Abstract
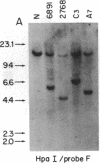
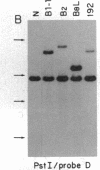
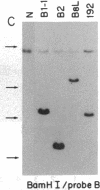
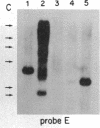
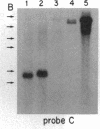
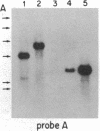
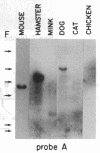
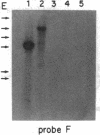
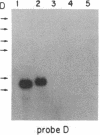
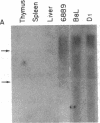
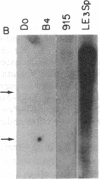
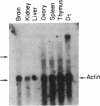
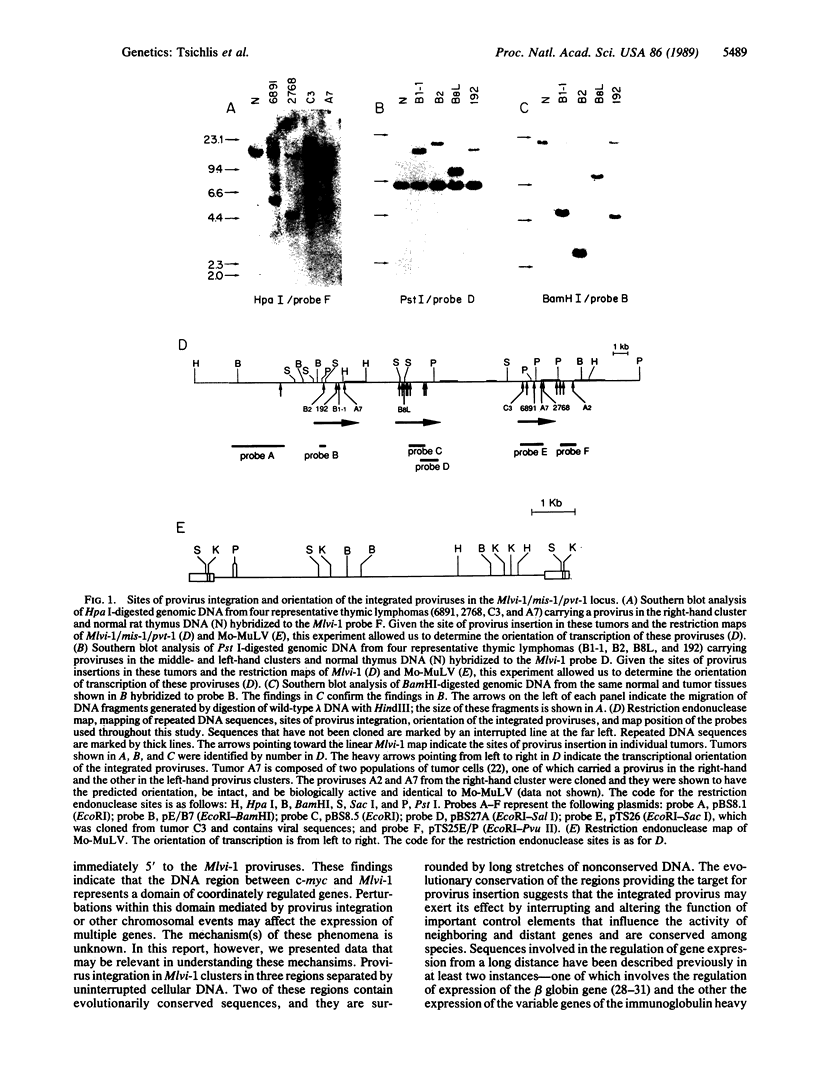
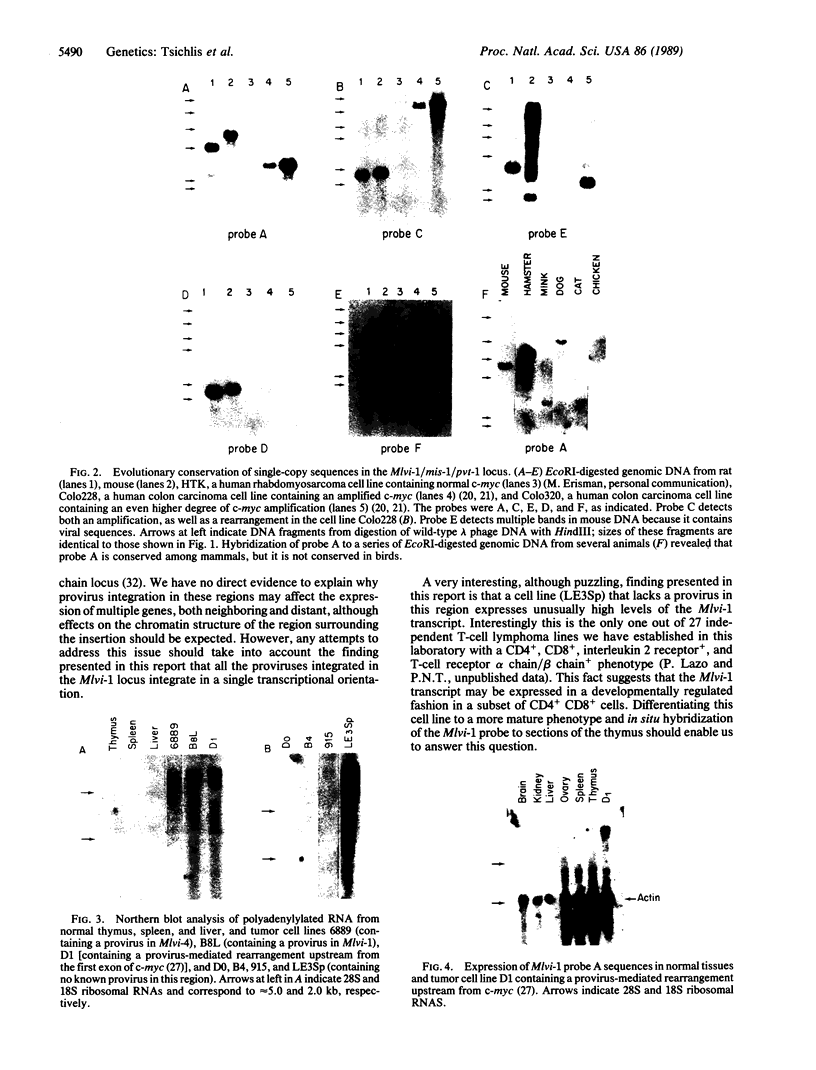
The Mlvi-1/mis-1/pvt-1 locus, located approximately 270 kilobase pairs 3' of the c-myc protooncogene, was originally discovered as a common region of provirus integration in Moloney murine leukemia virus-induced rat T-cell lymphomas. The same locus was shown subsequently to be coamplified with c-myc and to be involved in chromosomal translocations in a variety of human and animal neoplasms. Provirus integration in Mlvi-1 in Moloney murine leukemia virus-induced rat T-cell lymphomas activates the c-myc protooncogene. The studies reported here were aimed to determine whether, in addition to the activation of c-myc, provirus integration affected the expression of other neighboring genes. Provirus integration was shown to occur in three clusters separated by regions of uninterrupted DNA. The proviruses in all three clusters had integrated in a single-transcriptional orientation, and they appeared intact. Systematic hybridization of Mlvi-1 clones to rat, mouse, and human genomic DNA revealed three patches of evolutionarily conserved sequences. Two of them were mapped in regions targeted by the provirus, and the third was mapped immediately 5' to the provirus clusters. A probe derived from the conserved sequences 5' of the integrated proviruses detected a tumor-specific RNA transcript in tumors carrying a provirus in Mlvi-1 or in the neighboring Mlvi-4 and c-myc loci. The highest level of RNA transcript expression, however, was seen in a CD4+ CD8+ tumor cell line that was not carrying a provirus in this region. We conclude that provirus insertion in this region activates both c-myc and another gene that is located in the immediate vicinity of the integrated Mlvi-1 proviruses and may be developmentally regulated in T cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Koskinen P., Mäkelä T. P., Saksela K., Sistonen L., Winqvist R. myc oncogenes: activation and amplification. Biochim Biophys Acta. 1987 Apr 20;907(1):1–32. doi: 10.1016/0304-419x(87)90016-3. [DOI] [PubMed] [Google Scholar]
- Alitalo K., Schwab M. Oncogene amplification in tumor cells. Adv Cancer Res. 1986;47:235–281. doi: 10.1016/s0065-230x(08)60201-8. [DOI] [PubMed] [Google Scholar]
