Abstract
Modifications in the timing and dosage of immunosuppression can ameliorate the morbidity and mortality that has prevented widespread use of intestinal transplantation (ITx) in children. Thirty-six patients receiving ITx, aged 5 months to 20 years were given 2–3 mg(kg of rabbit anti-thymocyte globulin (rATG, thymoglobulin®) just before ITx, and 2–3 mg(kg postoperatively (total 5 mg(kg). Twice daily doses of tacrolimus (TAC) were begun enterally within 24 h after graft reperfusion with reduction of dose quantity or frequency after 3 months. Prednisone or other agents were given to treat breakthrough rejection. After 8–28 months follow-up (mean 15.8 ± 5.3), 1- and 2-year patient and graft survival is 100% and 94%, respectively. Despite a 44% incidence of acute rejection in the first month, 16 of the 34 (47%) survivors are on TAC (n = 14) or sirolimus (n = 2) monotherapy; 15 receive TAC plus low dose prednisone; one each receive TAC plus sirolimus, TAC plus azathioprine and TAC plus sirolimus and prednisone. There was a low incidence of immunosuppression-related complications. This strategy of immunosuppression minimized maintenance TAC exposure, facilitated the long-term control of rejection, decreased the incidence of opportunistic infections, and resulted in a high rate of patient and graft survival.
Keywords: Anti-thymocyte globulin, intestinal failure, intestinal transplantation, pediatric transplantation, short gut syndrome, tacrolimus
Introduction
Intestinal transplantation (ITx) has become the standard of care for children with intestinal failure who have complications of total parenteral nutrition (TPN) (1–3). As with other whole organs, short- and long-term survival is dependent on surgical technique, preservation technology and adequate immunosuppressive therapy. The immunosuppression must be sufficient to allow early graft survival. Yet, ultimate outcomes depend on the extent to which maintenance drug therapy is needed. Too much long-term immunosuppression is associated with drug toxicity, infections and tumors (4).
The cornerstone of immunosuppression for ITx has been TAC plus prednisone (1–4). In an effort to eliminate rejection, short courses of ‘third’ agents have been added in many centers (e.g. cyclophosphamide, anti-lymphocyte globulin (ALG), and more recently, daclizumab) and(or long-term maintenance has been augmented with azathioprine (AZA), mycophenolate mofetil (MMF) or sirolimus (SIR) (1). Although some of these protocols have yielded a very low incidence of rejection and marginally higher early patient and graft survival, they have been difficult to evaluate because of limited case numbers and short follow-up. Because of an unacceptable rate of early and late mortality and morbidity from drug toxicity per se, or from the well-known complications of global immune depression (e.g. infections and malignant tumors) (1,4), the 1- and 2-year survival of pediatric recipients of intestine has been 54% and 40% in registry compilations (International Intestinal Transplant Registry, May 31, 2003) (5).
It has been suggested that the conventional use of multiagent prophylactic immunosuppression could have the self-defeating effect of interdicting natural tolerogenic mechanisms, thereby making the patient permanently dependent on heavy maintenance treatment (6). To avoid this self-defeating effect of immunosuppression, two modifications in policy were instituted (7). First, rATG was administered peri-operatively instead of the usual practice of giving a course of 5–10 smaller daily postoperative doses. Our objective was to reduce global T cell immunity before exposure to donor antigen, and to thereby bring the anticipated donor-specific response into a more readily controllable range (6,7). The lymphoid depletion also made it practical to give much less post-transplant immunosuppression than before. The results with this strategy for ITx have been encouraging.
Material and Methods
Patient population
All 36 intestinal recipients at our center between March 2002 and November 2003 were included. The mean age at transplantation was 7.2 ±6.5 years (range 0.5–20 years); 61 % were male and 39% were female. The indications for ITx are listed in Table 1. Two (6%) of the 36 patients had a previous failed ITx. One of these failures was due to chronic rejection 6 years after a primary ITx alone. In the other, a multivisceral graft had been transplanted in a 17-year-old boy. At the age of 20, the graft was lost when an aortic conduit graft aneurysm was found to be unresectable, necessitating urgent retransplantation.
Table 1.
