Abstract
Aim
To investigate whether the emulsification of conventional silicone oils can be reduced by adding small amounts of silicone molecules of a very long chain length.
Methods
Siluron 1000, Siluron 2000, Siluron 5000, Acri.Sil-Ol 5000, Oxane 5700, Densiron 68 LV, Densiron 68 and Densiron 68 HV (0.5 ml) were each tested along with either plasma or serum (0.5 ml) in a glass cuvette. Emulsification was induced by sonication and documented by photography. The total area of emulsified oil was assessed using the ImageJ software.
Results
The addition of small amounts of very-long-chain silicone molecules significantly reduced the emulsification for 1000 cSt silicone oil (Siluron 2000) and for 1000 cSt silicone oil with an admixture of F6H8 (Densiron 68 HV).
Conclusion
New low-viscosity silicone oils show a reduced emulsification similar to that of 5000 cSt oils. In future, it may be possible to avoid using 5000 cSt oils. The findings may foster silicone oil surgery in general, and in particular the application of small-incision techniques.
Keywords: Silicone oil, polydimethylsiloxane, emulsification, vitreous, treatment surgery
Introduction
A major problem inherent in the use of silicone oil as vitreous tamponades is emulsification.1 Emulsification can generate oil droplets that may cause secondary glaucoma, keratopathy and subjective disturbances, even after the silicone oil is removed.2–5
The emulsification of silicone oil is influenced by many factors including viscosity and interface tension.6 Highly viscous silicone oil tends to be more stable in terms of emulsification than less viscous oil.7 Consequently, surgeons—at least in Europe—prefer high-viscosity silicone oils (5000 cSt), accepting extended filling and removal times. On the other hand, less viscous silicone oils allow better handling when using small-gauge instruments (25- or 23-gauge). It is therefore desirable to develop silicone oils that are resistant to emulsification and of low viscosity.
Silicone oils are composed of polymers and hence show the characteristics of non-Newtonian fluids. This means that the viscosity changes along with the shear rate. The shear viscosity does not fully explain the emulsification tendency, however. By mixing a small amount of very-long-chain silicone molecules into common silicone oil, the extensional viscosity may be increased while more or less maintaining the shear viscosity.8–10 The addition of very-long-chain silicone molecules increases the resistance to extensional deformation and therefore increasing its extensional viscosity. This increases the resistance to emulsification.11
In this study, using an in vitro test system, we compared the emulsification of new silicone oils that contain very-long-chain silicone molecules (Siluron 2000 and Densiron 68 HV) and various commonly used silicone oils (Siluron 1000, Siluron 5000, Densiron 68 LV, Densiron 68, AcriSil-Ol 5000 and Oxane 5700).
Material and methods
The different silicone oils were assessed using a modification of an in vitro model originally developed by Savion et al.12 In this model, oil droplets similar in diameter to those in patient eyes were generated. Two different established emulsifiers were used: serum and plasma.12
Emulsifier preparation
Blood samples were taken in compliance with the Declaration of Helsinki; informed consent was obtained. The samples were taken from a young, healthy male individual on one day. Blood samples were taken using Sarstedt tubes (S-Monovette, Sarstedt) filled with EDTA (for plasma samples) or with gel platelets (for serum samples). The blood samples were centrifuged at 1000 g for 10 min at 4°C. The supernatant was carefully collected (not disturbing the buffy coat) and stored at −80°C until use.
Silicone oil compositions
The compositions of the various silicone oils are summarised in table 1.
Table 1.
Compositions of various silicone oils
| Silicone oil | Composition |
| Siluron 1000 | PMDS 1000 mPas (MW 36 760 g/mol) |
| Siluron 2000 | 5% high-molecular-weight silicone oil (423 K PMDS)+95% Siluron 1000 |
| Siluron 5000 | PMDS 5000 mPas (MW 65 025 g/mol) |
| Acri.Sil-Ol 5000 | PMDS 5000 mPas |
| Oxane 5700 | PMDS 5700 mPas |
| Densiron 68 LV | 30.5% SFA+69.5% Siluron 1000 |
| Densiron 68 | 30.5% SFA+69.5% Siluron 5000 |
| Densiron 68 HV | 30.5% SFA+69.5% of (10% Sil 2500000+90% Siluron 1000) |
MW, molecular weight; PMDS, polydimethysiloxane; SFA, semifluorinated alkane.