- Berger S. L. Isolation of cytoplasmic RNA: ribonucleoside-vanadyl complexes. Methods Enzymol. 1987;152:227–234. doi: 10.1016/0076-6879(87)52024-9. [DOI] [PubMed] [Google Scholar]
- Dickson C., Smith R., Brookes S., Peters G. Tumorigenesis by mouse mammary tumor virus: proviral activation of a cellular gene in the common integration region int-2. Cell. 1984 Jun;37(2):529–536. doi: 10.1016/0092-8674(84)90383-0. [DOI] [PubMed] [Google Scholar]
- Dzierzak E. A., Papayannopoulou T., Mulligan R. C. Lineage-specific expression of a human beta-globin gene in murine bone marrow transplant recipients reconstituted with retrovirus-transduced stem cells. Nature. 1988 Jan 7;331(6151):35–41. doi: 10.1038/331035a0. [DOI] [PubMed] [Google Scholar]
- Forrester W. C., Takegawa S., Papayannopoulou T., Stamatoyannopoulos G., Groudine M. Evidence for a locus activation region: the formation of developmentally stable hypersensitive sites in globin-expressing hybrids. Nucleic Acids Res. 1987 Dec 23;15(24):10159–10177. doi: 10.1093/nar/15.24.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester W. C., Thompson C., Elder J. T., Groudine M. A developmentally stable chromatin structure in the human beta-globin gene cluster. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1359–1363. doi: 10.1073/pnas.83.5.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. K., Lewis W. G., Crittenden L. B., Kung H. J. Activation of the cellular oncogene c-erbB by LTR insertion: molecular basis for induction of erythroblastosis by avian leukosis virus. Cell. 1983 Jun;33(2):357–368. doi: 10.1016/0092-8674(83)90417-8. [DOI] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Jacobson A. Purification and fractionation of poly(A)+ RNA. Methods Enzymol. 1987;152:254–261. doi: 10.1016/0076-6879(87)52028-6. [DOI] [PubMed] [Google Scholar]
- Koehne C. F., Lazo P. A., Alves K., Lee J. S., Tsichlis P. N., O'Donnell P. V. The Mlvi-1 locus involved in the induction of rat T-cell lymphomas and the pvt-1/Mis-1 locus are identical. J Virol. 1989 May;63(5):2366–2369. doi: 10.1128/jvi.63.5.2366-2369.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazo P. A., Tsichlis P. N. Recombination between two integrated proviruses, one of which was inserted near c-myc in a retrovirus-induced rat thymoma: implications for tumor progression. J Virol. 1988 Mar;62(3):788–794. doi: 10.1128/jvi.62.3.788-794.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Beau M. M., Epstein N. D., O'Brien S. J., Nienhuis A. W., Yang Y. C., Clark S. C., Rowley J. D. The interleukin 3 gene is located on human chromosome 5 and is deleted in myeloid leukemias with a deletion of 5q. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5913–5917. doi: 10.1073/pnas.84.16.