Indications for intestinal transplantation
| Short gut syndrome 21 patients (58%) | |
| Volvulus | 11 |
| Intestinal atresia | 5 |
| Gastroschisis | 5 |
| Dysmotility disorders 10 patients (27%) | |
| Pseudoobstruction | 6 |
| Hirschsprung’s | 3 |
| Hypoperistalsis syndrome | 1 |
| Intestinal mucosal dysfunction 2 patients (6%) | |
| Microvillus inclusion disease | 2 |
| Previous ITx failure 2 patients (6%) | 2 |
| Budd Chiari disease 1 patient (3%) | 1 |
Patients requiring intestine-only transplantation were all UNOS (United Network for Organ Sharing) status I. The 18 patients whose allografts included a liver as well as the intestine had a mean PELD (pediatric end-stage liver disease) score at transplant of 23; 13 of the 18 were status I due to uncontrollable gastrointestinal bleeding. The allografts consisted of: small intestine only (n = 16), combined liver/intestine/pancreas (n = 12), all intraabdominal organs except colon and spleen (multivisceral, n = 6), the entire gastrointestinal tract without the liver (modified multivisceral, n = 1) and intestine and pancreas (n = 1). All donors were cadaveric, ABO identical and selected without regard for HLA match. Five patients (14%) had a positive cross-match. No ex vivo irradiation or other alteration of the donor organs was done: e.g. they were not irradiated or subjected to lymphoid depletion. Seven recipients (19%) received donor bone marrow augmentation (8). There was no selection based on CMV status of donor or recipient (there were seven CMV negative recipients who received a CMV-positive allograft).
Immunosuppression
rATG
All patients received a total of 5 mg/kg of rabbit anti-thymocyte Globulin (rATG, Thymoglobulin®). The potency and other characteristics of this broadly reacting polyclonal preparation are similar to other antilymphoid globulins (ALGs) that were first used clinically in 1966 (9). However, the T cell specificity is higher, and lot-to-lot reproducibility of the currently available rATG (Thymoglobulin®) has been greatly improved and used in steroid-sparing regimens (10–13). The half-life of the circulating thymoglobulin is approximately 7 days (14). Recovery of the depleted lineages begins in 1–2 weeks after a divided infusion of 5 mg/kg.
In adults, the infusion of 5 mg/kg rATG can be given over 4–6 h and can be completed before organ reperfusion. Because of concern that this rate of infusion would be too fast in children, the 5 mg/kg was routinely split into pre- and post-transplant doses. The first dose of 2–3 mg/kg was infused intravenously over 6–8 h before allograft reperfusion. Intravenous dexamethasone (0,4 mg/kg) was given as pre-medication for prophylaxis against possible cytokine release syndrome. As soon as the operation was completed and the patient was moved to the intensive care area, whatever remained of the 5 mg/kg (2–3 mg/kg) was infused over 6–8 h under a second umbrella of dexamethasone.
TAC
After the first postoperative day, maintenance monotherapy immunosuppression with TAC was begun enterally at a dose of 0.1 mg/kg every 12 h. Twelve-hour TAC trough levels of 10–15 ng/mL usually were achieved by about 3 days. The 12-h target trough blood level of 10–15 ng/mL was maintained for 3 months post-transplant, after which levels of 5–10 ng/mL were sought. The use of sirolimus or azathioprine was considered for patients with TAC-related complications.
For patients who were stable on twice daily oral dosing of TAC with no recent episodes of rejection, a ‘minimization’ strategy was implemented at three post-transplant months, with the objective of reducing TAC maintenance doses from two to one dose per day, or potentially to every other day. Overall management involved three steps.
Step 1 required that the patients were well enough to reliably ingest twice daily oral doses of 0.1 mg/kg. Throughout the first 3 months, doses were adjusted to achieve TAC levels of 10–15 ng/mL. No attempt was made to alter the dose timing until at least 3 months, or longer if the patients were not yet stable at 3 months. In step 2, patients who were stable on twice daily TAC dosing were changed to once daily oral doses. This was done by incorporating the twice daily doses into a single dose. For example, a patient who previously was receiving 2 mg TAC in the morning and evening would be given 4 mg as a single oral daily dose. Subsequent amounts were adjusted to achieve 24-h trough TAC levels of 5–10 ng/mL. Patients were followed carefully with appropriate laboratory parameters and clinical assessment every 2 weeks.
In step 3, a patient who was stable after 2 months on once per day dosing was considered to be a candidate for every other day treatment. For example, if the patient was receiving 2 mg once a day, the regimen change would be to 2 mg every other day. Patents were followed carefully with appropriate laboratory and clinical parameters monitored every 2 weeks. There is no minimum TAC level for this stage, since they will vary and are generally non-detectable.
Infection prophylaxis
Intravenous ganciclovir was used in all patients for 2 weeks after transplantation for CMV prophylaxis. EBV viral load and pp65 CMV antigenemia monitoring (15) were routinely performed for all ITx recipients. This consisted of testing every 2 weeks during the first 3 months, monthly for 3 months, every 2–3 months for another 6 months and every 3–4 months thereafter. In the event of increases in EBV viral load, or of CMV antigenemia, modifications of immunosuppression and the use of ganciclovir(hyperimmunoglobulin was considered (15,16).
