their inefficient processing in the absence of Nbs1. In addition, the Nbs1-null mutation abolishes proliferative expansion during positive and negative selection, which cannot be rescued by p53 deletion or overexpression of the TCRαβ transgene. All these mark the common but unique function of Nbs1 (MRN) compared to that of Atm. Thus, the study of T cells devoid of the entire Nbs1 protein reveals a physiological function of Nbs1 in repairing the Rag-induced DSBs during TCRβ recombination and proliferation, and both of which are essential for T-cell development.
Supplementary Material
Acknowledgments
We thank Lucien Frappart, Yufeng Liu, and Debra Weih for the histological examinations. We are grateful to Christopher Wilson for providing the Lck-Cre transgenic mice and André Nussenzweig for providing the Rag2+/− AND+ mice for this study. We also thank Simone Tänzer for cell sorting, Dominique Galendo and Christof Birch-Hirschfeld for their excellent assistance in the maintenance of the animal colonies, and Tjard Jörss for his excellent technical support. We also thank Wanjun Chen and Pierre-Olivier Frappart for their critical reading of the manuscript. Further thanks go to Eileen Stöckl for editing the manuscript. We are also grateful to many other members of our laboratory for helpful discussions.
Z.-Q.W. is supported by the Association for International Cancer Research, United Kingdom, and by the Deutsche Forschungsgemeinschaft, Germany.
Footnotes
Published ahead of print on 4 October 2010.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Barlow, C., S. Hirotsune, R. Paylor, M. Liyanage, M. Eckhaus, F. Collins, Y. Shiloh, J. N. Crawley, T. Ried, D. Tagle, and A. Wynshaw-Boris. 1996. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86:159-171. [DOI] [PubMed] [Google Scholar]
- 2.Bassing, C. H., W. Swat, and F. W. Alt. 2002. The mechanism and regulation of chromosomal V(D)J. recombination. Cell 109:S45-S55. [DOI] [PubMed] [Google Scholar]
- 3.Bird, A. W., D. Y. Yu, M. G. Pray-Grant, Q. Qui, K. E. Harmon, P. C. Megee, P. A. Grant, M. M. Smith, and M. F. Christman. 2002. Acetylation of histone H4 by Esa1 is required for DNA double-strand break repair. Nature 419:411-415. [DOI] [PubMed] [Google Scholar]
- 4.Buis, J., Y. Wu, Y. Deng, J. Leddon, G. Westfield, M. Eckersdorff, J. M. Sekiguchi, S. Chang, and D. O. Ferguson. 2008. Mre11 nuclease activity has essential roles in DNA repair and genomic stability distinct from ATM activation. Cell 135:85-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Candeias, S., K. Muegge, and S. K. Durum. 1996. Junctional diversity in signal joints from T cell receptor beta and delta loci via terminal deoxynucleotidyl transferase and exonucleolytic activity. J. Exp. Med. 184:1919-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao, C., E. M. Yang, and Y. Xu. 2000. Rescue of defective T cell development and function in Atm−/− mice by a functional TCR alpha beta transgene. J. Immunol. 164:345-349. [DOI] [PubMed] [Google Scholar]
- 7.Chen, H. T., A. Bhandoola, M. J. Difilippantonio, J. Zhu, M. J. Brown, X. Tai, E. P. Rogakou, T. M. Brotz, W. M. Bonner, T. Ried, and A. Nussenzweig. 2000. Response to RAG-mediated V(D)J cleavage by NBS1 and γ-H2AX. Nature 290:1962-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corneo, B., R. L. Wendland, L. Deriano, X. Cui, I. A. Klein, S. Y. Wong, S. Arnal, A. J. Holub, G. R. Weller, B. A. Pancake, S. Shah, V. L. Brandt, K. Meek, and D. B. Roth. 2007. Rag mutations reveal robust alternative end joining. Nature 449:483-486. [DOI] [PubMed] [Google Scholar]
- 9.D'Amours, D., and S. P. Jackson. 2002. The Mre11 complex: at the crossroads of DNA repair and checkpoint signalling. Nat. Rev. Mol. Cell Biol. 3:317-327. [DOI] [PubMed] [Google Scholar]
- 10.Deriano, L., T. H. Stracker, A. Baker, J. H. Petrini, and D. B. Roth. 2009. Roles for NBS1 in alternative nonhomologous end-joining of V(D)J recombination intermediates. Mol. Cell 34:13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Digweed, M., and K. Sperling. 2004. Nijmegen breakage syndrome: clinical manifestation of defective response to DNA double-strand breaks. DNA Repair (Amst.) 3:1207-1217. [DOI] [PubMed] [Google Scholar]
- 12.Dinkelmann, M., E. Spehalski, T. Stoneham, J. Buis, Y. Wu, J. M. Sekiguchi, and D. O. Ferguson. 2009. Multiple functions of MRN in end-joining pathways during isotype class switching. Nat. Struct. Mol. Biol. 16:808-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudley, D. D., J. Chaudhuri, C. H. Bassing, and F. W. Alt. 2005. Mechanism and control of V(D)J recombination versus class switch recombination: similarities and differences. Adv. Immunol. 86:43-112. [DOI] [PubMed] [Google Scholar]