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Beau M. M., Pettenati M. J., Lemons R. S., Diaz M. O., Westbrook C. A., Larson R. A., Sherr C. J., Rowley J. D. Assignment of the GM-CSF, CSF-1, and FMS genes to human chromosome 5 provides evidence for linkage of a family of genes regulating hematopoiesis and for their involvement in the deletion (5q) in myeloid disorders. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):899–909. doi: 10.1101/sqb.1986.051.01.103. [DOI] [PubMed] [Google Scholar]
- Lemay G., Jolicoeur P. Rearrangement of a DNA sequence homologous to a cell-virus junction fragment in several Moloney murine leukemia virus-induced rat thymomas. Proc Natl Acad Sci U S A. 1984 Jan;81(1):38–42. doi: 10.1073/pnas.81.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengle-Gaw L., Rabbitts T. H. A human chromosome 8 region with abnormalities in B cell, HTLV-I+ T cell and c-myc amplified tumours. EMBO J. 1987 Jul;6(7):1959–1965. doi: 10.1002/j.1460-2075.1987.tb02458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A. J., Caravatti M., Robert B., Cohen A., Daubas P., Weydert A., Gros F., Buckingham M. E. Mouse actin messenger RNAs. Construction and characterization of a recombinant plasmid molecule containing a complementary DNA transcript of mouse alpha-actin mRNA. J Biol Chem. 1981 Jan 25;256(2):1008–1014. [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Neil J. C., Forrest D. Mechanisms of retrovirus-induced leukaemia: selected aspects. Biochim Biophys Acta. 1987 Apr 20;907(1):71–91. doi: 10.1016/0304-419x(87)90019-9. [DOI] [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Goodwin R. G., Rottman F. M., Crittenden L. B., Raines M. A., Kung H. J. c-erbB activation in ALV-induced erythroblastosis: novel RNA processing and promoter insertion result in expression of an amino-truncated EGF receptor. Cell. 1985 Jul;41(3):719–726. doi: 10.1016/s0092-8674(85)80052-0. [DOI] [PubMed] [Google Scholar]
- Noori-Daloii M. R., Swift R. A., Kung H. J., Crittenden L. B., Witter R. L. Specific integration of REV proviruses in avian bursal lymphomas. Nature. 1981 Dec 10;294(5841):574–576. doi: 10.1038/294574a0. [DOI] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982 Nov;31(1):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Nusse R., van Ooyen A., Cox D., Fung Y. K., Varmus H. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature. 1984 Jan 12;307(5947):131–136. doi: 10.1038/307131a0. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Bishop J. M., Varmus H. E. Multiple arrangements of viral DNA and an activated host oncogene in bursal lymphomas. Nature. 1982 Jan 21;295(5846):209–214. doi: 10.1038/295209a0. [DOI] [PubMed] [Google Scholar]