Diagnosis of rejection
In all patients, allograft ileoscopy and biopsy were performed twice a week for the first 2 weeks, once a week for the next 2 weeks and then once a month for 2 months. After the third post-transplant month, intestinal allograft biopsies were obtained according to clinical indication. All episodes of acute cellular rejection (ACR) were graded as mild, moderate or severe (17), Mild ACR was treated with a 6-day course of dexamethasone beginning with an infusion of 2 mg/kg dexamethasone on the first day. On the second day, 1 mg/kg was given, and on days 3–6, the doses were reduced to 0.8, 0.6, 0.4 and 0.2 mg/kg. As soon as steroid therapy could be provided orally, further treatment was with enteral prednisone. Steroid-resistant rejection or more severe episodes of first time rejection (such as moderate to severe histologic rejection, however, with evidence of significant ulceration at endoscopy) were treated with anti-lymphocyte antibody therapy (OKT3 or rATG). In addition, close monitoring of TAC levels was done in all cases of ACR to assure that trough levels of ≥15 ng/dL were achieved until the episode was resolved.
Diagnosis of graft versus host disease (GVHD)
With clinical suspicion of GVHD, an attempt was made to confirm the diagnosis with skin biopsy or other means including a search with flow cytometry for donor leukocyte macrochimerism in peripheral blood.
Determination of chimerism
In cases of a sex mismatch between donor and recipient, donor cells in the blood or tissues were identified by fluorescence in-situ hybridization (FISH) for X and Y chromosomes. Otherwise, immunostaining for donor HLA markers was done when possible, or as the percentage of recipient HLA-negative cells when appropriate antibodies for donor HLA were not available (18,19).
Statistical analysis
Actuarial patient and graft survival was determined with Kaplan-Meier methodology. Data are expressed as mean ±SD unless otherwise specified.
Results
Patient and graft survival
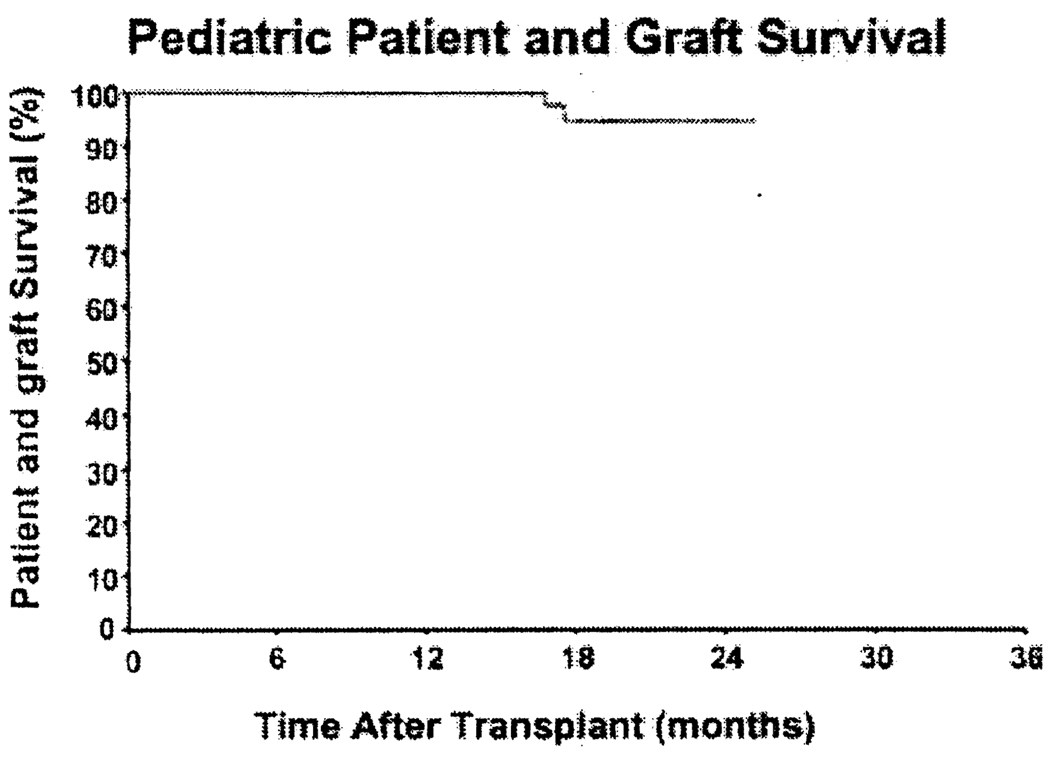
With a mean follow-up of 15.8 ±5.3 months (median 15.7; range 8–28), the actuarial patient and graft survival (Kaplan Meier) is 100% for the first year and 94% for the second year (Figure 1). The attempt to augment chimerism with adjunct bone marrow cells (20) in 7 (19%) of the 36 recipients had no identifiable influence on outcome.
Figure 1.
Pediatric patient and graft survival under rATG immunosuppresion.