Silicone oil emulsification procedures
The emulsifier (0.5 ml) was placed into a glass cuvette with a flat base (inner dimensions: 4×10×40 mm). Silicone oil (0.5 ml) was added carefully to allow phase separation. The whole glass cuvette was then centrifuged at 5000 g to eliminate air bubbles. Since the emulsification procedures occur in the border between the two phases, no air bubbles were involved. The following silicone oils were used: Siluron 1000 (1000 mPas, Lot SIL1 170708), Siluron 2000 (2000 mPas, Lot SIL2 120808), Siluron 5000 (5000 mPas, Lot SIL5 191007), Acri.Sil-Ol 5000 (5000 mPas, Lot 3282), Oxane 5700 (5700 mPas, Lot 31908), Densiron 68 LV (300 mPas, Lot D68-LV 120207), Densiron 68 (1400 mPas, Lot D68 101207) and Densiron 68 HV (1300 mPas). Siluron 1000, Siluron 2000, Siluron 5000, Densiron 68 LV, Densiron 68 and Densiron 68 HV were provided by Fluoron GmbH (Neu-Ulm, Germany). Acri.Sil-Ol 5000 was obtained from Acri.Tec GmbH (Hennigsdorf, Germany), and Oxane 5700 was obtained from Bausch & Lomb Surgical (Berlin, Germany).
The test was performed in duplicate for each oil type and for each emulsifier. For the negative control, the emulsifier was replaced with distilled water. The glass cuvette was held in the centre of a sonication device (Sonorex TK 30, Bandelin). The water in the sonication device was kept at 20–24°C. Sonication was performed for 3 min to induce emulsification. Thereafter, the glass cuvette was centrifuged at 5000 g for 30 min.
Assessment of the emulsification
The emulsification was assessed every 10 min after centrifugation. The cuvette was photographed together with a ruler on its side, and the amount of emulsified oil was then measured on the photograph using the ImageJ software (National Institutes of Health, Bethesda, Maryland). The ratio of emulsified silicone oil was determined by comparing with the whole area of the aqueous phase, emulsified oil and non-emulsified oil. The emulsification area as a percentage was calculated: emulsification area (%)=(area of emulsified oil/total area)×100. Total area=area of aqueous area+area of emulsified oil+area of non-emulsified oil. Each sample (n=2) was measured 10 times. The mean ratio was calculated from all 10 measurements. All data were calculated from the emulsification area after 30 min of centrifugation.
Statistical methods
For statistical analysis, a t test was performed using the NCSS (number cruncher statistical system) software (version 2004). The means of all 10 measurements were used. p Values <0.05 were considered to be statistically significant. All values are shown as mean±SD.
Results
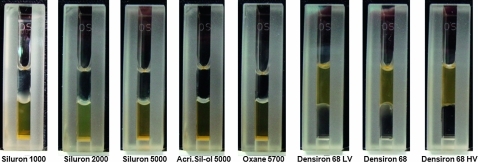
In the negative control using distilled water, a small amount of oil droplets was detected after sonication; the oil droplets disappeared after centrifugation. After the emulsification procedure using silicone oils with an emulsifier, three phases were seen in the glass cuvettes (figure 1). An aqueous phase at the bottom, emulsified silicone oil in the middle and non-emulsified silicone oil on top were seen for Siluron 1000, Siluron 2000, Siluron 5000, AcriSil-Ol 5000 and Oxane 5700. Heavier-than-water silicone oils showed non-emulsified silicone oil at the bottom, emulsified silicone oil in the middle and an aqueous phase on top. During the sonication procedures, the emulsified oil bubbles are created at the border between the aqueous and oil phase. No air bubbles were involved in this process. The emulsification area was assessed every 10 min after centrifugation, and no statistical difference was found among them. Therefore, all data shown use the data after 30 min of centrifugation.
Figure 1.