- 14.Frappart, P. O., W. M. Tong, I. Demuth, I. Radovanovic, Z. Herceg, A. Aguzzi, M. Digweed, and Z. Q. Wang. 2005. An essential function for NBS1 in the prevention of ataxia and cerebellar defects. Nat. Med. 474-475. [DOI] [PubMed]
- 15.Harfst, E., S. Cooper, S. Neubauer, L. Distel, and U. Grawunder. 2000. Normal V(D)J recombination in cells from patients with Nijmegen breakage syndrome. Mol. Immunol. 37:915-929. [DOI] [PubMed] [Google Scholar]
- 16.Hayday, A. C., and D. J. Pennington. 2007. Key factor in the organized chaos of early T cell development. Nat. Immunol. 8:137-144. [DOI] [PubMed] [Google Scholar]
- 17.Helmink, B. A., A. L. Bredemeyer, B. S. Lee, C. Y. Huang, G. G. Sharma, L. M. Walker, J. J. Bednarski, W. L. Lee, T. K. Pandita, C. H. Bassing, and B. P. Sleckman. 2009. MRN complex function in the repair of chromosomal Rag-mediated DNA double-strand breaks. J. Exp. Med. 206:669-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herceg, Z., H. Li, C. Cuenin, V. Shukla, M. Radolf, P. Steinlein, and Z. Q. Wang. 2003. Genome-wide analysis of gene expression regulated by the HAT cofactor Trrap in conditional knockout cells. Nucleic Acids Res. 31:7011-7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hopfner, K. P., L. Craig, G. Moncalian, R. A. Zinkel, T. Usui, B. A. Owen, A. Karcher, B. Henderson, J. L. Bodmer, C. T. McMurray, J. P. Carney, J. H. Petrini, and J. A. Tainer. 2002. The Rad50 zinc-hook is a structure joining Mre11 complexes in DNA recombination and repair. Nature 418:562-566. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh, C. L., C. F. Arlett, and M. R. Lieber. 1993. V(D)J recombination in ataxia telangiectasia, Bloom's syndrome, and a DNA ligase I-associated immunodeficiency disorder. J. Biol. Chem. 268:20105-20109. [PubMed] [Google Scholar]
- 21.Huang, C. Y., G. G. Sharma, L. M. Walker, C. H. Bassing, T. K. Pandita, and B. P. Sleckman. 2007. Defects in coding joint formation in vivo in developing ATM-deficient B and T lymphocytes. J. Exp. Med. 204:1371-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.International Nijmegen Breakage Syndrome Study Group. 2000. Nijmegen breakage syndrome. Arch. Dis. Child. 82:400-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang, J., R. T. Bronson, and Y. Xu. 2002. Targeted disruption of NBS1 reveals its roles in mouse development and DNA repair. EMBO J. 21:1447-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaye, J., M. L. Hsu, M. E. Sauron, S. C. Jameson, N. R. Gascoigne, and S. M. Hedrick. 1989. Selective development of CD4+ T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature 341:746-749. [DOI] [PubMed] [Google Scholar]
- 25.Kracker, S., Y. Bergmann, I. Demuth, P. O. Frappart, G. Hildebrand, R. Christine, Z.-Q. Wang, K. Sperling, M. Digweed, and A. Radbruch. 2005. Nibrin functions in Ig class-switch recombination. Proc. Natl. Acad. Sci. U. S. A. 102:1584-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavin. 2007. ATM and the Mre11 complex combine to recognize and signal DNA double-strand breaks. Oncogene 26:7749-7758. [DOI] [PubMed] [Google Scholar]
- 27.Lee, J. H., and T. T. Paull. 2005. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science 308:551-554. [DOI] [PubMed] [Google Scholar]
- 28.Lieber, M. R. 2010. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu. Rev. Biochem. 79:181-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyons, A. B., J. Hasbold, and P. D. Hodgkin. 2001. Flow cytometry analysis of cell division history using dilution of carboxyfluorescein diacetate succinimidyl ester, a stably integrated fluorescent probe. Methods Cell Biol. 63:375-398. [DOI] [PubMed] [Google Scholar]
- 30.Michalkiewicz, J., C. Barth, K. Chrzanowska, H. Gregorek, M. Syczewska, C. M. B. Weemaes, K. Madalinski, D. Dzierzanowska, and J. Stachowski. 2003. Abnormalities in the T and NK lymphocyte phenotype in patients with Nijmegen breakage syndrome. Clin. Exp. Immunol. 134:482-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mickelsen, S., C. Snyder, K. Trujillo, M. Bogue, D. B. Roth, and K. Meek. 1999. Modulation of terminal deoxynucleotidyltransferase activity by the DNA-dependent protein kinase. J. Immunol. 163:834-843. [PubMed] [Google Scholar]