One patient died with an anatomically(histologically normal and functioning graft 16 months after multivisceral transplant from catheter sepsis. The other death was from a post-transplant lymphoproliferative disorder (PTLD) 17 months after multivisceral transplantation.
Morbidity
The array of complications of ITx and the management of these problems in our early experience (2,4) and more recently (1) have been reported elsewhere. In a current example of a non-transplant complication, one of the patients with preexisting achalasia suffered a perforated esophagus during a laparoscopic procedure 8 months after transplantation. The injury required repair through open thoracotomy. A subsequent stricture is being treated with esophageal dilatation. Such non-transplant complications may require return to TPN for variable periods.
Renal function
Two of our 34 surviving recipients developed renal failure after 12 and 24 months from multifactorial causes that included years of intermittent sepsis and antibiotic treatment while on hyperalimentation; however, tacrolimus nephrotoxicity was assumed to be additive. These two patients are awaiting a kidney transplant. The mean creatinine level in the other 32 survivors is 0.62 ±0.35 mg/dL Four (12.5%) of these patients are being treated with a single hypertensive agent.
Pancreatic endocrine function
None of the patients developed diabetes mellitus.
Neurotoxicity
The incidence of neuropathic side effects has been zero.
Infections
There were 22 patients presenting positive blood cultures after transplantation: bacterial only (n = 15), bacterial and fungal (yeast organisms only) (n = 7). There were no cases of invasive fungal infection (soft tissue disease). Fifteen asymptomatic patients had positive blood tests for EBV (detected by PCR; n = 12) or CMV (detected by pp65 antigenemia; n = 3). These patients were treated preemptively (16). Clinical disease developed in only one of these patients. The exception was a recipient of a multivisceral allograft (CMV-positive donor and recipient) who developed CMV enteritis, which was controlled with ganciclovir. The patient subsequently died from a widely disseminated PTLD.
Intestinal graft function
Thirty-four patients (94% of recipients) are independent of TPN; two (6%) of the 34 currently obtain 50% of their nutritional support by infusion at 10 and 20 months post-transplant, respectively. These two patients are not yet considered to be treatment failures, first, because the grafted intestine appears histopathologically normal, and second, because we have had other examples of patients with extremely delayed graft function who subsequently recovered.
Ten of the 34 (29%) surviving patients are free of all fluid, electrolyte and nutritional support: i.e. they have no need for venous access. Another 22 (65%) receive no TPN, but require daily or every other day calorie-free infusions, either because of poor electrolyte absorption, or because of high enteric output. Most of these 22 patients still have ileostomies that either have not been closed, or cannot ever be closed (e.g. patients with Hirschsprung’s disease).
Rejection rate (30 days)
Within the first 30 days after ITx, 16 (44%) of the 36 patients experienced ACR which was mild in seven, moderate in eight and severe in one. The episodes occurred between 6 and 22 days (median 14).
Out of 18 patients who received a composite graft which included the liver, six patients presented with ACR of the intestinal component of the allograft; among these six rejectors of the intestinal allograft, two patients also presented simultaneous ACR of the liver component of the allograft (11 %). None of these 18 recipients of a composite graft had an isolated liver ACR. Eleven (69%) of the rejections responded to intravenous administration of dex-amethasone, while the other five (31%) required a 5-day course of muromonab-CD3 (OKT3). Of the patients requiring OKT3, three patients had gross and histologic evidence significant enough to require primary therapy with OKT3, and two patients received it after a course of intravenous steroids. Of the 18 patients with combined transplants, six experienced acute rejection of the intestinal graft. This was not statistically different from the 10 of 18 patients with acute rejection in the isolated intestinal grafts.
Rejection (after 30 days)
At 31–90 days intestinal rejection occurred in seven patients (three recurrent and four de novo), thus resulting in an overall rejection incidence of 55% at 90 days post-transplant. There were eight patients (three recurrent and five de novo) who presented rejection after 90 days. All but one episode was mild. These late rejections were treated in the same way as those occurring in the first month (see below under ‘Immunosuppression Dosing’). There has been no histopathologic or clinical evidence to date of chronic intestinal rejection.
Graft versus host disease
Histologically-proven GVHD, manifesting primarily as dermatitis, was seen in four patients (11 %) 1–2 months after transplantation (Table 2). Six additional patients (17%), who had peripheral blood donor chimerism measurable with flow cytometry, presented after 9 days to 2.5 months with non-specific signs and symptoms that included fever, arthralgias and skin reactions. Although skin biopsies did not permit an unqualified diagnosis of GVHD, their symptoms prompted testing for peripheral blood cell chimerism. The syndrome was attributed to graft versus host reactions (GVHR) (Table 3). None of the 10 patients had been given an adjunct bone marrow cell infusion. Asymptomatic patients were not tested for chimerism.
Table 2.
