Abstract
Here, we probe the response to calcium during growth on a surface and show that calcium influences the transcriptome and stimulates motility and virulence of Vibrio parahaemolyticus. Swarming (but not swimming) gene expression and motility were enhanced by calcium. Calcium also elevated transcription of one of the organism's two type III secretion systems (T3SS1 but not T3SS2) and heightened cytotoxicity toward host cells in coculture. Calcium stimulation of T3SS gene expression has not been reported before, although low calcium is an inducing signal for the T3SS of many organisms. EGTA was also found to increase T3SS1 gene expression and virulence; however, this was demonstrated to be the consequence of iron rather than calcium chelation. Ectopic expression of exsA, encoding the T3SS1 AraC-type regulator, was used to define the extent of the T3SS1 regulon and verify its coincident induction by calcium and EGTA. To begin to understand the regulatory mechanisms modulating the calcium response, a calcium-repressed, LysR-type transcription factor named CalR was identified and shown to repress swarming and T3SS1 gene expression. Swarming and T3SS1 gene expression were also demonstrated to be linked by LafK, a σ54-dependent regulator of swarming, and additionally connected by a negative-feedback loop on the swarming regulon propagated by ExsA. Thus, calcium and iron, two ions pertinent for a marine organism and pathogen, play a signaling role with global consequences on the regulation of gene sets that are relevant for surface colonization and infection.
Bacteria can sense calcium and respond in diverse ways (reviewed in references 10 and 39). Fluxes in the intracellular calcium level influence chemotactic behavior in Bacillus subtilis and Escherichia coli (42, 52). The concentrations of MgCl2 and CaCl2 that are found in seawater enhance the swimming motility of Vibrio fischeri (43). Calcium regulates spore germination of Streptomyces coelicolor and heterocyst differentiation of Anabaena sp. PCC 7120 (54, 60). Biofilm thickness of Pseudomonas aeruginosa can increase more than 10-fold in the presence of 10 mM CaCl2 compared to that during growth in medium without CaCl2 supplementation (46). The formation of some types of Vibrio cholerae biofilms is dependent upon calcium, and biofilms dissolve when the medium is depleted of calcium, perhaps mimicking the transition from seawater to fresh water and playing a role in dissemination of the organism (24). Other types of V. cholerae biofilms are repressed by calcium (2). Thus, calcium influences bacterial behavior, differentiation, and environmental survival.
The availability of calcium also affects virulence. Some type III secretion systems (T3SS) that are used to inject virulence effectors into hosts are induced under calcium-depleted conditions. In Yersinia pestis, 2.5 mM calcium prevents T3SS secretion, and it does so in part by modulating gene expression and protein activity (7, 9, 14, 56). Similarly to Yersinia strains, secretion of P. aeruginosa T3SS effectors can be induced when calcium is chelated from the medium (8). The absence of calcium may reflect a particular environmental cue and/or mimic an unknown signal generated upon contact with host cells, perhaps by destabilizing the injectisome (reviewed in references 8, 15, and 59).
The genome-wide transcriptional response to calcium has been studied in a few organisms. The effects of calcium (1.38 mM CaCl2) and other minerals on the transcriptome of sporulating B. subtilis have been examined because ions in the growth medium were found to profoundly affect the timing of development and resistance properties of spores (41). Approximately 300 genes, including spore coat- and biofilm-pertinent genes, showed >2-fold expression changes in response to calcium. Microarray transcriptome analysis of E. coli grown in 10 mM CaCl2 and 5 mM EGTA identified 110 genes that were differentially regulated (P < 0.0001) (38). Some of these encoded potential transport components, outer membrane proteins, and enzymes. Comparison of the high-calcium (5 mM CaCl2) and low-calcium (5 mM EGTA) gene expression profiles of P. aeruginosa revealed 318 genes showing differential expression (>1.5-fold) (57). Among these were genes encoding the T3SS and a number of potential regulatory and signal transduction proteins. When V. cholerae was grown in the presence of 10 mM calcium, 76 genes were considered differentially regulated (>1.5-fold) compared to levels during growth in unsupplemented medium; some of these genes encoded regulators and extracellular polysaccharide (2). Taken together, the evidence suggests that calcium plays a signaling role that globally regulates gene expression in bacteria.
In this work, we begin to investigate the role of calcium in another Vibrio species, Vibrio parahaemolyticus. This ubiquitous marine and estuarine organism is a leading worldwide cause of seafood-borne gastroenteritis (reviewed in references 21 and 37). Thus, high and fluctuating calcium concentrations are pertinent to its lifestyle. V. parahaemolyticus is a highly motile bacterium that can swim in aqueous environments, swarm efficiently over solid surfaces, and form robust biofilms (13, 31, 32). Swarming motility requires the production of hundreds of lateral flagella, which are structurally different from the single polar flagellum used for swimming; therefore, the bacterium has two distinct flagellar motility systems, the polar and the lateral. While the polar organelle is produced continuously, the lateral system is induced only during growth on surfaces or in viscous environments. As for all members of the Vibrionaceae, the V. parahaemolyticus genome is organized in two circular chromosomes, and the motility systems are encoded on different chromosomes. Polar genes are found on chromosome 1, and the lateral genes are on chromosome 2. In addition to these independent flagellar systems, some strains possess two distinct virulence type III secretion systems (designated T3SS1 and T3SS2), and these are also encoded on separate chromosomes (27).
Here, we examine motility, cytotoxicity, and the genome-wide transcriptional response to calcium in V. parahaemolyticus. We show that high calcium increases the rate of swarming motility and that this is the consequence of effects on lateral flagellar gene expression. Our microarray analyses reveal a set of strongly regulated calcium-responsive genes. Unexpectedly, among the calcium-induced genes are the T3SS1 genes. Calcium induction of V. parahaemolyticus T3SS1 seems unique and opposite to the T3SS calcium response in other bacteria. EGTA was also found to enhance T3SS1 transcription and virulence; however, this seemed the consequence of iron and not calcium chelation. To begin to unravel the molecular mechanism by which calcium influences gene expression, we have identified a transcription factor, named CalR, that modulates lateral flagellar gene and T3SS1 transcription in response to calcium.
MATERIALS AND METHODS
Bacterial strains, media, and nomenclature.
The bacterial strains and plasmids used in this work are described in Table 1 and are derivatives of BB22 (1). V. parahaemolyticus undergoes translucent (TR)/opaque (OP) phase variation between a swarm-competent, virulent cell type and a swarm-defective, avirulent cell type (16, 20, 33). All parental strains in this work are TR due to mutations in opaR; all are swarm proficient and virulent in cytotoxicity assays and form translucent colonies. V. parahaemolyticus strains were routinely grown at 30°C and on plates unless otherwise indicated. Heart infusion (HI) broth contained 25 g heart infusion (Difco) and 15 g NaCl per liter; heart infusion plates contained HI broth with 20 g/liter granulated agar (Difco), except for swarming motility plates, which contained 15 g/liter Bacto (Difco) agar. 2216 marine medium contained 28 g/liter 2216 marine broth (Difco). Supplements were used at the following concentrations: kanamycin at 50 μg/ml, gentamicin at 25 μg/ml, tetracycline at 10 μg/ml, 2,2′-dipyridyl at 100 μM, FeCl3 and isopropyl-β-d-1-thiogalactopyranoside (IPTG) at 50 μM, and 1,2-bis-(2-aminophenoxyethane)-N,N,N′,N′-tetraacetic acid tetrapotassium salt (BAPTA; Fisher Scientific) as indicated. CaCl2 and EGTA (RPI Corp.) were used at a 4 mM concentration unless otherwise indicated. VP locus tag designations refer to genes found on chromosome 1, and VPA locus tag designations refer to genes found on chromosome 2.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Parent (reference) or source |
|---|---|---|
| V. parahaemolyticus strains | ||
| LM5191 | ΔopaR1 | LM5674 (13) |
| LM5197 | ΔopaR1 calR1::Tn5 (Kanr) | LM5191 |
| LM5674 | ΔopaR1 | 13 |
| LM5738 | opaR2 flgB1L::Tn5 lux (Kanr) | LM5431 (51) |
| LM5949 | ΔopaR1 flgC3016L::lacZ (Camr) | LM5674 |
| LM6151 | opaR2 VPA0979::Tn5 luxa (Kanr) | LM5431 |
| LM7035 | ΔopaR1 ΔVP1672::Camr | LM5674 |
| LM7120 | ΔopaR1 flgC3016L::lacZ (Camr) calR2::Tn5 lux (Kanr) | LM5949 |
| LM7789 | ΔopaR1 ΔlafK2::Camr | LM5674 |
| LM7915 | ΔopaR1 calR2::Tn5 lux (Kanr) flgC3016L::lacZ (Camr)/pLM2795 (calR+) | LM7120 |
| LM7916 | ΔopaR1 calR2::Tn5 lux (Kanr) flgC3016L::lacZ (Camr)/pLM1877 | LM7120 |
| LM8863 | ΔopaR1/pLM1877 | LM5674 |
| LM8864 | ΔopaR1/pLM3650 (exsA+) | LM5674 |
| LM9217 | ΔopaR1 flgB1L::Tn5 lux (Kanr)/pLM3650 (exsA+) | LM5738 |
| LM9218 | ΔopaR1 flgB1L::Tn5 lux (Kanr) pLM1877 | LM5738 |
| LM9372 | ΔopaR1 ΔVP1672::Camr/pLM1877 | LM7035 |
| LM9373 | ΔopaR1 ΔVP1672::Camr/pLM3650 (exsA+) | LM7035 |
| LM9596 | ΔopaR1 lafK1::lacZ (Genr) | LM5191 |
| LM9649 | ΔopaR1/pLM3940 | LM5191 |
| LM9650 | ΔopaR1 calR1::Tn5 (Kanr)/pLM3940 | LM5197 |
| Plasmids | ||
| pLM1877 | Apr Genr, IPTG-inducible vector | 3 |
| pLM2795 | Apr GenrcalR+ (VP0350), IPTG inducible | pLM1877 |
| pLM3034 | Tetr cosmid with lafK1::lacZ (Genr) | 51 |
| pLM3298 | Tetr cosmid covering VP1672 to VP1692 | This work |
| pLM3650 | Apr GenrexsA+ (VP1699), IPTG inducible | pLM1877 |
| pLM3940 | Tetr pLM3298 with VP1675::Tn5 lux (Kanr) | pLM3298 |
Or iutA::Tn5 lux.