- 32.Paull, T. T., and M. Gellert. 1998. The 3′ to 5′ exonuclease activity of Mre11 facilitates repair of DNA double-strand breaks. Mol. Cell 1:969-979. [DOI] [PubMed] [Google Scholar]
- 33.Qi, L., M. A. Strong, B. O. Karim, M. Armanios, D. L. Huso, and C. W. Greider. 2003. Short telomeres and ataxia-telangiectasia mutated deficiency cooperatively increase telomere dysfunction and suppress tumorigenesis. Cancer Res. 63:8188-8196. [PubMed] [Google Scholar]
- 34.Rass, E., A. Grabarz, I. Plo, J. Gautier, P. Bertrand, and B. S. Lopez. 2009. Role of Mre11 in chromosomal nonhomologous end joining in mammalian cells. Nat. Struct. Mol. Biol. 16:819-824. [DOI] [PubMed] [Google Scholar]
- 35.Reina-San-Martin, B., M. C. Nussenzweig, A. Nussenzweig, and S. Difilippantonio. 2005. Genomic instability, endoreduplication, and diminished Ig class-switch recombination in B cells lacking Nbs1. Proc. Natl. Acad. Sci. U. S. A. 102:1590-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinkai, Y., G. Rathbun, K. P. Lam, E. M. Oltz, V. Stewart, M. Mendelsohn, J. Charron, M. Datta, F. Young, A. M. Stall, et al. 1992. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68:855-867. [DOI] [PubMed] [Google Scholar]
- 37.Stucki, M., and S. P. Jackson. 2006. gammaH2AX and MDC1: anchoring the DNA-damage-response machinery to broken chromosomes. DNA Repair (Amst.) 5:534-543. [DOI] [PubMed] [Google Scholar]
- 38.Touvrey, C., E. Borel, P. N. Marche, E. Jouvin-Marche, and S. M. Candeias. 2006. Gene-specific signal joint modifications during V(D)J recombination of TCRAD locus genes in murine and human thymocytes. Immunobiology 211:741-751. [DOI] [PubMed] [Google Scholar]
- 39.Touvrey, C., C. Couedel, P. Soulas, R. Couderc, M. Jasin, J. P. de Villartay, P. N. Marche, E. Jouvin-Marche, and S. M. Candeias. 2008. Distinct effects of DNA-PKcs and Artemis inactivation on signal joint formation in vivo. Mol. Immunol. 45:3383-3391. [DOI] [PubMed] [Google Scholar]
- 40.Uziel, T., Y. Lerenthal, L. Moyal, Y. Andegeko, L. Mittelman, and Y. Shiloh. 2003. Requirement of the MRN complex for ATM activation by DNA damage. EMBO J. 22:5612-5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vacchio, M. S., A. Olaru, F. Livak, and R. J. Hodes. 2007. ATM deficiency impairs thymocyte maturation because of defective resolution of T cell receptor alpha locus coding end breaks. Proc. Natl. Acad. Sci. U. S. A. 104:6323-6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weemaes, C. M., D. F. Smeets, and C. J. van der Burgt. 1994. Nijmegen breakage syndrome: a progress report. Int. J. Radiat. Biol. 66:S185-S188. [PubMed] [Google Scholar]
- 43.Williams, R. S., G. Moncalian, J. S. Williams, Y. Yamada, O. Limbo, D. S. Shin, L. M. Groocock, D. Cahill, C. Hitomi, G. Guenther, D. Moiani, J. P. Carney, P. Russell, and J. A. Tainer. 2008. Mre11 dimers coordinate DNA end bridging and nuclease processing in double-strand-break repair. Cell 135:97-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie, A., A. Kwok, and R. Scully. 2009. Role of mammalian Mre11 in classical and alternative nonhomologous end joining. Nat. Struct. Mol. Biol. 16:814-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu, Y., E. M. Yang, J. Brugarolas, T. Jacks, and D. Baltimore. 1998. Involvement of p53 and p21 in cellular defects and tumorigenesis in Atm−/− mice. Mol. Cell. Biol. 18:4385-4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan, C. T., C. Boboila, E. K. Souza, S. Franco, T. R. Hickernell, M. Murphy, S. Gumaste, M. Geyer, A. A. Zarrin, J. P. Manis, K. Rajewsky, and F. W. Alt. 2007. IgH class switching and translocations use a robust non-classical end-joining pathway. Nature 449:478-482. [DOI] [PubMed] [Google Scholar]
- 47.Yang, Y. G., A. Saidi, P. O. Frappart, W. Min, C. Barrucand, V. Dumon-Jones, J. Michelon, Z. Herceg, and Z. Q. Wang. 2006. Conditional deletion of Nbs1 in murine cells reveals its role in branching repair pathways of DNA double-strand breaks. EMBO J. 25:5527-5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeo, T. C., D. Xia, S. Hassouneh, X. O. Yang, D. E. Sabath, K. Sperling, R. A. Gatti, P. Concannon, and D. M. Willerford. 2000. V(D)J rearrangement in Nijmegen breakage syndrome. Mol. Immunol. 37:1131-1139. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, Y., J. Zhou, and C. U. Lim. 2006. The role of NBS1 in DNA double strand break repair, telomere stability, and cell cycle checkpoint control. Cell Res. 16:45-54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.