Patients presenting confirmed GVHD
| Pt# | Type ITx | Sex | Donor sex |
Donor BM |
Site of GVHD |
Presentation (Post-ITx days) |
Grade GVHD* |
Flow cytometry at symptoms** (Post-ITx days) |
Flow cytometry follow-up** (Post-ITx days) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | L/SB/P | M | M | N | Skin | 24 | I | 16.1 (21) | 2.5 (62) |
| 2 | L/SB/P | M | M | N | Skin | 23 | I | 14.1 (20) | 6.7 (64) |
| 3 | SB | F | M | N | Skin | 60 | II | 0.9 (27) | 3.5(47)/15(61) |
| 4 | SB | M | F | N | Skin | 30 | I | 6.8 (52) | ND |
Histologic classification of skin changes in GVHD (Grade I–IV).
Percentage of donor derived cells in peripheral blood of recipient in one or two separate measurements.
ND = not done.
Table 3.
Patients presenting with GVHR
| Pt# | Type ITx | Sex | Donor sex |
Donor BM |
Presentation (Post-ITx days) |
Flow cytometry* at symptoms (Post-ITx days) |
Flow cytometry* at follow-up (Post-ITx days) |
|---|---|---|---|---|---|---|---|
| 1 | SB | M | F | N | 32 | 6.3(14) | 0.0 (36) |
| 2 | SB | M | M | N | 72 | 16.1 (13) | 1.0(22)/0.0(356) |
| 3 | MV | M | F | N | 13 | 2.6(13) | 0.0 (300) |
| 4 | MV | M | M | N | 9 | 2.6 (9) | ND |
| 5 | SB | M | M | N | 10 | 2 (26) | ND |
| 6 | MV | F | F | N | 9 | 20.6 (27) | 35.9(35)727.1 (235) |
Percentage of donor derived cells in peripheral blood of these patients. ND = not done.
These patients did not meet criteria for a diagnosis of GVHD.
Treatment of the 10 patients was limited to the use of oral prednisone for symptomatic dermatitis. In addition, exposure to TAC was minimized by reducing the total dose by half and by administering the TAC as a single daily dose until 24-h trough levels were at or below 5 ng/mL. With this regimen, all skin rashes resolved despite the presence of up to 30% donor cells in peripheral blood. None of these 10 patients developed late rejection.
Immunosuppression dosing
First 3 months
At the end of 3 months, the mean trough level of the whole group was 10.1 ± 4.2 ng/mL. Thirty-five of the 36 patients were on TAC: 23 were on twice daily doses (trough level 11.5 ± 4.0 ng/mL) 11 were on one dose/day (trough 7.68 2.6 ng/mL) and one was on one dose every other day (trough 4.1 ng/mL). The 36th recipient had been converted to twice daily sirolimus because of TAC-associated hemolysis.
Throughout the 3 months, steroids were temporarily added only for treatment of biopsy-proved rejection of either the allograft intestine or liver allograft, or because of some other immunological event (e.g. GVHD). At the 3 month milestone, only six (17%) of the 36 recipients were on prednisone (range 2.5–10 mg/day). However, only 12 (33%) of the 36 patients were considered to have reached a stable state of minimal monotherapy: 11 on once per day TAC doses, and the 12th on an every other day schedule.
After 3 months
Patients who developed intestinal allograft rejection thereafter had highly individualized treatment, most commonly with prednisone, but in some cases with muromonab-CD3 or the brief addition of sirolimus or MMF. The ultimate goal was to reach a state of monotherapy. This was accomplished in 16 of the 34 patients. After 8–28 months, immunosuppression of the 34 surviving patients consists of TAC only (n = 14), TAC plus prednisone (n = 15), TAC plus sirolimus (n = 2), TAC plus AZA in one and TAC plus sirolimus and prednisone in one. The mean maintenance prednisone doses in the 15 patients who are not steroid free are 0.3 ±0.22 mg/kg. Those patients on TAC monotherapy (n = 14) are receiving it twice daily (n = 4), once daily (n = 9) and every other day (n = 1).
Discussion
The strategy reported here has been associated with improved patient and graft survival, and effective control of rejection, despite minimization of drug treatment during the first three postoperative months and subsequently. In addition, the incidence of drug toxicity was modest and there was a reduction of infections and malignancies. Relative to our historical experience with infants and children (1), the 3% incidence of CMV disease and PTLD has been dramatically reduced. The one case of PTLD, however, was severe and was refractory to rituximab infusions and radiation/chemotherapy.