Microarray growth conditions.
To examine V. parahaemolyticus grown on plates with CaCl2 or EGTA, a single colony was streaked as a lawn over the entire surface of a fresh HI plate. The following day, 5 ml of HI broth was used to suspend the cells on the plate, which were then diluted to an optical density at 600 nm (OD600) of 0.05. Fifty microliters of the diluted cells was spread onto an HI plate or an HI plate amended with 4 mM CaCl2 (HI Ca) or 4 mM EGTA (HI EGTA). After 8 h of growth, cells were harvested from the plate and diluted to an OD600 of 1.0 by using RNAprotect (Qiagen) that had been diluted 2-fold in 1× Dulbecco's phosphate-buffered saline (DPBS), pH 7.1 (Gibco). To examine strains bearing the exsA expression plasmid or vector control, a single colony was streaked as a lawn on HI plates with gentamicin. The following day, these plates were suspended in sterile HI broth and diluted to an OD600 of 0.2. Fifty microliters of the diluted cells was spread onto an HI plate with gentamicin and 1 mM IPTG. After 4 h of growth, cells were harvested using RNAprotect as described above.
RNA isolation and cDNA synthesis, labeling, and hybridization.
Cells in RNAprotect were centrifuged and lysed by using lysozyme, proteinase K, and QIAzol (Qiagen) according to the manufacturer's protocol (Qiagen RNAprotect bacterial reagent handbook). For each RNA preparation, 2 ml of plate-grown cells was extracted and the RNA purified using 4 RNeasy minicolumns (Qiagen), which yielded ∼50 μg RNA. This RNA was treated twice with RQ1 DNase (12 U; Promega) at 37°C for 1 h and purified after treatment using an RNeasy minikit. RNA was checked for DNA contamination by PCR (with and without reverse transcriptase) and for degradation by using 1% agarose gels. cDNA was synthesized according to the Affymetrix prokaryotic target preparation protocol using a Superscript II reverse transcriptase kit (Invitrogen) and pD(N)6 random hexamers (GE Healthcare). Approximately 36 μg of RNA was converted to ∼7 μg cDNA, which was purified with a MinElute PCR purification kit (Qiagen) and fragmented with RQ1 DNase to yield fragments between 50 and 200 bp. cDNA was labeled with biotin-ddUTP by using a BioArray terminal labeling kit (Enzo). Samples (∼4.5 μg) were hybridized to the chips in the DNA Core Facility at the University of Iowa according to the standard Affymetrix protocols for E. coli.
Microarray analyses.
Custom GeneChips (rhofispaa52026F; 11-μm feature size) were constructed by Affymetrix Inc. (Santa Clara, CA) and were designed as described by the manufacturer in the GeneChip Custom Express microarray design guide. Chip annotation conforms to the original genome annotation (27). The GeneChip contains 5,161 probe sets designed by using the V. parahaemolyticus RIMD2210633 genome. These probe sets include the 4,912 open reading frames (ORFs) identified by Makino et al. (27), 141 ORFs identified by TIGR (http://cmr.jcvi.org/cgi-bin/CMR/GenomePage.cgi?org=ntvp01), and 23 additional ORFs identified by our analyses, as well as the five small quorum RNAs identified by Lenz et al. (26) and 40 intergenic regions greater than 500 bp. Multiple probe sets were designed for some of the very large or very small genes, and there were 22 genes for which suitable probes could not be produced.
Each condition used to query the chips was repeated at least 2 times using independently isolated RNA samples. Analysis was done using the following software, available at the University of Iowa DNA facility: Affymetrix GeneChip operating software 1.2.1 and Affymetrix GeneChip genotyping analysis software (GTYPE) 4.0. Using the raw signal output generated by GTYPE, processing and comparisons were made by using R version 2.8.1 GUI 1.27 Tiger build 32-bit (5301) (http://www.r-project.org) within Bioconductor software for bioinformatics (http://www.bioconductor.org). The complete data set was preprocessed by using the GCRMA (GC content robust multichip averaging) method to perform optical adjustment, background adjustment, normalization, and summarization functions (58). Comparisons of the output expression values were made by analysis of variance (ANOVA), and the criteria for inclusion in the list of significantly regulated genes were a false discovery rate (FDR) of ≤0.03 and a change in gene expression of 2-fold or greater.
Cytotoxicity assays.
Chinese hamster ovary (CHO) cells (ATCC CCL-61) were maintained in T-75 tissue culture flasks in Ham's F-12 nutrient medium supplemented with 10% fetal bovine serum and 10 U/ml penicillin and streptomycin at 37°C and 5% CO2. For coculture experiments, CHO cells were seeded in 24-well tissue culture plates without antibiotics at a density of 8 × 104 cells/well 20 to 22 h before the start of the assay, which yielded ∼1 × 105 cells/well at the time of infection. The medium was aspirated, and the cells were washed once with Ham's F-12 without serum or antibiotics prewarmed to 37°C before addition of bacteria. The V. parahaemolyticus inocula for the cytotoxicity experiments were grown under the same conditions and timing as the cells that were grown for microarray analysis. Bacterial cells were then suspended in prewarmed Ham's F-12 without serum or antibiotics at 1.5 × 106 cells/ml to yield an approximate multiplicity of infection (MOI) of 15. For cytotoxicity experiments measuring the effect of exsA expression, 25 μM IPTG was added to the tissue culture medium during infection to maintain induction. The cocultures were incubated at 37°C and 5% CO2 for 5 h. The bacterial inoculum was also serially diluted and plated to calculate the actual MOI. Cytotoxicity was assayed by measuring release of lactate dehydrogenase (LDH) by using a CytoTox 96 nonradioactive cytotoxicity kit (Promega Corp., Madison, WI). Five replicate wells were measured per data point. Percent lysis was calculated by comparison to the total lysis obtained in control reactions using 0.9% Triton X-100; control wells without bacteria were used to calculate the background level of lysis. The maximal HI-grown cytotoxicity for the wild-type strain was usually ∼70% lysis compared to detergent-induced lysis. All cytotoxicity experiments were repeated 3 or more times.
Swarming motility assays.
Two microliters of an overnight culture in HI broth was spotted onto the center of a swarm plate with or without amendments, and the diameter of the expanding colony was measured each hour for up to 12 h. Plates were incubated at 30°C. Four replicate plates were measured for each condition, and each experiment was repeated at least three times.
β-Galactosidase and luminescence assays.
Overnight cultures grown on HI plates were suspended and diluted to an OD600 of 0.05 in HI broth, and 50 μl was spread on plates with supplements as indicated in the figure legends. Cells were periodically harvested from these plates by suspension in 5 ml of medium. β-Galactosidase measurements were performed according to Miller (35), except that Koch's lysis solution was used to permeabilize the cells (45). Bioluminescence was measured by using a luminometer (TD20/22; Turner Designs) and is reported as specific light units (SLU), which are relative total light units per min normalized to OD600. Assays were performed in triplicate, and each experiment was performed at least three times, with similar results.
Statistical analysis.
Tests for statistical significance were conducted by using Student's t test (two-tailed distribution with two-sample, equal-variance calculations).
Microarray data accession number.
Microarray data have been submitted to the NCBI and assigned GEO accession number GSE22190.
RESULTS
Calcium regulates swarming motility and lateral flagellar gene expression.
Observations of swarming on different types of media suggested that calcium might influence swarming motility; e.g., more-proficient swarming was observed on plates made with Bacto agar, which contains more calcium than other types of agar, and on plates made with 2216 marine medium, which contains 12 mM Ca2+. To examine the swarming response to calcium, the effect of calcium supplementation was quantified using our standard swarming agar, which is based upon HI medium. Cells were inoculated in the center of HI swarm agar with and without 4 mM CaCl2. The swarm colony diameter was measured periodically, and rates of radial expansion were calculated (Fig. 1 A). Swarm colony diameter expanded at a rate of 4.5 mm/h on HI, and the rate increased to 6.3 mm/h on HI with 4 mM CaCl2. Calcium supplementation had no effect on swimming motility (analyzed in 0.3% agar semisolid motility plates by using a Laf− strain with only a functional polar system [data not shown]).
FIG. 1.