Emulsification of various silicone oils using plasma as emulsifier. When testing lighter-than-water silicone oils, three phases can be seen: non- emulsified silicone oil (top), emulsified silicone oil (middle) and aqueous phase (bottom). When testing heavier-than-water silicone oils, the aqueous phase was on top, emulsified silicone oil in the middle and non-emulsified oil at the bottom. Densiron 68 LV emulsifies easily and somewhat disperses, creating a hazy appearance, as compared with prior sonication.
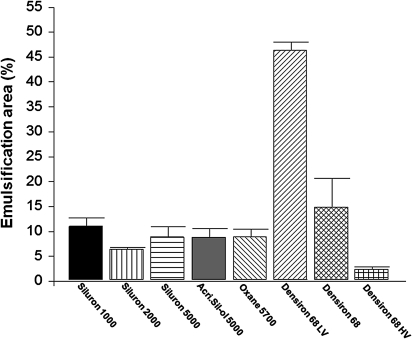
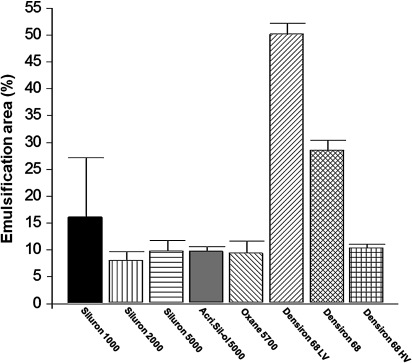
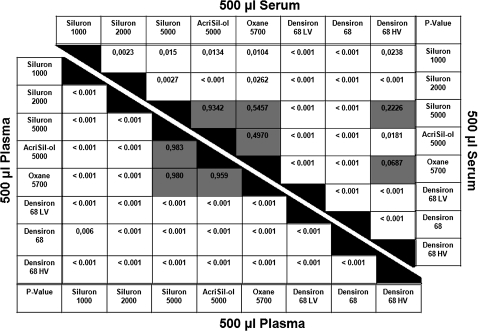
Overall, the emulsification areas of the silicone oils tested were higher in the serum than in the plasma group (table 2). The lowest emulsification areas were found for Siluron 2000 in the serum group (8.2%) and Densiron 68 HV in the plasma group (2.4%). A summary of the emulsification areas in both groups is presented in figures 2, 3. The p values of all groups are presented in figure 4. Overall, no statistical significance was seen between AcriSil-Ol 5000, Oxane 5700 and Siluron 5000. Compared with other lighter-than-water silicone oils, Siluron 2000 has less emulsification, which is also statistically significant. Densiron 68 HV has also less emulsification compared with other heavier-than-water silicone oils. In the serum group, Densiron 68 HV has about the same emulsification as Siluron 5000 and Oxane 5700.
Table 2.
Emulsification area (%; mean±SD) of silicone oils determined using an in vitro test
| Silicone oil | ||||||||
| Emulsifier | Siluron 1000 | Siluron 2000 | Siluron 5000 | AcriSil-Ol 5000 | Oxane 5700 | Densiron 68 LV | Densiron 68 | Densiron 68 HV |
| Plasma | 11.0±1.7 | 6.5±0.4 | 8.9±2.0 | 8.9±1.8 | 8.9±1.6 | 46.3±1.6 | 14.9±5.7 | 2.4±0.5 |
| Serum | 16.4±11.1 | 8.2±1.6 | 10.0±1.9 | 9.9±0.8 | 9.6±2.2 | 50.3±2.0 | 28.7±1.8 | 10.5±0.7 |
Figure 2.
Emulsification areas of various silicone oils using plasma as emulsifier.
Figure 3.
Emulsification areas of various silicone oils using serum as emulsifier.
Figure 4.
Probability level (p values) of differences between the silicone oils tested using plasma and serum as emulsifiers. Non-significant values are shown in grey shaded boxes.
Discussion
In this study, we present a quantitative analysis of the emulsification of silicone oil. We modified the method originally developed by Savion et al12 by using a glass cuvette with a flat base, which allowed us also to measure heavier-than-water silicone oils. We also determined the area of the emulsified oils instead of the height, which increased the precision of the test.