The nature of ITx has seen a continuous decline in survival (principally within the first few years). Innovations in management strategies have addressed multiple surgical, clinical and immunosuppressive issues in sequential and at times overlapping periods. Consequently, developmental strides have been slow and incremental. The immunosuppressive strategy reported here incorporates an attempt to modify the immunosuppressive load within the first post-transplant year, and is measurable by the steroid-free rate and ability to minimize TAC dosing. Therefore, a rejection free rate of 56% and 45% at 30 and 90 days, respectively, reflects a significant improvement over our previous experience (1). These results must, however, be taken in light of the ‘uncontrolled series’ nature of the trial. Longer-term results and the possibility of future multicenter randomized trials may help to validate these initial observations. The incidence of late rejection (after 90 days) in eight (22%) patients, of which five were first time episodes, reflects the stability of this strategy, after the induction effects of the ATG have subsided. It is during this period that acceptance of the allograft under lower exposure levels of immunosuppression has occurred in over 50% of survivors. The impact of rejection at this stage is yet to be determined; however, the more ominous spectre of exfoliative on chronic rejection has not been seen.
Use of this protocol has required considerable flexibility of management. Factors influencing absorption and metabolism are highly variable at different stages after ITx. Consequently, dose adjustments are necessary at different stages of convalescence to avoid breakthrough rejection at one extreme, or toxic drug levels at the other. Nevertheless, the combination of lymphoid depletion at the outset followed by minimal maintenance immunosuppression has allowed the elimination of steroids in the majority of patients and minimal steroid dependence in most of the others.
Morbidity associated with this protocol included the occurrence of renal failure (6%) and hypertension (11 %), which reflect a combination of long-term illness, peri-operative fluid issues and drug toxicities which includes the use of tacrolimus. Since the induction phase TAC dosing parallels previous standard guidelines, any changes are expected to be modest. However, we are hopeful that the ability to minimize TAC dosing with this protocol will provide better long-term renal function. Blood-borne infection with bacteria and fungi was frequent (61 %). However, this is commonly seen after ITx for two principal reasons: the transplantation of a contaminated intestinal graft, and the need to maintain venous catheter access for periods of as long as 1 year. Consequently, the frequency of such infections would be expected to be similar under any protocol; reports by other centers have documented infection rates of 90%, with a significant mortality rate (21). It is difficult to ascribe the frequency observed in this series to the immunosuppressive protocol utilized herein; however, the occurrence of only one mortality from catheter associated sepsis is encouraging.
The true measure of the impact of immunosuppressive drug therapy remains the frequency and severity of infection with invasive fungi (not seen in this series) and viruses, principally EBV and CMV which occurred with a frequency of 33% and 8%, respectively. The majority of cases were asymptomatic and detected by routine viral monitoring, and were successfully treated preemptively. However, progression to life-threatening viral disease (PTLD) occurred in one patient (3%); this reflects a dramatic decrease from our previous experience (1). In an uncontrolled clinical trial, we can only speculate that such a beneficial effect may be due to this immunosuppressive protocol, i.e. the elimination of steroids in 47% of patients, the intent to minimize TAC exposure and a lower incidence of rejection. However, the rate of patients at risk (as revealed by a positive EBV-PCR) has remained comparatively unchanged (1). Consequently, intestinal transplant recipients must still be considered susceptible to developing EBV-related complications. Therefore, the beneficial effect of preemptive antiviral therapy continues to play an important role with ITx (16).
‘Intestinal failure requires the use of parenteral nutrition as long as it persists’ (22). Consequently, the standard for success after ITx has been freedom from TPN; this has been achieved in 95% of the recipients. Of concern, however, has been the need for intravenous fluid support, which has been required for up to 1 year after ITx, both historically and in this report. As with our previous cohorts of patients, the need for such support decreases over time (as the enteral nutritional intake is optimized), and as the patients progress to closure of their intestinal stomas. However, with improvements in short- and long-term survival, the ‘cure’ of intestinal failure will have to be measured by a higher standard which addresses this and other issues such as native gut dysmotility, pancreatitis, diabetes mellitus and quality of life. Consequently, we maintain cautious optimism, and realize that the standards of success must now take into account all levels of medical support. These will be criteria to assess both historic and future innovations.
The donor lymphoid population of the intestinal allograft and its fate in the recipient after ITx has been a point of continuous experimental study and clinical inquiry (23). The intestine contains a large and highly mobile lymphoid cell population which, after transplantation, are replaced by recipient cells. It has been suggested that such immunocytes may enhance intestinal allograft immunogenicity/alloreactivity, however, others have theorized such a phenomenon may be responsible for varying degrees of graft acceptance and even tolerance (24,25). The clinical translations of these phenomena have been rejection and GVHD. Historical concern about the threat of GVHD after ITx (26,27) prompted strategies to deplete the donor immunocyte population of the allograft or change its lineage composition (20). In clinical experience, however, the incidence of GVHD has been approximately 5% (28). The unexpected low rate may be explained by the reciprocal induction of clonal activation → exhaustion → deletion of coexisting immune competent donor and recipient cells (29).