Calcium regulates swarming motility and lateral flagellar gene expression. (A) Calcium affects rate of swarming. Strain LM5674 was spotted in the center of HI plates or HI plates supplemented with 4 mM CaCl2. Rates of radial expansion were calculated by periodically measuring the diameter of the swarming colony. Error bars represent standard deviations for at least 4 replica plates at each time point from one representative experiment. The slopes were calculated over the linear portion for each condition, from 3 to 7 h. The rate of expansion was 4.5 mm/h (R2 = 0.99) on HI and 6.3 mm/h (R2 = 0.99) on HI Ca. (B) Calcium affects laf::lux expression. Strain LM5738, with a lux fusion in a lateral flagellar gene (laf::lux), was inoculated on HI plates or HI plates supplemented with 4 mM CaCl2, 4 mM MgCl2, or 4 mM BAPTA. Plates were harvested periodically by suspending the cells to measure luminescence. Luminescence measured for each growth condition with supplement was calculated to be significantly different from that for growth in HI without supplement from 6 to 9 h (P < 0.001). (C) Ion specificity and laf::lux expression. Light production by LM5738 harvested from plates with the indicated CaCl2 or MgCl2 concentration was determined at 8 h. The statistical significance of the differences with and without supplement was calculated at P values of <0.0001 for 4 and 10 mM Ca and <0.001, <0.002, and <0.03 for 4, 10, and 40 mM Mg, respectively. The differences elicited by 40 and 4 mM Mg compared to 10 mM Mg were not considered statistically significant (P > 0.07). Luminescence is expressed as SLU, which are total light units per min normalized to OD600. Error bars represent standard deviations for triplicate light measurements from a representative experiment. Experiments were repeated at least 3 times.
To distinguish the mechanism by which calcium stimulated swarming motility, lateral flagellar (laf) gene expression was measured by using the luminescent reporter strain LM5738, which contains a lux fusion in the lateral flagellar gene flgBL, encoding a rod component of the basal body. (The subscript L designates lateral flagellar genes, in distinction to polar genes.) Luminescence was quantified during a time course for cells grown on HI plates or HI plates with 4 mM CaCl2 or 4 mM BAPTA, a highly specific calcium chelator (Fig. 1B). Reporter activity increased starting at ∼4 h after inoculation and peaked between 7 and 8 h. A maximum of 183,000 SLU (total light units per min normalized to OD600) was achieved when the reporter strain was grown on HI. Addition of 4 mM calcium stimulated laf::lux expression ∼2.5-fold compared to that with HI (maximum of 462,000 SLU). BAPTA decreased lateral flagellar gene expression ∼6-fold compared to that with HI (29,800 maximum SLU), and the chelator had no effect on growth (data not shown). Hence, the difference in maximal lateral flagellar gene expression between conditions with and without calcium was ∼15-fold.
Ion specificity was investigated by adding magnesium to the medium. It increased laf::lux gene expression, albeit not as well as calcium amendment, i.e., ∼1.5-fold induction with 4 mM MgCl2 compared to that with HI (Fig. 1B). Supplementation of the medium with increasing concentrations of CaCl2 further induced laf gene expression, but increasing levels of MgCl2 up to 40 mM, which is the concentration of Mg2+ in seawater, did not cause a fold change greater than that which was observed at 4 mM (Fig. 1C).
Calcium regulates global gene expression in V. parahaemolyticus.
To explore the global role of calcium in V. parahaemolyticus gene expression, the transcriptional profiles of strain LM5674 grown on HI plates with 4 mM CaCl2 (HI Ca), HI, or HI with 4 mM EGTA (HI EGTA) were captured and compared by using microarrays. These particular concentrations of CaCl2 and EGTA were chosen in order to produce the best-matched growth, as higher concentrations of either amendment decreased the growth rate. EGTA was used as the calcium chelator because it has been used in other microarray analyses and has a known role in regulating T3SS gene expression in other organisms (38, 57). RNA was prepared from cells grown on plates and harvested at 8 h, the time corresponding to the peak of laf gene expression (as shown in Fig. 1B). Tests for differential gene expression were performed using an ANOVA model, and results were considered significant at or above a 2-fold discriminator, with a false discovery rate (FDR) of 0.03. When HI Ca was compared to HI, 50 genes showed changes in expression: 35 of these showed increased expression (2- to 11-fold), and 15 showed decreased expression (2- to 24-fold). When HI was compared to HI EGTA, 267 genes showed changes: 118 of these showed increased expression, and 149 showed decreased expression. When HI Ca was compared to HI EGTA, 122 genes showed changes in expression: 60 of these showed increased expression, and 62 showed decreased expression. Table S1 in the supplemental material provides the fold changes (with P values and q values) for these differentially regulated genes; the q value provides a measure of significance in terms of the false discovery rate.
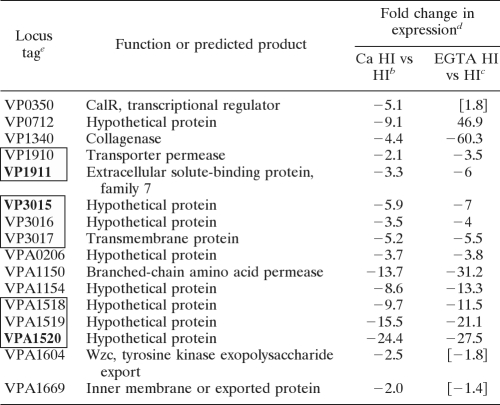
Expression profiles of individual genes shown in Fig. 2 suggest a complex response to EGTA. One pattern, exemplified by VP1657 and VPA0451, which are both involved in T3SS1, showed increased expression with HI Ca and HI EGTA compared to that with HI. In fact, the majority of the T3SS1 genes displayed this expression profile (Table 2 ). In contrast, VPA1520 (encoding a hypothetical protein) and VP3017 (encoding an outer membrane protein) were repressed by calcium and EGTA compared to HI. Other profiles appeared to respond to a gradient of calcium availability. For example, VP0350, encoding a transcriptional regulator, and VP0712, encoding a hypothetical protein, were repressed by calcium and induced by EGTA. Other genes were repressed by calcium and not significantly regulated by EGTA, such as VPA1604, encoding a putative tyrosine kinase similar to the Wzc protein involved in polysaccharide transport, and VPA1669, encoding a potential membrane or exported protein. A number of genes were regulated by EGTA but not by calcium. Some of these were induced by EGTA, e.g., VP0302 (encoding a hypothetical protein), and some were repressed, e.g., VP0167 (encoding a component of a TonB transport system). Further analysis must be done to determine which of these EGTA-regulated genes are bona fide members of the calcium regulon. It may be that some calcium-regulated genes are triggered by the levels of calcium available in HI and thus do not undergo a change in expression in HI versus HI Ca; however, we suspect that these include more heterogeneously regulated genes, as EGTA can chelate ions in addition to calcium (48). For example, the product of VP0302 contains a predicted zinc-binding site, suggesting that the effect of EGTA encompasses more than a response to lowered calcium concentrations.
FIG. 2.
Microarray expression profiles of selected genes. LM5674 was grown on HI plates or HI plates with 4 mM CaCl2 or 4 mM EGTA. Expression levels are log2 values of the normalized expression value for each gene under each condition. Locus tag designations are given, with the predicted products in parentheses. Error bars represent the standard errors from two replicates for each condition.
TABLE 2.
Genes induced by ExsA and their responses to calcium and EGTAa
Inclusion in this list was determined by a change in expression of 4-fold, with an FDR of ≤0.03, when exsA was overexpressed compared to the expression with the vector control. VP1675 is the exception and was included because it is part of a potential operon in which all other genes met the criteria.
b Fold change in gene expression comparing the exsA overexpression strain LM8864 to the vector control strain LM8863.
c Fold change in gene expression comparing strain LM5674 grown on HI with 4 mM CaCl2 to LM5674 grown on HI. A change in expression of 2-fold or greater and an FDR of ≤0.03 were the criteria for significant change.
d Fold change in gene expression comparing strain LM5674 grown on HI with 4 mM EGTA to LM5674 grown on HI. A change in expression of 2-fold or greater and an FDR of ≤0.03 were the criteria for significant change.
e A bracketed number indicates that the fold change or the FDR value did not meet the criteria.
f Boxes indicate putative operons, with the promoter-proximal gene in bold.
g NT01 genes were annotated by TIGR after the original annotation was performed.
Most of the lateral flagellar genes in the swarming regulon were not observed to be regulated differentially in the array comparison. The reason for this failure to discriminate the laf genes became apparent on inspection of the expression values in the microarray data, namely, that these genes were so highly expressed under the HI condition that the capacity for the chips to report a significantly increased signal was exceeded. For example, VPA1548, encoding the lateral flagellin LafA, had an average log2 expression value of 12.5 when the cells were grown in HI; VPA0267, encoding FlgEL, had an average log2 expression value of 11.4 in HI (Fig. 2). These values are at the upper limit of detection by the microarray analysis.
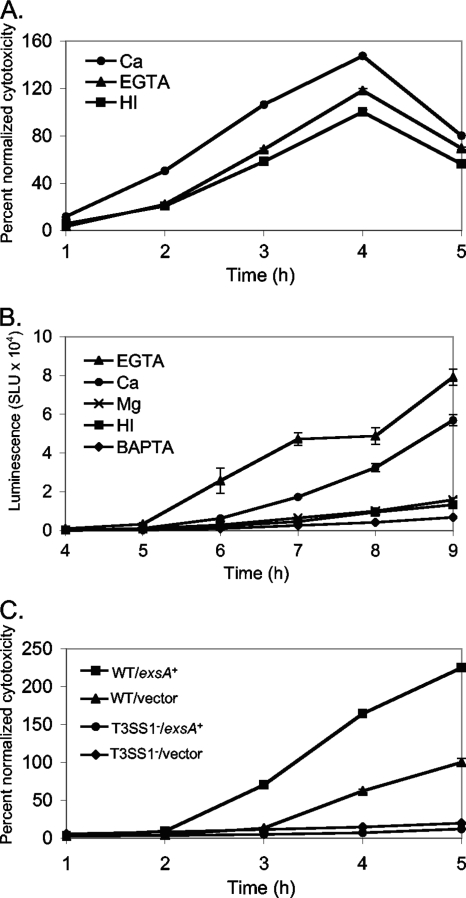
Calcium and EGTA increase cytotoxicity.
