Introduction
During the development of cardiac hypertrophy, heart failure, and ischemia reperfusion challenge, the heart accumulates misfolded proteins as a result of cellular stresses 1–4. While the compensatory increases in chaperones/co-chaperones work to prevent misfolding, refold denatured proteins and/or target them for degradation, this system can become overwhelmed, leading to worsening of cardiac function. In fact, recent studies have demonstrated experimentally that increasing the burden of misfolded proteins in the heart can contribute to the development of cardiac dysfunction 5. In this review, we discuss the role of heat shock proteins in common cardiac diseases including cardiac hypertrophy, heart failure, and ischemia/reperfusion injury. Furthermore, we delineate the many specific mechanisms by which these chaperones, co-chaperones, and heat shock factor (HSF) transcription factors have been found to be cardioprotective in experimental models. Lastly, we review recent studies involving drugs that are being developed (and currently used) to increase the expression (and presumably function) of chaperone/co-chaperone systems that may be applicable to the treatment of common cardiac diseases as well as familial cardiac diseases whose etiology includes a major component of misfolded proteins (e.g. desminopathies).
Chaperones enhance productive protein folding and refolding and prevent protein aggregation
There are several general families of molecular chaperones in the cytoplasm of mammalian cells, including heat shock protein (HSP) 90, HSP70, CCT (also called TRiC), and small HSP (sHSP) family proteins (Figure 1A). Members of the HSP90 family of chaperones are the most abundant chaperones located in the cytosol. They form dimers consisting of HSP90α and HSP90β subunits and are inducible with stress, although are quite abundant without stress also 8, 9. HSP90 assists many proteins involved with signal transduction, including more than 40 kinases and many steroid hormone receptors, with a supporting role in conformational changes involved in ATP hydrolysis 10, 11. The HSP70 chaperone family consists of 6 member proteins that are found in the cytosol 12, including HSP70 and the cognate of HSP70 (HSC70). Like HSP90, HSP70 proteins are inducible with stress, however they are also highly abundant without stress. HSP70 family members are functionally highly homologous, recognizing hydrophobic surfaces of unfolded proteins and partially folded intermediates. Their activity is controlled by their hydrolysis activity as well as by their ability to bind ATP 13. HSP90 and HSP70 proteins both inhibit protein aggregation, thereby promoting productive folding of proteins (for comprehensive reviews, see Young et al.14–16).
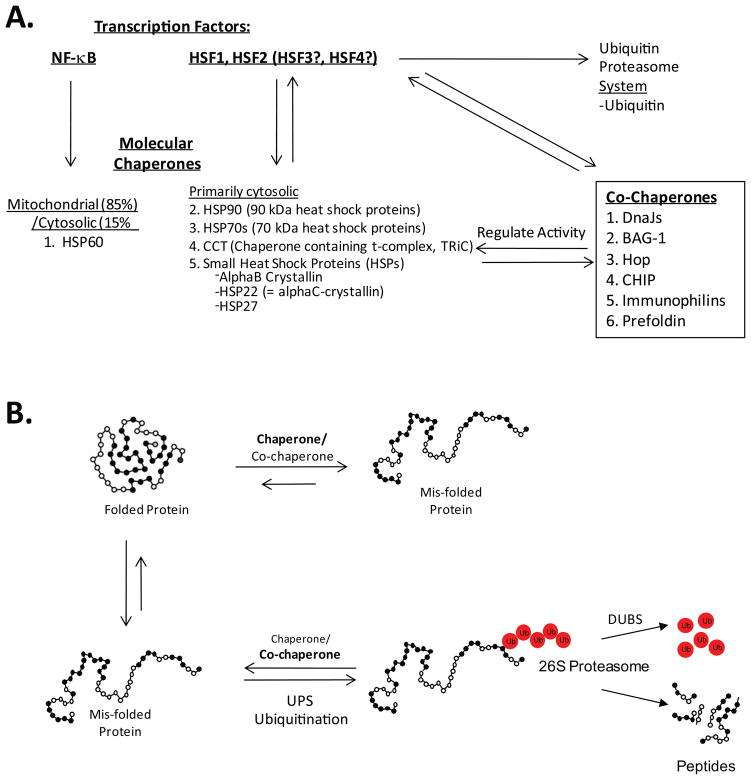
Figure 1. The regulation of the protein quality control and chaperone systems in the heart that protect against the toxicity of misfolded proteins.