Siluron 5000, Acri.Sil-Ol 5000 and Oxane 5700 are the tamponade agents most frequently used in Germany and are considered the standard silicone oils against which all other oils are tested. Using our in vitro model, there were no statistically significant differences between these three oils with regard to their emulsifications (figure 4). As expected, a significantly higher degree of emulsification was found for 1000 cSt silicone oil (Siluron 1000). This difference was more prominent when using serum as emulsifier rather than plasma.
The difference between serum and plasma is the content of thrombocytes. Serum lacks thrombocytes, while plasma does not. Overall, a slightly higher emulsification was seen, using serum instead of plasma. One explanation for this might be the activation of the thrombocytes in the serum tubes during the coagulation process. During this thrombocytes secreted various coagulation proteins such as serotonin, platelet factor 4, platelet derived growth factor, fibrinogen, etc. These might serve as an emulsifier as well, explaining the slightly higher emulsification rate in the serum group. The role of each protein secreted in emulsification is still speculative though.
Siluron 2000 (1000 cSt silicone oil plus long-chain silicone oil) emulsified significantly less than Siluron 1000 (1000 cSt conventional silicone oil), and even less than Siluron 5000, Acri.Sil-Ol 5000 and Oxane 5700 (figures 3, 4). This shows that the emulsification can be reduced by adding very-long-chain silicone molecules. The shear viscosity of Siluron 1000 (1000 cSt) increased to 2000 cSt in the case of Siluron 2000.
Most objections against heavier-than-water silicone oil are based on the higher degree of emulsification of Densiron 68 (Siluron 5000+F6H8).1 13–15 We speculate that the addition of semifluorinated fluorocarbon not only renders silicone more hydrophilic but also makes it more susceptible to the splitting off of small droplets into an aqueous environment. It is fortunate that the addition of long-chain silicone oil molecules (Densiron 68 HV) to Densiron 68 LV (Siluron 1000+F6H8) significantly reduces emulsification compared with either Densiron 68 LV or Siluron 1000. When compared with the three 5000 cSt oils, Densiron 68 HV shows about the same emulsification in the serum group but shows a lower emulsification in the plasma group (figure 4). The shear viscosities of Densiron 68 (1400 mPas) and Densiron 68 HV (1300 mPas) are rather similar. One may suggest therefore giving preference to Densiron 68 HV over Densiron 68; studies on retinal tolerance are pending, however.
The higher the probability of emulsification for the conventional silicone oils, the more pronounced was the lowering effect of adding long chains of silicone. This effect is best shown when comparing Densiron 68 LV (mixture of Siluron 1000 and F6H8) with Densiron 68 HV (mixture of Siluron 1000, F6H8, and very-long-chain silicone molecules). The emulsification of Densiron 68 HV is lower, and the difference to Densiron 68 LV is strikingly significant. We conclude that adding small amounts of very-long-chain silicone oil molecules has a large influence on reducing the emulsification. The reason for this is the increased extensional viscosity of a non-Newtonian fluid, which determines the propensity for emulsification.
The in vitro results presented here require verification by future studies using an animal model. Still, some difficulties using animal models for testing emulsification and toxicity of silicone oil are yet to be overcome. In the in vivo model, various amounts of emulsifiers or various intensity of eye movement in the animal eye might produce different amounts of emulsification for the same silicone oil. Also, no objective method is currently known for quantifying emulsification in vivo. In terms of toxicity, models in some animals, such as rabbits, have been shown to be inadequate. This is because of the inability to induce complete posterior vitreous detachment, causing the retina to be protected from the silicone oil by the rest of the vitreous.16 Proper animal models for testing emulsification and toxicity are still to be developed. Nevertheless, we show in this paper that the emulsification of silicone oils can be influenced by adding very-long-chain silicone oil. The in vitro model shown in this paper is even more important, since it shows that with same amount of emulsifier and same energy used for inducing emulsification, there is less emulsification with Siluron 2000 and Densiron 68 HV than with other silicone oils. This is an important step before moving on to in vivo models. Also, in an in vivo model, different proportions of oil and emulsifiers would be expected.