Induction of T3SS1 genes by calcium as well as EGTA was an unexpected result. Calcium has not been reported as a T3SS inducing signal, although EGTA induces T3SS gene expression in organisms such as P. aeruginosa and Yersinia spp. (8, 9). To examine whether the calcium- and EGTA-induced changes in gene expression were meaningful with respect to the virulence potential of the organism, the wild-type strain was grown on plates and harvested under the same conditions used for microarray analysis (i.e., HI, HI EGTA, and HI Ca) and used to infect Chinese hamster ovary (CHO) cells at an MOI of approximately 15. Cytotoxicity was measured over time postinfection as the amount of released host lactate dehydrogenase (LDH). This assay has been used as a measure of V. parahaemolyticus T3SS1 activity (44), and we demonstrate that this assay can be used for the CHO cell line (Fig. 3 A). V. parahaemolyticus cells grown in the presence of EGTA caused 118% maximal lysis compared to the maximal lysis produced by cells grown with HI, and cells grown with calcium caused 147% maximal lysis compared to that for cells grown with HI (Fig. 3A). In summary, the increased expression of T3SS1 genes observed in the microarray analysis correlated with the observed change in the virulence phenotype when cells were grown with EGTA or CaCl2.
FIG. 3.
Calcium, EGTA, and ExsA induce T3SS1 cytotoxicity and gene expression. (A) Ca and EGTA affect cytotoxicity. Strain LM5191 was grown and harvested from HI plates or plates with 4 mM CaCl2 or 4 mM EGTA, as per the microarray growth conditions, and used to infect Chinese hamster ovary (CHO) cells at an MOI of 15. Cytotoxicity was assayed by measuring release of host cell lactate dehydrogenase (LDH) at 1-h intervals postinfection and is expressed as percent lysis normalized to the maximum lysis for LM5191 grown on HI. Values for the Ca and EGTA conditions were significantly different from those for HI at 3 to 5 h (P < 0.0006). (B) T3SS1::lux expression. Strain LM9649 carrying the T3SS1 reporter plasmid (with T3SS1::lux) was grown on HI kanamycin plates or HI kanamycin plates with 4 mM CaCl2, 4 mM MgCl2, 4 mM EGTA, or 4 mM BAPTA. Plates were harvested periodically by suspending the cells to measure luminescence. Luminescence is expressed as SLU from one representative experiment. Error bars represent the standard deviations for triplicate measurements at each time point. Values for each condition with supplement (Ca, EGTA, and BAPTA) were significantly different from those for HI at 6 to 9 h (P < 0.003); the Mg condition was not significantly different from HI. (C) ExsA affects cytotoxicity. The effect of exsA expression on cytotoxicity was measured by using strains grown and harvested as per the microarray experiment to infect CHO cells at an MOI of 15. The cytotoxicity of wild-type LM5674 carrying the exsA expression plasmid (pLM3650) or the T3SS1 mutant strain LM7035 (ΔVP1672::Camr) carrying the vector (pLM1877) or the exsA expression plasmid was significantly different from that of the wild-type strain carrying the vector at 4 and 5 h (P < 0.001). Cytotoxicity is expressed as percent lysis normalized to the maximum lysis for LM5674 with the vector. For the cytotoxicity experiments with results shown in panels A and C, error bars indicate standard errors of the means from 5 individual samples at each time point for a representative experiment. All experiments were repeated at least three times.
Calcium and EGTA increase T3SS1 gene expression, as does ExsA.
To further analyze T3SS1 gene expression, a lux reporter fusion in a T3SS1 gene (VP1675, encoding a YscU/EscU-type T3SS component protein) was isolated. This T3SS1::lux reporter was carried on a low-copy-number cosmid containing ∼15 kb of V. parahaemolyticus DNA from VP1672 through VP1692. The strain containing this reporter plasmid was grown on HI, HI Ca, and HI EGTA plates (and kanamycin to maintain the cosmid). Ca or EGTA amendment increased T3SS1 gene expression throughout the time course of growth (Fig. 3B); therefore, the reporter gene induction was consistent with the fold changes observed for T3SS1 transcription in the microarray analysis (Table 2).
To probe whether calcium and EGTA were inducing signals for the entire T3SS1 and define the full spectrum of T3SS1 genes, VP1699, encoding the transcriptional regulator ExsA, was cloned into an IPTG-inducible expression vector. VP1699 shows 40% amino acid similarity to ExsA of P. aeruginosa and has been shown to positively regulate expression of a subset of type III secretion genes in V. parahaemolyticus (61). Strains carrying either the vector or the exsA plasmid were grown on HI plates for 4 h with 1 mM IPTG (and gentamicin to maintain the plasmid), harvested as per the microarray conditions, and used to infect CHO cells at an MOI of approximately 15. Cytotoxicity was assessed at each hour of growth in coculture by measuring released LDH. IPTG-induced expression of exsA increased the maximal observed cytotoxicity ∼225% compared to that for the strain carrying the vector control plasmid (Fig. 3C). The observed LDH release was dependent on a functional T3SS1, as no lysis was observed upon induction of exsA in a T3SS1− mutant strain with a deletion of VP1672, which encodes a YscR/EscR-type ortholog.
Ectopic expression of exsA was used as a condition to query the microarray, and expression of 67 genes increased at least 4-fold upon exsA induction (Table 2). Of these, 43 were located in the region predicted to encode T3SS1 from VP1656 to VP1701 on chromosome 1. (We note that expression of four genes in this region, VP1676 to VP1679, was unaffected.) Two genes located on chromosome 2, VPA0450 and VPA0451, forming a predicted operon, were induced strongly (>50-fold); these have previously been characterized as encoding a type III effector and cognate chaperone pair that are dependent on T3SS1 for secretion (40). Of the genes predicted by homology to play a role in T3SS1, only VP1675 was not induced significantly by ExsA, calcium, or EGTA. VP1675 occurs at the end of a potential 8-gene operon and was demonstrated to be induced by calcium and EGTA by using a reporter fusion (Fig. 3B). The majority (40 of 69) of the genes induced upon exsA overexpression were also induced under calcium and/or EGTA growth conditions (Table 2). We suggest that the set of genes induced by exsA as well as calcium and EGTA consists of the genes belonging to T3SS1.
Some ExsA-regulated genes showed small changes in expression provoked by calcium or EGTA (i.e., not passing our fold and FDR criteria). Two of these genes, VP1681 and VP1685, are located within the T3SS1 pathogenicity island. They encode very small hypothetical proteins (35 and 57 amino acids [aa], respectively) that do not occur in predicted operons. Twenty-three genes induced by exsA are unlinked to the T3SS1 pathogenicity island and have no obvious role in type III secretion. None were induced by calcium, and only three were induced, between 4- and 7-fold, by EGTA (VP2376, VP2591, and VPA0256). We suspect that some may not be bona fide members of T3SS1.
ExsA represses laf gene expression and swarming motility.
The ExsA microarray analysis also revealed 66 genes showing >4-fold-decreased transcription upon exsA induction (Table 3). The majority (78%) of these genes were lateral flagellar (laf) genes and genes known to be part of the swarming regulon. The repression of laf genes was confirmed by examining the effect of ectopic exsA expression in the flgBL::lux reporter strain (Fig. 4 A). IPTG-mediated induction of exsA repressed flgBL expression throughout the time course. To examine the effect of ExsA on swarming motility, strains carrying the exsA expression plasmid and the vector were spotted on swarm plates with IPTG (Fig. 4B). Induction of exsA inhibited swarming motility in a wild-type background as well as in a strain containing a deletion mutation in gene VP1672, encoding the EscR-type T3SS1 component critical for production of the T3SS1 apparatus. Thus, the inhibition of swarming motility and gene expression by exsA induction was not simply a consequence of the overproduction of many T3SS1 organelles on the cell surface.
TABLE 3.
Genes repressed by overexpression of exsAa
Inclusion in this list was determined by a change in expression of 4-fold, with an FDR of ≤0.03, when exsA was overexpressed compared to the expression with the vector control.
b Boxes indicate putative operons, with the promoter-proximal gene in bold.
c NT01 genes were annotated by TIGR after the original annotation was performed.
FIG. 4.
ExsA inhibits lateral flagellar gene expression and swarming motility. (A) ExsA affects laf::lux expression. The lateral flagellar gene reporter fusion strain LM5738 (laf::lux) carrying the vector or the IPTG-inducible exsA clone was grown on HI gentamicin plates with 1 mM IPTG and harvested periodically to measure luminescence, which is reported as SLU. Error bars represent standard deviations for triplicate light measurements from each sample. Light values for these strains were significantly different from each other at 5 to 7 h (P < 0.001). A representative experiment of three is shown. (B) ExsA affects swarming. Wild-type (WT) LM5674 or T3SS1 mutant strain LM7035 (ΔVP1672::Camr) carrying the vector or an IPTG-inducible exsA expression construct was spotted on HI gentamicin swarm plates with IPTG and incubated overnight.
Iron limitation also affects T3SS gene expression as it influences lateral flagellar gene expression.