A. Both NF-kB and HSF transcription factors regulate the expression of key molecular chaperones in the mitochondria and cytosol, respectively. Stress-activated HSF1 and HSF2 also increase the expression of co-chaperones and ubiquitin expression. B. The upregulation of both chaperones and ubiquitin help maintain protein quality control by either refolding (chaperone) the protein or ubiquitinating misfolded proteins (co-chaperone, e.g. CHIP). This is a dynamic process particularly important during cardiac stress. Adapted from: Kubota 2009 6 and Wang et al. 7
Other molecular chaperones present in the cytosol include TRiC (TCP1-ring complex, or chaperonin containing TCP1 (CCT)) 17, 18. The central cavity of TRiC uses a lid-like structure to encapsulate substrate proteins to allow it to trap and fold target proteins. This encapsulation prevents aggregation and allows changes in conformation that ensure correct folding (in an ATP-dependent manner) before substrates are released. The family of sHSPs also maintain protein confirmation in an ATP-independent manner 19. Over 10 sHSP chaperone proteins have been characterized and all function by maintaining an equilibrium between the dimer and large oligomer states of their target proteins 20. In contrast to HSP90 and 70 though, only a few sHSPs (including HSP27, HSP22, and alphaB-crystallin) are increased in response to stress 21. HSP27 and alphaB-crystallin (CryAB) are abundant in cardiac and skeletal muscles and increase in response to stress in order to protect against insults such as ischemia 22. Both these proteins associate with actin and are vital to muscle development and assembly 23. CryAB also interacts with several other cytoskeletal proteins such as desmin to maintain protein folding and prevent aggregation 24–27.
In addition to the molecular chaperones that are found predominantly in the cytosol, there are also chaperones that are known to maintain proteins in other compartments of the cell, in particular the mitochondria. HSP60 is a chaperone originally identified in the mitochondria 28 but which is also found in the cytosol 29. HSP60 is responsible for refolding and transportation of proteins between the mitochondrial matrix and the cytoplasm of the cell 30 and associates with a number of cytosolic proteins involved in apoptosis such as B-cell lympohoma-1-assocated X protein (BAX), B-cell lymphoma (BCL)-xl and BCL-2 homologous antagonist/killer (BAK) 31, 32. HSP60 is believed to be a homolog of the bacterial heat shock protein groEL and, as such, it is believed that HSP60 assists in folding linear amino acids into their three dimensional structures in ways that have been described for groEL 33.
Co-chaperones control the activity of chaperones: DnaJ, BAGs, Hop, CHIP, Immunophilins, and Prefoldin
As their name implies, co-chaperones are proteins that assist chaperone functions, including in protein folding. There are over 40 genes in the HSP40/DnaJ protein family, with the different members playing diverse roles in regulating protein folding, assembly, translocation, and even degradation. DnaJ proteins bind to the ATPase binding domain of HSP70 proteins to enhance their ATPase activity 34. They also bind substrate proteins to modulate folding in a substrate-specific manner. Bcl2-associated athanogene (BAG) proteins all have a conserved BAG region that also binds the ATPase domain of HSP70 proteins to affect the rate of substrate binding and release 35. Conversely, Hop, carboxy terminus of HSP70-binding protein (CHIP) and immunophilins all bind both HSP70 and HSP90 via tetra-tricopeptide repeat (TPR) domains, which allow the transfer of substrates between them 8, 11. CHIP is critical to quality control processes and ubiquitinates misfolded proteins when correct folding cannot be achieved (see Figure 1B) 36, 37, while immunophilins are necessary for the functions of the p23 steroid aporeceptor-associated protein 11. Lastly, prefoldin, also known as GimC, helps TriC/CCT-dependent folding of tubulin and actin by way of its 6 tentacle-like processes that trap unfolded substrates and assist with folding in collaboration with HSP70/HSP9038, 39.
The transcription factors HSF1 and HSF2 regulate chaperone and co-chaperone expression in the cell
The number of misfolded proteins increases during times of cellular stress, including oxidative stress and proteasome inhibition. Therefore, many chaperones are induced at the transcriptional level in the presence of these conditions to protect against the toxicity of misfolded proteins. There are at least 4 transcription factors (HSF1-4) that regulate heat shock proteins. HSF1 is the primary transcription factor involved in this process and binds heat shock response elements (HSE) in the promoter regions of stress-induced genes. HSF1 is found throughout the cytosol as a monomer that binds HSP90 to inhibit its chaperone activity. During stress, denatured proteins competitively bind HSP90 40, effectively releasing HSF1, thereby allowing it to translocate to the nucleus as trimers 41. In the nucleus, increased HSF1 upregulates the expression of chaperones, including HSP70. HSP70 then binds HSF1, which in turn, attenuates the HSF response, indicating a feedback mechanism 42. Interestingly, part of the stress response induced by HSF1 includes the upregulation of ubiquitin, suggesting that it regulates the ubiquitin proteasome system to enhance the cell’s capacity to degrade proteins during stress (recently reviewed by Willis et al.43). The roles of the remaining three HSFs in the stress response are less well studied. Studies have shown that HSF2 contributes to the inducible expression of heat shock protein genes by interacting with HSF1 44. The roles of HSF3 and HSF4 as factors that regulate the expression of non-classical and sHSPs are just beginning to be understood 45, 46.