In this context, the acute interaction of the two hema-tolymphopoietic cell populations is central to alloengraftment. Thus, the frequent presence in these patients of enough donor leukocyte chimerism to be detectable by flow cytometry, even when associated with clinical manifestations of GHVD, was not viewed with panic. The presence of GVHD was interpreted as achieving a level of ‘chimerism’ whereby minimization of TAC could be initiated as part of clinical management. In fact, the symptoms usually were readily controlled with steroids and/or by reducing the doses of baseline TAC immunosuppression. Patients with GVHR (non-specific signs and symptoms not diagnostic of GVHD) responded in a similar fashion. Long-term follow up of these and the asymptomatic patients (for which no chimeric data are available) will be necessary in order to assess the significance of persistent donor cells in the host. Likewise, allograft rejection, while occurring in up to 44% of children within the first month, has usually been sensitive to steroids and has not been associated with the development of chronic rejection. In fact, chronic intestinal allograft rejection has not yet been encountered in any of our patients treated with our current protocol. Though the significance of stable donor cell chimerism is currently unknown, we speculate that, in the context of this protocol, it may reflect donor-specific hyporesponsiveness; this has been reported by others (30).
Although the follow-up is still short, patient and graft survival to this point has improved relative to our historical experience and to that of multicenter registries. We postulate that our low incidence of infectious complications is primarily due to the reduced cumulative exposure to immunosuppression. The low rate of nephrotoxicity, new onset diabetes and hypertension also is consistent with this hypothesis.
In conclusion, perioperative lymphoid depletion may allow significant successful improvement in survival in children who require ITx. Engraftment can be achieved without the use of steroids as a maintenance drug in more than 50% of cases. Long-term follow-up and immune monitoring in these children may lead to further understanding of the immunologic events allowing for a minimization strategy. More importantly, however, further experience with long-term stability, as well as late and chronic rejection, is required.
References
- 1.Reyes J, Mazariegos GV, Bond GMD, et al. Pediatric intestinal transplantation: historical notes, principles and controversies. Pediatr Transplant. 2002;6:193–207. doi: 10.1034/j.1399-3046.2002.02003.x. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Elmagd K, Bond G, Reyes J, Fung JJ. Intestinal transplantation: a coming of age. Adv Surg. 2002;36:65–101. [PubMed] [Google Scholar]
- 3.Kaufman SS, Atkinson JB, Bianchi A, et al. Indications for pediatric transplantation: a position paper of the American Society of Transplantation. Pediatr Transplant. 2001;5:80–87. doi: 10.1034/j.1399-3046.2001.005002080.x. [DOI] [PubMed] [Google Scholar]
- 4.Todo S, Reyes J, Furukawa H, Abu-Elmagd K, et al. Outcome analysis of 71 clinical intestinal transplantations. Ann Surg. 1995;222:270–282. doi: 10.1097/00000658-199509000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Transplant Registry. 2003 May 31; ( www.lhsc.on.ca/itr )
- 6.Starzl TE, Zinkemagel R. Transplantation tolerance from a historical perspective. Nat Rev Immunol. 2001;3:2339. doi: 10.1038/35105088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Starzl TE, Murase N, Abu-Elmagd K, et al. Tolerogenic immunosuppression for organ transplantation. Lancet. 2003;361:1502–1510. doi: 10.1016/s0140-6736(03)13175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abu-Elmagd K, Reyes J, Todo S, et al. Clinical intestinal transplantation: new perspectives and immunologic considerations. J Am Coll Surg. 1998;186:512–527. doi: 10.1016/s1072-7515(98)00083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starzl TE, Porter KA, Iwasaki Y, Marchioro TL, Kashiwagi N. In: The use of antilymphocyte globulin in human renal homotranspiantation. Wolstenholme GEW, O’Connor M, editors. London: Antilymphocytic serum J and A Churchill Limited; 1967. p. 4. [Google Scholar]
- 10.Brennan DC, Flavin K, Lowell J, et al. A randomized, double-blinded comparison of Thymoglobulin vs ATGAM for induction immunosuppression therapy in adult renal transplant recipients. Transplantation. 1999;67:1011–1018. doi: 10.1097/00007890-199904150-00013. [DOI] [PubMed] [Google Scholar]