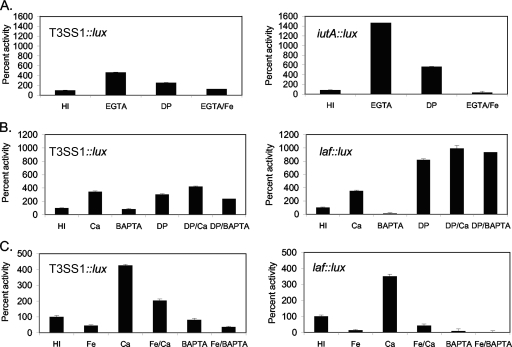
EGTA and calcium inducing T3SS gene expression seemed paradoxical. Although EGTA has a high affinity for Ca2+, it can chelate other ions, e.g., Mg2+ and Fe2+ or Fe3+ (29, 48). To probe ion specificity, the T3SS1 reporter strain was grown with MgCl2 and the specific calcium chelator BAPTA (Fig. 3B). MgCl2 had little effect on T3SS1::lux; cells grown on plates in HI and HI Mg produced similar amounts of light throughout a time course experiment. BAPTA inhibited expression of T3SS1::lux ∼2-fold compared to the level of expression with HI. As a consequence, it seemed likely that the effect of EGTA was the result of chelation of cations other than Ca2+ and Mg2+. To examine iron availability in the presence of EGTA, we used a second lux reporter strain with a fusion in the iron acquisition gene VPA0979, or iutA (Fig. 5 A). Luminescence was induced ∼7-fold by growth of this strain under mildly iron-limiting conditions (HI and 100 μM 2,2′-dipyridyl) compared to growth with HI. The iutA::lux fusion was induced 47-fold in the presence of EGTA compared to HI, consistent with removal of iron from the medium by EGTA; furthermore, when the iutA::lux strain was grown in EGTA with iron (50 μM FeCl3), luminescence was repressed. The T3SS1::lux reporter behaved similarly, i.e., EGTA-induced luminescence could be repressed by iron supplementation, and addition of the iron chelator dipyridyl to HI induced T3SS1::lux expression ∼3-fold (Fig. 5A). These data suggest that induction of T3SS1 by EGTA was not the result of calcium starvation but rather iron starvation. Consistent with these findings, cytotoxicity was enhanced by pregrowth under iron-limiting conditions (i.e., HI broth versus HI broth with 50 μM dipyridyl) (data not shown).
FIG. 5.
Iron and calcium affect lateral flagellar and T3SS1 gene expression. (A) EGTA limits iron availability. (B) Effects of limiting iron in the presence or absence of calcium. (C) Effects of excess iron in the presence or absence of calcium. Reporter strains were grown on HI plates or plates with 4 mM EGTA, 50 μM FeCl3, 100 μM 2,2′-dipyridyl (DP), 4 mM CaCl2, or 4 mM BAPTA. Plates were harvested periodically, and results from one time point (9 h) are shown; however, trends were consistent throughout the time course. Reporter gene activity is expressed as the percent luminescence (calculated as SLU) produced under a given growth condition normalized to that produced under the HI condition. Error bars represent the standard deviations from triplicate measurements. The reporters were in strains LM9649 (T3SS1::lux), LM5738 (laf::lux), and LM6151 (ferric aerobactin receptor, iutA::lux). The experiments were repeated three times. All conditions with supplements elicited reporter expression significantly different from that with HI alone (P < 0.03), except for the EGTA/Fe and BAPTA effects compared to the HI effects for T3SS::lux, which were not considered different. The differences in expression for each reporter between EGTA and EGTA with Fe, Ca and Ca with Fe, and BAPTA and BAPTA with Fe were significant (P < 0.001).
The interplay between the calcium and iron signals was investigated in a series of experiments using combinations of conditions (Fig. 5B and C). Lateral flagellar gene expression was examined in addition to T3SS gene expression because iron limitation is one known signal inducing swarming (30). The observed trends (i.e., induction or repression under a particular set of conditions) were similar for both kinds of reporters. Calcium supplementation induced gene expression in the presence or absence of iron, iron limitation induced gene expression in the presence or absence of calcium, and iron repressed gene expression in the presence or absence of calcium. Consequently, for both laf and T3SS1 regulation, each inducing signal appeared to work independently.
CalR is a calcium-regulated transcription factor.
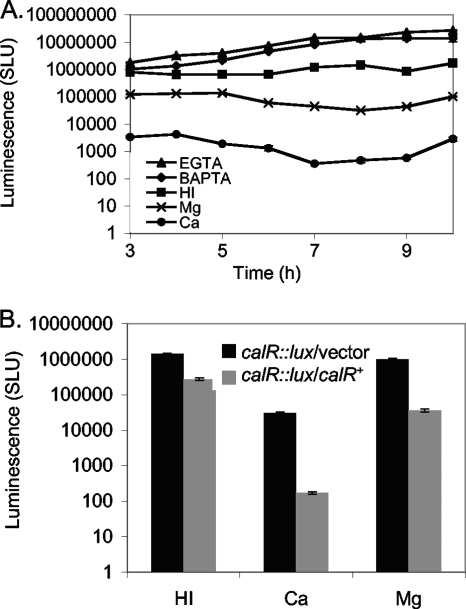
The data revealed that calcium plays a signaling role in V. parahaemolyticus that has global consequences in terms of gene expression and phenotype. Moreover, the microarray analysis identified some candidate transcriptional regulators that might participate in the calcium response. Of the 16 genes repressed by calcium (Table 4), one (VP0350) encodes a LysR-type regulator that was repressed 5.1-fold in the presence of calcium, and its regulation seemed responsive to a gradient of calcium, as the gene was induced 1.8-fold under the EGTA condition (Fig. 2 and Table 4). We had several existing transposon mutants in our strain collection with insertions in VP0350. One contained a luminescence reporter fusion that had been isolated previously in a transposon mutant screen for lux fusions responsive to calcium (K. H. Shaw, L. L. McCarter, and J. L. Enos-Berlage, unpublished data). This LM7120 mutant was used to analyze the expression of VP0350. The reporter strain was grown on HI or HI with EGTA, Ca, Mg, or BAPTA, and luminescence was monitored over time (Fig. 6 A). Light output was generally constant during growth and responsive to a gradient of calcium. At 8 h, calcium repressed VP0350::lux expression ∼3,000-fold, MgCl2 reduced expression ∼50-fold, and BAPTA and EGTA increased expression ∼9- and 10-fold, respectively, compared to HI alone. (We note that 2 mM EGTA is sufficient to chelate the amount of calcium in HI, as LM7120 was responsive to EGTA amendment in a titration experiment up to 2 mM [data not shown].) Due to its clear calcium responsiveness, we have named this gene calR.
TABLE 4.
Genes repressed by calciuma
Inclusion in this list was determined by a change in expression of 2-fold or greater, with an FDR of ≤0.03, during growth on HI with 4 mM CaCl2 versus growth on HI.
b Fold change in gene expression when the wild type was grown on HI with 4 mM CaCl2 versus on HI alone.
c Fold change in gene expression when the wild type was grown on HI with 4 mM EGTA versus on HI alone.
d A bracketed number indicates that the fold change or the FDR value did not meet the criteria.
e Boxes indicate putative operons, with the promoter-proximal gene in bold.
FIG. 6.
The LysR-type transcription factor CalR is calcium regulated. (A) calR::lux expression. Strain LM7120 (calR::lux) was inoculated on HI plates and HI plates with 4 mM CaCl2, 4 mM MgCl2, 4 mM EGTA, or 4 mM BAPTA. Cells were harvested periodically to measure luminescence, reported as SLU. Error bars represent standard deviations from triplicate samples for one representative experiment of three. calR::lux expression levels were significantly different between HI and all other conditions from 6 to 9 h (P < 0.001). (B) CalR autoregulation. Luminescence is shown for LM7915 (calR::lux calR+) and LM7916 (calR::lux vector) grown in HI broth (with gentamicin and 50 μM IPTG) alone or supplemented with 4 mM CaCl2 or 4 mM MgCl2. calR was supplied in trans by using the IPTG-inducible plasmid pLM2795. Error bars represent standard deviations from triplicate samples for one representative experiment of three. For each growth condition, calR::lux expression levels were significantly different between strains carrying the calR expression plasmid and strains carrying the vector (P < 0.001).
Since LysR-type regulators often show autoregulation (47), we examined the ability of CalR to regulate the calR::lux chromosomal reporter fusion. An IPTG-inducible expression vector containing calR was introduced into the calR::lux reporter strain LM7120 to yield strain LM7915, and the corresponding vector control strain LM7916 was also constructed. These strains were grown in HI broth with 50 μM IPTG and 4 mM CaCl2 or 4 mM MgCl2. Light production was measured periodically and is shown after 2 h of IPTG induction (Fig. 6B). CalR repressed calR::lux expression 5.1-fold in HI. BAPTA diminished this repression to about 2-fold (data not shown). When CaCl2 was added to the medium, IPTG-controlled CalR repression of calR::lux was 180-fold compared to the amount of light produced by the strain carrying the vector. CalR activity was somewhat responsive to MgCl2; repression by CalR in the presence of magnesium was 27-fold compared to the level for the strain carrying the vector. Thus, calR appeared negatively autoregulated, and the CalR repression activity was potentiated by calcium; however, calR expression was also repressed by high calcium through a CalR-independent mechanism.
CalR inhibits cytotoxicity, T3SS1 gene expression, swarming motility, and laf gene expression.
To define the role of this new regulator, the calR phenotype was explored. The calR mutant LM5197 displayed enhanced cytotoxicity compared to that of its parental strain LM5191 (Fig. 7 A). The highest cytotoxicity was observed when the calR mutant was pregrown with calcium. These results were confirmed by using a second calR mutant that was isolated as part of a different mutant isolation (data not shown). calR mutants were derepressed with respect to cytotoxicity; however, they retained some capacity to respond to calcium. The cytotoxicity phenotype correlated with gene expression changes observed using the plasmid-borne T3SS1 luminescence reporter. Expression of T3SS1::lux in the calR mutant was elevated compared to that in the parental strain, and both strains had higher levels of T3SS1 gene expression when grown in the presence of added CaCl2 (Fig. 7B).