- Selten G., Cuypers H. T., Berns A. Proviral activation of the putative oncogene Pim-1 in MuLV induced T-cell lymphomas. EMBO J. 1985 Jul;4(7):1793–1798. doi: 10.1002/j.1460-2075.1985.tb03852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Ong G. L., Morse H. C., 3rd, Potter M., Mushinski J. F. Two modes of c-myb activation in virus-induced mouse myeloid tumors. Mol Cell Biol. 1986 Feb;6(2):380–392. doi: 10.1128/mcb.6.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsichlis P. N., Lohse M. A., Szpirer C., Szpirer J., Levan G. Cellular DNA regions involved in the induction of rat thymic lymphomas (Mlvi-1, Mlvi-2, Mlvi-3, and c-myc) represent independent loci as determined by their chromosomal map location in the rat. J Virol. 1985 Dec;56(3):938–942. doi: 10.1128/jvi.56.3.938-942.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsichlis P. N., Strauss P. G., Hu L. F. A common region for proviral DNA integration in MoMuLV-induced rat thymic lymphomas. 1983 Mar 31-Apr 6Nature. 302(5907):445–449. doi: 10.1038/302445a0. [DOI] [PubMed] [Google Scholar]
- Tsichlis P. N., Strauss P. G., Lohse M. A. Concerted DNA rearrangements in Moloney murine leukemia virus-induced thymomas: a potential synergistic relationship in oncogenesis. J Virol. 1985 Oct;56(1):258–267. doi: 10.1128/jvi.56.1.258-267.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve L., Rassart E., Jolicoeur P., Graham M., Adams J. M. Proviral integration site Mis-1 in rat thymomas corresponds to the pvt-1 translocation breakpoint in murine plasmacytomas. Mol Cell Biol. 1986 May;6(5):1834–1837. doi: 10.1128/mcb.6.5.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. F., Calame K. The endogenous immunoglobulin heavy chain enhancer can activate tandem VH promoters separated by a large distance. Cell. 1985 Dec;43(3 Pt 2):659–665. doi: 10.1016/0092-8674(85)90238-7. [DOI] [PubMed] [Google Scholar]
- Webb E., Adams J. M., Cory S. Variant (6 ; 15) translocation in a murine plasmacytoma occurs near an immunoglobulin kappa gene but far from the myc oncogene. Nature. 1984 Dec 20;312(5996):777–779. doi: 10.1038/312777a0. [DOI] [PubMed] [Google Scholar]
- Weinstein Y., Ihle J. N., Lavu S., Reddy E. P. Truncation of the c-myb gene by a retroviral integration in an interleukin 3-dependent myeloid leukemia cell line. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5010–5014. doi: 10.1073/pnas.83.14.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M. C., Wada M., Satoh H., Yoshida T., Sakamoto H., Miyagawa K., Yokota J., Koda T., Kakinuma M., Sugimura T. Human HST1 (HSTF1) gene maps to chromosome band 11q13 and coamplifies with the INT2 gene in human cancer. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4861–4864. doi: 10.1073/pnas.85.13.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]