- 11.Agha IA, Rueda J, Alvarez A, et al. Short course induction immunosuppression with Thymoglobulin for renal transplant recipients. Transplantation. 2002;73:473–475. doi: 10.1097/00007890-200202150-00025. [DOI] [PubMed] [Google Scholar]
- 12.Buchler M, de Ligny BH, Madec C, Lebranchu Y. Induction therapy by anti-thymocute globulin (rabbit) in renal transplantation: a 1-yr follow-up of safety and efficacy. Clin Transplant. 2003;17:539–545. doi: 10.1046/j.1399-0012.2003.00102.x. [DOI] [PubMed] [Google Scholar]
- 13.Eason JD, Nair S, Cohen AJ, Blazek JL, Loss GE., Jr Steroid-free liver transplantation using rabbit antitymocyte globulin and early tacrolimus monotherapy. Transplantation. 2003;75:1396–1399. doi: 10.1097/01.TP.0000062834.30922.FE. [DOI] [PubMed] [Google Scholar]
- 14.Mueller TF. Thymoglobulin: an immunologic overview. Current Opin Organ Transplant. 2003;8:305–312. [Google Scholar]
- 15.Rowe D, Weber S, Schauer EM, Reyes J, Green M. Epstein-Barr virus load monitoring: its role in the prevention and management of post-transplant lymphoproliferative disease. Transpl Infect Dis. 2001;3:79–87. doi: 10.1034/j.1399-3062.2001.003002079.x. [DOI] [PubMed] [Google Scholar]
- 16.Green M, Reyes J, Weber S, Rowe D. The role of antiviral and immunoglobulin therapy in the prevention of Epstein-Barr virus and post-transplant lymphoproliferative disease following solid organ transplantation. Transpl Infect Dis. 2001;3:97–103. doi: 10.1034/j.1399-3062.2001.003002097.x. [DOI] [PubMed] [Google Scholar]
- 17.White FV, Reyes J, Jaffe R, Yunis EJ. Pathology of intestinal transplantation in children. Am J Surg Pathol. 1995;19:687–698. doi: 10.1097/00000478-199506000-00009. [DOI] [PubMed] [Google Scholar]
- 18.van Tol MJD, Langlois van den Bergh R, Mesker W, et al. Simultaneous detection of X and Y chromosomes by two-color fluorescence in situ hybridization in combination with immunophenotyp-ing of single cells to document chimerism after sex mismatched bone marrow transplantation. Bone Marrow Tranpl. 1998;21:497–503. doi: 10.1038/sj.bmt.1701122. [DOI] [PubMed] [Google Scholar]
- 19.Metes D, Logar A, Rudert WA, et al. Four-color cytometric analysisi of peripheral blood donor cell chimerism. Hum Immunol. 2003;64:787–795. doi: 10.1016/s0198-8859(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abu-Elmagd K, Reyes J, Bond G, et al. Clinical intestinal transplantation: a decade of a single center experience. Ann Surg. 2001:404–416. doi: 10.1097/00000658-200109000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loinaz C, Kato T, Nishida S, et al. Bacterial infections after intestinal and multivisceral transplantation. Transplant Proc. 2003;35:1929–1930. doi: 10.1016/s0041-1345(03)00728-0. [DOI] [PubMed] [Google Scholar]
- 22.Goulet O, Jan D. Intestinal failure: causes and management in children. Curr Opin Transplantation. 2004;9:192–200. [Google Scholar]
- 23.Fryer JP, Newell KA. Small bowel transplantation: a work in progress. Current Opin Organ Transplant. 2004;9:225–232. [Google Scholar]
- 24.Frezza EE, Gerunda GE, Fassina A, et al. NK activity during graft-versus-host disease and graft rejection in rats following intestinal semiallogenic and allogenic transplantation with or without mesenteric lymphadenectomy. Transplantation. 1994;58:698–701. [PubMed] [Google Scholar]
- 25.Loffeler S, Meyer D, Otto C, et al. Different kinetics of donor cell populations after isolated liver and combined liverfsmall bowel transplantation. Transpl Int. 2000;13 Suppl. 1:S537. doi: 10.1111/j.1432-2277.2000.tb02099.x. [DOI] [PubMed] [Google Scholar]
- 26.Monchik GJ, Russell PS. Transplantation of small bowel in the rat: technical and immunological considerations. Surgery. 1971;70:693–702. [PubMed] [Google Scholar]
- 27.Murase N, Demetris AJ, Woo J, et al. Graft-versus-host disease after brown Norway-to-Lewis and Lewis-to-Brown Norway rat intestinal transplantation under FK506. Transplantation. 1993;55:1–7. doi: 10.1097/00007890-199301000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazariegos GV, Abu-Elmagd K, Jaffe R, et al. Graft versus host disease in intestinal transplantation. Am J Transplant. 2004;4:1459–1465. doi: 10.1111/j.1600-6143.2004.00524.x. [DOI] [PubMed] [Google Scholar]
- 29.Starzl TE, Demetris AJ, Murase N, lldstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pirenne J, Koshiba T, Geboes K, et al. Complete freedom from rejection after intestinal transplantation using a new tolerogenic protocol combined with low immunosuppression. Transplantation. 2002;73:966–968. doi: 10.1097/00007890-200203270-00024. [DOI] [PubMed] [Google Scholar]