FIG. 7.
CalR inhibits cytotoxicity, T3SS1 gene expression, swarming motility, and laf gene expression. (A) calR mutant cytotoxicity. Strain LM5191 and the congenic calR mutant LM5197 were grown on HI plates with or without 4 mM CaCl2 and used to infect CHO cells at an MOI of 15. Cytotoxicity is reported as percent lysis normalized to the maximum lysis for the wild type grown on HI. Error bars represent standard errors of the means from 5 replicates for one representative experiment of three. At 2 to 4 h postinfection, levels of LDH release were significantly different between the wild type grown on HI and all other conditions (P < 0.008). (B) calR mutant T3SS1::lux expression. Strains LM9649 and LM9650 (constructed from LM5191 and LM5197, respectively), each carrying the T3SS1::lux reporter on a plasmid, were grown on HI plates with or without 4 mM CaCl2. Luminescence was measured periodically. Error bars represent standard deviations from 3 light measurements for one representative experiment of three. At 6 to 9 h, T3SS1::lux expression levels were significantly different between the wild-type strain grown in calcium and grown in HI, between the calR strain grown in calcium and grown in HI, and between the calR strain and the wild-type strain grown in HI or HI Ca (P < 0.001). (C) calR mutant swarming rates. Strains used as described for panel A were spotted in the center of HI swarm plates with or without 4 mM CaCl2. Colony expansion was measured periodically. Error bars represent standard deviations from at least 4 replicates at each point for one representative experiment of three. Rates of radial expansion on HI Ca were 7.1 mm/h (R2 = 0.99) for LM5197 (calR) and 6.9 mm/h (R2 = 0.99) for LM5191 (WT). Rates on HI were 5.7 mm/h (R2 = 0.99) for LM5197 and 3.9 mm/h (R2 = 0.99) for LM5191. (D) calR mutant laf::lacZ expression. Strain LM5949 (laf::lacZ) and strain LM7120 (laf::lacZ calR::Tn5 lux) were grown on HI plates with or without 4 mM CaCl2. β-Galactosidase activity was measured periodically and is expressed in Miller units. From 6 to 9 h, LacZ activity for the wild type grown on Ca and the calR mutant grown on HI or HI Ca was significantly different from that for the wild type grown on HI (P < 0.001). Error bars represent standard deviations from triplicate assays for one representative experiment of three.
Swarming motility was assessed by measuring the diameters of swarm colonies of the wild-type and calR mutant strains on HI swarm plates or plates with added CaCl2 (Fig. 7C). The radial expansion rates on HI were 5.7 mm/h for the calR mutant and 3.9 mm/h for the wild-type strain. Both strains swarmed faster and/or earlier on plates with CaCl2 than on HI (7.1 and 6.9 mm/h for calR and wild-type strains, respectively). This result was confirmed by using a second, independently isolated calR mutant strain (data not shown). The role of calR in the regulation of lateral flagellar gene expression was examined in a flgCL::lacZ reporter strain. The calR mutant showed higher levels of gene expression than the wild type, and as was the case with swarming motility, both strains showed increases in gene expression in the presence of added CaCl2 (Fig. 7D). Taken together, these results are consistent with the gene expression profile of calR. Calcium repressed calR expression, and CalR negatively regulated laf and T3SS1 transcription. Thus, under high calcium, CalR levels are predicted to be low, and swarming and T3SS1 transcription levels are predicted to be elevated. These phenotypes were even more profound when CalR was eliminated by mutation.
LafK is required for the calcium-mediated increase of T3SS1 gene expression and cytotoxicity.
The preceding data demonstrated that CalR played a role in the regulation of swarming motility and T3SS1 in response to calcium. Calcium regulation mediated by CalR could act directly to modulate expression of each regulon or through a common regulatory pathway. LafK is a key σ54-dependent regulator required for swarming motility (51). A lafK strain and its parental strain were grown in the presence of increasing levels of calcium and used to infect CHO cells (Fig. 8 A). The parental strain showed an enhanced profile of cytotoxicity with increasing calcium; however, the lafK mutant did not respond similarly. Although there was a modest response to calcium, even in the presence of 10 mM CaCl2, the cytotoxicity of the lafK mutant was lower than the cytotoxicity of the wild type grown in the absence of calcium. To examine T3SS1 gene expression, the T3SS1::lux reporter cosmid was introduced into the lafK mutant and parent strains. T3SS1 gene expression was enhanced by increasing calcium concentrations in the growth medium in the wild-type and lafK strains; however, even in the presence of 10 mM calcium, the lafK mutant failed to produce as much light as the wild-type strain grown in the absence of calcium (Fig. 8B). Consequently, LafK appeared to be one regulatory link between swarming and T3SS1 gene expression and was required to mediate the major portion of the T3SS1 response to calcium.
FIG. 8.
Calcium-enhanced cytotoxicity and T3SS1 gene expression require LafK. (A) lafK mutant cytotoxicity. Strain LM5674 and the congenic lafK mutant LM7789 were grown on HI plates or HI plates with 4 mM CaCl2 or 10 mM CaCl2. Cells were suspended in HI broth from these plates and used to infect CHO cells at an MOI of 15. Cytotoxicity, measured in the LDH release assay, is reported as percent lysis normalized to the maximum lysis for the wild type grown on HI. Error bars represent standard errors from 5 replicates for one representative experiment of three. From 3 to 5 h postinfection, the lafK mutant cytotoxicity was statistically different from that for the wild type grown under each condition (P < 0.01). (B) T3SS1::lux in the lafK mutant. T3SS1 gene expression was measured in strain LM5191 and the congenic lafK mutant strain LM9596, carrying a plasmid with a T3SS1::lux reporter. Strains were grown on HI plates with tetracycline (to maintain the reporter) or HI plates with 4 mM CaCl2 or 10 mM CaCl2. Cells were harvested periodically for luminescence measurements, expressed as SLU. Error bars represent standard deviations from triplicate assays at each time point for one representative experiment of three. From 7 to 9 h, expression in the lafK mutant was statistically different from expression in the wild type under each condition (P < 0.001).
DISCUSSION
In this work, we demonstrate that metal ions play distinct roles in modulating gene expression and behavior in V. parahaemolyticus: high-calcium and low-iron growth conditions promote the induction of swarming and T3SS regulons. These inducing signals are not surprising for a bacterium that is found in marine and estuarine environments, which can have very high and varying levels of calcium as well as low iron availability. The natures of the two gene sets regulated by these conditions also seem consistent, for each of these regulons encodes complex organelles that function on surface contact. Swarming is a particular kind of flagellum-mediated, surface-adapted behavior that allows bacteria to move over and colonize surfaces (reviewed in reference 22). Type III secretion gene expression and activity are activated by contact with host cells (reviewed in reference 18).
One initially surprising response, however, was that the predicted T3SS1 regulon was induced not only by growth in medium supplemented with calcium but also by use of the metal ion chelator EGTA, which has a high but not exclusive affinity for calcium. Overexpression of exsA, encoding the master T3SS1 transcriptional regulator (61), was used to independently define the extent of the T3SS1 regulon. Calcium and EGTA, two apparently opposing signals, were confirmed as regulating a core set of genes that were regulated by ExsA. This initially confounding observation was resolved upon finding that the EGTA induction was the consequence of iron and not calcium chelation by EGTA. Swarming is also under iron control (30). Iron restriction seems an appropriate signal for both the induction of swarming motility during growth in a crowded community on a surface where diffusion of iron seems limiting and virulence in a vertebrate host. Iron limitation has been identified as an inducing signal for the type III secretion systems in Shigella dysenteriae and Salmonella enterica serovar Typhimurium (12, 36).
Ectopic expression of exsA also repressed expression of some genes, and the majority of these ExsA-repressed genes were lateral flagellar genes and other genes known to be part of the swarming regulon. We suspect that repression of the entire swarming regulon upon upregulation of T3SS1 is a physiologically relevant response, because the observed repression was highly specific to the swarming genes; moreover, it seems a functionally efficient response. The induction of type III secretion systems typically shows a feed forward regulation in which translocation competence triggers enhanced gene expression (reviewed in reference 4). Presumably, repression of the swarming regulon is manifested when T3SS1 is highly activated; it would seem counterproductive for a cell to engage simultaneously in swarming motility and virulence effector secretion. During swarming, hundreds of lateral flagella are produced per cell, so many that they might effectively prevent efficient host cell contact and effector delivery via the T3SS1 organelles. Thus, although some of the same environmental signals, e.g., calcium and iron limitation, seem to potentiate swarming and T3SS1 transcription, other signals must certainly influence the balance between production of lateral and T3SS organelles. Regulatory interactions between flagella and the T3SS have been observed in other organisms. For example, flagellar regulatory mutants of Salmonella enterica serovar Typhi reduce Salmonella pathogenicity island 1 (SPI-1) T3SS gene expression (11), whereas P. aeruginosa flagellum-defective mutations affect the T3SS by enhancing gene expression and cytotoxicity, and overexpression of exsA decreases motility and expression of some, but not all, flagellar genes (50, 57).