In conclusion, using an in vitro technique, we quantified the emulsification of various silicone oils. The method presented in this study provides a very effective tool for the accurate determination of emulsification and comparison of different silicone oils. We also showed that, by adding long-chain silicone oil molecules, the emulsification of silicone oil can be reduced, as in the case of Siluron 2000 and Densiron 68 HV.
Footnotes
Funding: Supported in part by the Forschung für das Sehen eV.
Competing interests: Materials, that is silicone oils, were provided by Fluoron GmbH, Neu-Ulm, Germany.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Engelmann K, Herbrig E. [Different endotamponade agents and their clinical indications]. Klin Monbl Augenheilkd 2008;225:138–45 [DOI] [PubMed] [Google Scholar]
- 2.Blodi FC. Injection and impregnation of liquid silicone into ocular tissues. Am J Ophthalmol 1971;71:1044–51 [DOI] [PubMed] [Google Scholar]
- 3.Kirchhof B, Tavakolian U, Paulmann H, et al. Histopathological findings in eyes after silicone oil injection. Graefes Arch Clin Exp Ophthalmol 1986;224:34–7 [DOI] [PubMed] [Google Scholar]
- 4.Manschot WA. Intravitreal silicone injection. Adv Ophthalmol 1978;36:197–207 [PubMed] [Google Scholar]
- 5.Ni C, Wang WJ, Albert DM, et al. Intravitreous silicone injection. Histopathologic findings in a human eye after 12 years. Arch Ophthalmol 1983;101:1399–401 [DOI] [PubMed] [Google Scholar]
- 6.Joussen AM, Wong D. The concept of heavy tamponades-chances and limitations. Graefes Arch Clin Exp Ophthalmol 2008;246:1217–24 [DOI] [PubMed] [Google Scholar]
- 7.Heidenkummer HP, Kampik A, Thierfelder S. Experimental evaluation of in vitro stability of purified polydimethylsiloxanes (silicone oil) in viscosity ranges from 1000 to 5000 centistokes. Retina 1992;12(3 Suppl):S28–32 [DOI] [PubMed] [Google Scholar]
- 8.Barnes HA. Handbook of elementary rheology. Wales: University of Wales, Institute of non-Newtonian Fluid Mechanics, 2000:204 [Google Scholar]
- 9.Landau LD, Lifhitz EM. Fluid mechanics (course of theoretical physics). 2nd edn Oxford, UK: Butterworth-Heinemann, 1987:552 [Google Scholar]
- 10.Happel J, Brenner H. Low Reynolds number hydrodynamics: with special applications to particulate media (mechanics of fluids and transport processes). 1st edn The Hague: Martinus Nijhoff Publishers, 1973:570 [Google Scholar]
- 11.Williams RL, Day M, Garvey MJ, et al. Increasing the extensional viscosity of silicone oil reduces the tendency for emulsification. Retina 2009 [DOI] [PubMed] [Google Scholar]
- 12.Savion N, Alhalel A, Treister G, et al. Role of blood components in ocular silicone oil emulsification. Studies on an in vitro model. Invest Ophthalmol Vis Sci 1996;37:2694–9 [PubMed] [Google Scholar]
- 13.Heimann H, Stappler T, Wong D. Heavy tamponade 1: a review of indications, use, and complications. Eye 2008;22:1342–59 [DOI] [PubMed] [Google Scholar]
- 14.Majid MA, Hussin HM, Biswas S, et al. Emulsification of Densiron-68 used in inferior retinal detachment surgery. Eye 2008;22:152–7 [DOI] [PubMed] [Google Scholar]
- 15.Lai WW, Wong D, Li KK, et al. Emulsification and inverted hypopyon formation of oxane HD in the anterior chamber. Graefes Arch Clin Exp Ophthalmol 2008;246:1633–5 [DOI] [PubMed] [Google Scholar]
- 16.Mackiewicz J, Maaijwee K, Luke C, et al. Effect of gravity in long-term vitreous tamponade: in vivo investigation using perfluorocarbon liquids and semi-fluorinated alkanes. Graefes Arch Clin Exp Ophthalmol 2007;245:665–75 [DOI] [PubMed] [Google Scholar]