LafK, which is a σ54-dependent regulator of genes in the V. parahaemolyticus swarming regulon, including the lateral flagellar genes (51), was found to participate in linking T3SS and swarming gene expression. This regulator is required to fully activate T3SS1 in response to calcium. We emphasize that the lafK mutation modulates but does not abrogate cytotoxicity. Stationary-phase wild-type and lafK strains grown on plates and used to infect host cells are equally toxic (data not shown). A similar observation, namely, no observed difference in pathogenicity between stationary-phase wild-type and lafK strains, was made for Aeromonas hydrophila (53).
Many strains of V. parahaemolyticus possess two distinct type III secretion systems, one found on each chromosome, with the genes for each system generally organized in clusters (6, 27, 44). Both systems contribute to pathogenicity; however, they may do so with different proficiencies and target hosts (17). We note that only T3SS1 was observed to be regulated by calcium and EGTA; genes in the T3SS2 locus showed no upregulation by calcium, and some but not all of the T3SS2 genes in the region between VP1352 and VP1367 showed enhanced expression, between 2- and 5-fold, in the presence of EGTA. Whether this suggests that T3SS2 is regulated in a meaningful way in response to calcium chelation (or another signal) remains to be deciphered. The secretion of some V. parahaemolyticus T3SS1 effectors has recently been reported to be repressed by addition of supplemental calcium (25); however, this analysis was quite different from the experiments we report here with respect to outputs measured, strain differences, and growth conditions, including medium composition, temperature, and growth in broth culture. We suspect that the last condition, i.e., growth in liquid versus on plates, may be one critical variable impinging on T3SS regulation, in part because lafK is expressed only during surface colonization (51). We note that the role of calcium signaling in enteropathogenic E. coli (EPEC) also seems complex, i.e., secretion of the T3SS effectors EspB and EspD increases when the calcium chelator EGTA is added to the medium, whereas other T3SS effectors, such as Tir, are secreted at higher levels in the presence of calcium (19, 23).
The global calcium response was found to be mediated in part by a newly identified LysR-type transcription factor. CalR represses swarming and T3SS1 gene expression as well as its own transcription. LysR family members are known to autoregulate and also to bind a wide range of cofactors (47); it therefore seems possible that CalR could be a calcium-binding protein. Importantly, CalR-dependent repression of the calR::lux fusion was enhanced in the presence of calcium; however, whether this occurs directly or indirectly remains to be determined. Although there is no precedence for bacterial transcription factors that bind calcium, other kinds of bacterial proteins are known to bind calcium, and calcium regulates the expression of transcription factors in other organisms (2, 54, 57, 60). We are currently investigating the ability of CalR to bind calcium. Alternately, a calcium-pertinent coregulator might be involved or CalR could control transcription of a gene involved in calcium transport. Nevertheless, even in the absence of the CalR protein, calR gene expression is regulated by calcium availability. Thus, other mechanisms promoting calcium-responsive gene expression must also exist.
In summary, calcium plays a vital signaling role in V. parahaemolyticus. We have identified regulators—LafK and CalR—that participate in coordinating this response during growth on a surface (Fig. 9), although how calcium works to promote global changes in gene expression is not clear. Millimolar concentrations of calcium could act intracellularly or extracellularly to elicit changes that have consequences on the lifestyle of the organism. Iron availability also seems key to regulating activity of these gene sets. Although the environmental cues studied in this work are clearly of importance to a marine organism, the calcium concentrations used in these studies also seem pertinent for a gastrointestinal pathogen. The calcium level in the human intestine is reported to be between ∼0.5 and 4.5 mM (49, 55). Iron limitation as well seems a relevant signal for a gastrointestinal pathogen (5, 28, 34). Numerous additional environmental conditions most certainly also converge to regulate swarming and type III secretion. Deciphering the relevant signals, including surface contact-elicited responses, host-specific factors, and the way in which diverse signals become integrated at the level of gene control networks, will provide important clues for understanding the particular ecological niches and mechanisms of pathogenesis of this organism.
FIG. 9.
Calcium regulates swarming and type III secretion. The availability of calcium affects global gene expression positively and negatively in V. parahaemolyticus. Two large gene sets displaying calcium-induced gene expression encode the swarming and T3SS1 regulons. This regulation is mediated in part by the calcium-repressed, LysR-type transcription factor CalR. Calcium represses calR transcription in CalR-dependent and -independent manners. calR mutants show enhanced swarming motility, laf gene expression, cytotoxicity, and T3SS1 gene expression. As a consequence, calR is repressed in high calcium, and swarming and virulence increase. The swarming regulator LafK is required to mediate a major portion of the T3SS1 response to calcium. In addition, evidence suggests a capacity for feedback regulation of swarming upon upregulation of exsA expression. Thus, during growth on a surface, calcium seems to act as a colonization signal influencing the capacity to swarm and be pathogenic; however, regulatory mechanisms also exist to link and modulate the expression of swarming and T3SS1 genes. Although not depicted, iron availability has been identified as an additional signal operating independently to influence expression of these two regulons; iron-limiting conditions induce swarming and T3SS1.
Supplementary Material
Acknowledgments
We thank David Weiss and Ryan Kustusch for help with the microarray data collection and Patrick Breheny for designing the analysis of the microarray data. We thank the Microbial Genetics Laboratory class for discovering some of the calcium-pertinent mutants and, in particular, Komal H. Shah for helping to elucidate the role of CalR. We also thank Jodi Enos-Berlage for many stimulating discussions and fruitful collaboration and Tim Yahr for his encouraging and critical interest.
This work was supported by NIH grant 5 R21 AI065526, NSF grant 0817593, and a Medical Research Initiative grant from the Carver College of Medicine. C.J.G.-P. was supported by NIH training grant 5 T32 GM077973 “Statistics in Microbiology, Infectious Diseases & Bioinformatics.”
Footnotes
Published ahead of print on 17 September 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Belas, R., M. Simon, and M. Silverman. 1986. Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus. J. Bacteriol. 167:210-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilecen, K., and F. H. Yildiz. 2009. Identification of a calcium-controlled negative regulatory system affecting Vibrio cholerae biofilm formation. Environ. Microbiol. 11:2015-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boles, B. R., and L. L. McCarter. 2000. Insertional inactivation of genes encoding components of the sodium-type flagellar motor and switch of Vibrio parahaemolyticus. J. Bacteriol. 182:1035-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brutinel, E. D., and T. L. Yahr. 2008. Control of gene expression by type III secretory activity. Curr. Opin. Microbiol. 11:128-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler, A. 2005. Marine microbial iron mobilization: new marine siderophores, abstr. U893. Abstr. Papers Am. Chem. Soc., vol. 229.
- 6.Caburlotto, G., M. Gennari, V. Ghidini, M. Tafi, and M. M. Lleo. 2009. Presence of T3SS2 and other virulence-related genes in tdh-negative Vibrio parahaemolyticus environmental strains isolated from marine samples in the area of the Venetian lagoon, Italy. FEMS Microbiol. Ecol. 70:506-514. [DOI] [PubMed] [Google Scholar]
- 7.Cheng, L. W., O. Kay, and O. Schneewind. 2001. Regulated secretion of YopN by the type III machinery of Yersinia enterocolitica. J. Bacteriol. 183:5293-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dasgupta, N., A. Ashare, G. W. Hunninghake, and T. L. Yahr. 2006. Transcriptional induction of the Pseudomonas aeruginosa type III secretion system by low Ca2+ and host cell contact proceeds through two distinct signaling pathways. Infect. Immun. 74:3334-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeBord, K. L., N. S. Galanopoulos, and O. Schneewind. 2003. The ttsA gene is required for low-calcium-induced type III secretion of Yop proteins and virulence of Yersinia enterocolitica W22703. J. Bacteriol. 185:3499-3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dominguez, D. C. 2004. Calcium signalling in bacteria. Mol. Microbiol. 54:291-297. [DOI] [PubMed] [Google Scholar]
- 11.Eichelberg, K., and J. E. Galan. 2000. The flagellar sigma factor FliA (sigma(28)) regulates the expression of Salmonella genes associated with the centisome 63 type III secretion system. Infect. Immun. 68:2735-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellermeier, J. R., and J. M. Slauch. 2008. Fur regulates expression of the Salmonella pathogenicity island 1 type III secretion system through HilD. J. Bacteriol. 190:476-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enos-Berlage, J. L., Z. T. Guvener, C. E. Keenan, and L. L. McCarter. 2005. Genetic determinants of biofilm development of opaque and translucent Vibrio parahaemolyticus. Mol. Microbiol. 55:1160-1182. [DOI] [PubMed] [Google Scholar]
- 14.Ferracci, F., F. D. Schubot, D. S. Waugh, and G. V. Plano. 2005. Selection and characterization of Yersinia pestis YopN mutants that constitutively block Yop secretion. Mol. Microbiol. 57:970-987. [DOI] [PubMed] [Google Scholar]
- 15.Francis, M. S., H. Wolf-Watz, and A. Forsberg. 2002. Regulation of type III secretion systems. Curr. Opin. Microbiol. 5:166-172. [DOI] [PubMed] [Google Scholar]
- 16.Henke, J. M., and B. L. Bassler. 2004. Quorum sensing regulates type III secretion in Vibrio harveyi and Vibrio parahaemolyticus. J. Bacteriol. 186:3794-3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiyoshi, H., T. Kodama, T. Iida, and T. Honda. 2010. Contribution of Vibrio parahaemolyticus virulence factors to cytotoxicity, enterotoxicity, and lethality in mice. Infect. Immun. 78:1772-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ide, T., S. Michgehl, S. Knappstein, G. Heusipp, and M. A. Schmidt. 2003. Differential modulation by Ca2+ of type III secretion of diffusely adhering enteropathogenic Escherichia coli. Infect. Immun. 71:1725-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaques, S., and L. L. McCarter. 2006. Three new regulators of swarming in Vibrio parahaemolyticus. J. Bacteriol. 188:2625-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joseph, S. W., R. R. Colwell, and J. B. Kaper. 1982. Vibrio parahaemolyticus and related halophilic Vibrios. Crit. Rev. Microbiol. 10:77-124. [DOI] [PubMed] [Google Scholar]
- 22.Kearns, D. B. 2010. A field guide to bacterial swarming motility. Nat. Rev. Microbiol. 8:634-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenny, B., A. Abe, M. Stein, and B. B. Finlay. 1997. Enteropathogenic Escherichia coli protein secretion is induced in response to conditions similar to those in the gastrointestinal tract. Infect. Immun. 65:2606-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kierek, K., and P. I. Watnick. 2003. The Vibrio cholerae O139 O-antigen polysaccharide is essential for Ca2+-dependent biofilm development in sea water. Proc. Natl. Acad. Sci. U. S. A. 100:14357-14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kodama, T., C. Yamazaki, K.-S. Park, Y. Akeda, T. Iida, and T. Honda. 2010. Transcription of Vibrio parahaemolyticus T3SS1 genes is regulated by a dual regulation system consisting of the ExsACDE regulatory cascade and H-NS. FEMS Microbiol. Lett. 283:176-181. [DOI] [PubMed] [Google Scholar]
- 26.Lenz, D. H., K. C. Mok, B. N. Lilley, R. V. Kulkarni, N. S. Wingreen, and B. L. Bassler. 2004. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell 118:69-82. [DOI] [PubMed] [Google Scholar]
- 27.Makino, K., K. Oshima, K. Kurokawa, K. Yokoyama, T. Uda, K. Tagomori, Y. Iijima, M. Najima, M. Nakano, A. Yamashita, Y. Kubota, S. Kimura, T. Yasunaga, T. Honda, H. Shinagawa, M. Hattori, and T. Iida. 2003. Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V. cholerae. Lancet 361:743-749. [DOI] [PubMed] [Google Scholar]
- 28.Markel, T. A., P. R. Crisostomo, M. J. Wang, C. M. Herring, K. K. Meldrum, K. D. Lillemoe, and D. R. Meldrum. 2007. The struggle for iron: gastrointestinal microbes modulate the host immune response during infection. J. Leukoc. Biol. 81:393-400. [DOI] [PubMed] [Google Scholar]
- 29.Martell, A., and R. Smith. 1982. Critical stability constants. Plenum Press, New York, NY.
- 30.McCarter, L., and M. Silverman. 1989. Iron regulation of swarmer cell differentiation of Vibrio parahaemolyticus. J. Bacteriol. 171:731-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarter, L., and M. Silverman. 1990. Surface-induced swarmer cell differentiation of Vibrio parahaemolyticus. Mol. Microbiol. 4:1057-1062. [DOI] [PubMed] [Google Scholar]
- 32.McCarter, L. L. 2004. Dual flagellar systems enable motility under different circumstances. J. Mol. Microbiol. Biotechnol. 7:18-29. [DOI] [PubMed] [Google Scholar]
- 33.McCarter, L. L. 1998. OpaR, a homolog of Vibrio harveyi LuxR, controls opacity of Vibrio parahaemolyticus. J. Bacteriol. 180:3166-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mekalanos, J. J. 1992. Environmental signals controlling expression of virulence determinants in bacteria. J. Bacteriol. 174:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 36.Murphy, E. R., and S. M. Payne. 2007. RyhB, an iron-responsive small RNA molecule, regulates Shigella dysenteriae virulence. Infect. Immun. 75:3470-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nair, G. B., T. Ramamurthy, S. K. Bhattacharya, B. Dutta, Y. Takeda, and D. A. Sack. 2007. Global dissemination of Vibrio parahaemolyticus serotype O3:K6 and its serovariants. Clin. Microbiol. Rev. 20:39-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naseem, R., K. T. Wann, I. B. Holland, and A. K. Campbell. 2009. ATP regulates calcium efflux and growth in E. coli. J. Mol. Biol. 391:42-56. [DOI] [PubMed] [Google Scholar]
- 39.Norris, V., S. Grant, P. Freestone, J. Canvin, F. N. Sheikh, I. Toth, M. Trinei, K. Modha, and R. I. Norman. 1996. Calcium signalling in bacteria. J. Bacteriol. 178:3677-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ono, T., K. S. Park, M. Ueta, T. Iida, and T. Honda. 2006. Identification of proteins secreted via Vibrio parahaemolyticus type III secretion system 1. Infect. Immun. 74:1032-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oomes, S. J., M. J. Jonker, F. R. Wittink, J. O. Hehenkamp, T. M. Breit, and S. Brul. 2009. The effect of calcium on the transcriptome of sporulating B. subtilis cells. Int. J. Food Microbiol. 133:234-242. [DOI] [PubMed] [Google Scholar]
- 42.Ordal, G. W. 1977. Calcium ion regulates chemotactic behaviour in bacteria. Nature 270:66-67. [DOI] [PubMed] [Google Scholar]
- 43.O'Shea, T. M., C. R. Deloney-Marino, S. Shibata, S. Aizawa, A. J. Wolfe, and K. L. Visick. 2005. Magnesium promotes flagellation of Vibrio fischeri. J. Bacteriol. 187:2058-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park, K. S., T. Ono, M. Rokuda, M. H. Jang, K. Okada, T. Iida, and T. Honda. 2004. Functional characterization of two type III secretion systems of Vibrio parahaemolyticus. Infect. Immun. 72:6659-6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Putnam, S. L., and A. L. Koch. 1975. Complications in the simplest cellular enzyme assay: lysis of Escherichia coli for the assay of beta-galactosidase. Anal. Biochem. 63:350-360. [DOI] [PubMed] [Google Scholar]
- 46.Sarkisova, S., M. A. Patrauchan, D. Berglund, D. E. Nivens, and M. J. Franklin. 2005. Calcium-induced virulence factors associated with the extracellular matrix of mucoid Pseudomonas aeruginosa biofilms. J. Bacteriol. 187:4327-4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schell, M. A. 1993. Molecular biology of the LysR family of transcriptional regulators. Annu. Rev. Microbiol. 47:597-626. [DOI] [PubMed] [Google Scholar]
- 48.Schroder, K. H. 1963. Stability of the ferric complex with EGTA. Acta Chem. Scand. 17:1509-1514. [Google Scholar]
- 49.Sheikh, M. S., A. Ramirez, M. Emmett, C. Santa Ana, L. R. Schiller, and J. S. Fordtran. 1988. Role of vitamin D-dependent and vitamin D-independent mechanisms in absorption of food calcium. J. Clin. Invest. 81:126-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soscia, C., A. Hachani, A. Bernadac, A. Filloux, and S. Bleves. 2007. Cross talk between type III secretion and flagellar assembly systems in Pseudomonas aeruginosa. J. Bacteriol. 189:3124-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stewart, B. J., and L. L. McCarter. 2003. Lateral flagellar gene system of Vibrio parahaemolyticus. J. Bacteriol. 185:4508-4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tisa, L. S., and J. Adler. 1995. Cytoplasmic free-Ca2+ level rises with repellents and falls with attractants in Escherichia coli chemotaxis. Proc. Natl. Acad. Sci. U. S. A. 92:10777-10781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vilches, S., N. Jimenez, J. M. Tomas, and S. Merino. 2009. Aeromonas hydrophila AH-3 type III secretion system expression and regulatory network. Appl. Environ. Microbiol. 75:6382-6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang, S. L., K. Q. Fan, X. Yang, Z. X. Lin, X. P. Xu, and K. Q. Yang. 2008. CabC, an EF-hand calcium-binding protein, is involved in Ca2+-mediated regulation of spore germination and aerial hypha formation in Streptomyces coelicolor. J. Bacteriol. 190:4061-4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wensel, R. H., C. Rich, A. C. Brown, and W. Volwiler. 1969. Absorption of calcium measured by intubation and perfusion of the intact human small intestine. J. Clin. Invest. 48:1768-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams, A. W., and S. C. Straley. 1998. YopD of Yersinia pestis plays a role in negative regulation of the low-calcium response in addition to its role in translocation of Yops. J. Bacteriol. 180:350-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolfgang, M. C., V. T. Lee, M. E. Gilmore, and S. Lory. 2003. Coordinate regulation of bacterial virulence genes by a novel adenylate cyclase-dependent signaling pathway. Dev. Cell 4:253-263. [DOI] [PubMed] [Google Scholar]
- 58.Wu, Z., R. Irizarry, R. Gentleman, F. Martinez-Murillo, and F. Spencer. 2004. A model-based background adjustment for oligonucleotide expression arrays. J. Am. Stat. Assoc. 99:909-917. [Google Scholar]
- 59.Yahr, T. L., and M. C. Wolfgang. 2006. Transcriptional regulation of the Pseudomonas aeruginosa type III secretion system. Mol. Microbiol. 62:631-640. [DOI] [PubMed] [Google Scholar]
- 60.Zhao, Y., Y. Shi, W. Zhao, X. Huang, D. Wang, N. Brown, J. Brand, and J. Zhao. 2005. CcbP, a calcium-binding protein from Anabaena sp. PCC 7120, provides evidence that calcium ions regulate heterocyst differentiation. Proc. Natl. Acad. Sci. U. S. A. 102:5744-5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou, X. H., D. H. Shah, M. E. Konkel, and D. R. Call. 2008. Type III secretion system 1 genes in Vibrio parahaemolyticus are positively regulated by ExsA and negatively regulated by ExsD. Mol. Microbiol. 69:747-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.