Cells have a diverse array of molecular chaperones available to them in the cytosol and mitochondrial compartments that are regulated by the transcription factors NF-κB and HSF1-4. While these chaperones primarily play a role in refolding proteins, some co-chaperones cross talk with the ubiquitin proteasome system to directly ubiquitinate proteins for subsequent degradation by the proteasome. While much of the work on molecular chaperones has been in non-cardiovascular systems, their role in the heart during health and disease is becoming more greatly appreciated.
Cardiac chaperones are regulated in the pathophysiology of a number of common cardiac diseases
Cardiac hypertrophy
Cardiac chaperones such as HSP70, CryAB, and HSP22 (alphaC-crystallin) increase in expression during the development of cardiac hypertrophy (Table 1). HSP70 can be induced by a variety of hypertrophic stimuli, including aortic banding, angiotensin II infusion, isoproterenol infusion and swimming 57. CryAB can be induced in rat cardiomyocytes in response to endothelin-1 induced cardiomyocyte hypertrophy, resulting in a 2-fold increase in CryAB expression. The DNA binding activity of HSF1 is heightened as a result of increasing pre-load in heart 71. Since cardiac hypertrophy is a response to a number of stress stimuli, this general increase in cardiac chaperone expression in cardiac hypertrophy is not surprising. However, the purpose behind this increased chaperone expression is much less well understood. Mice with transgenic overexpression of CryAB gene challenged with 2 weeks of aortic banding to induce pathologic cardiac hypertrophy exhibit a significant reduction in NFAT (nuclear factor of activated T cells) transactivation and attenuated hypertrophy 86. This contrasts to CryAB null mice, which display an enhanced NFAT transactivation at baseline, and an accelerated development of heart failure in response to pressure overload induced by aortic banding 86. These studies demonstrate that cardiac CryAB plays a role in suppressing cardiac hypertrophic response, possibly through inhibiting NFAT signaling.
Table 1. A summary of the known regulation of chaperones, co-chaperones, and transcription factors involved in the heat shock protein response in cardiac hypertrophy, heart failure, and ischemia reperfusion injury.
N.D.=not determined.
| Example substrate recognition/Other | Cardiac Hypertrophy | Heart Failure | Ischemia reperfusion injury | |
|---|---|---|---|---|
| Chaperones | ||||
| 1. HSP90 | Actin, tubulin, TGFbeta1, CHIP 47, 48 | N.D. | HSP90 in DCM=IHD=Normal Controls 60 | Rat cardiac ischemia-reperfusion results in increased HSP90 65 Rat cardiac ischemia-reperfusion results in increased HSP70 65 |
| 2. HSP70 | Calcineurin, HDAC6, CHIP49, 50 | Hypertrophic stimuli (aortic banding, isoproterenol, AngII, swimming) induce HSP70 expression57 HSP70 support pro-hypertrophic signaling via interaction with Hdac2 57 |
HSP72 not increased in heart failure induced by coronary artery ligation in rats for 9–12 weeks 7 No difference in HSP72 expression8 weeks post-coronary artery occlusion compared to controls.61 HSP70 and HSC70 in DCM=IHD=Normal controls 60 |
HSP72 increased at 1 week post-coronary artery occlusion 61 |
| 3. CCT (TRiC) | TRiC binds Actin 51 | N.D. | N.D. | N.D. |
| 4. Small HSPs | ||||
| -AlphaB-Crystallin (=HSPB5) | AlphaB-Crystallin binds desmin 52 | AlphaB-Crystallin increases 2 fold in ET-1 treated neonatal rat cardiomyocytes 58 | N.D. | N.D. |
| -HSP20 (=HSPB6) | HSP20 binds actin and alpha-actinin53 | N.D. | N.D. | HSP20 increased in I/R, regulated, in part by miR-32066. |
| -HSP22 (=HSPB8, H11 kinase, alphaC-Crystallin) | HSP22 binds lipid membranes 54 | HSP22 expression increases ~3 fold in cardiac hypertrophy induced in dogs by aortic banding 59 | N.D. | HSP22 expression is increased ~3 fold 1 hour after reperfusion following ischemia in a pig model 67 In human hibernating myocardium and swine model of is hibernating myocardial, HSP22 increased 68 |
| -HSP27 (=HSPB1, HSPB2) | HSP27 binds IκB 55 | N.D. | No change in HSP27 expression at 8 weeks post-coronary artery occlusion (rat)/Increased HSP27 at 1 week post-coronary artery occlusion 61 HSP27 increased in human DCM compared to controls 60 |
N.D. |
| 5. Mitochondrial HSPs | ||||
| -HSP60 | HSP60 binds Bax and Bak 56 | HSP60 decreased 13 fold in ET-1 treated neonatal rat cardiomyocytes 58 | HSP60 increased 8+ weeks (but not at 1 week) post-coronary artery occlusion (rat) 7, 61, 62 There is increased expression of cardiac HSP60 in heart failure (coronary artery ligation in rats for 9–12 weeks); may be driven by NF-κB activation 7. HSP60 doubled in human DCM and IHD compared to controls60 HSP60 moves from cytoplasm to mitochondria in DCM and IHD 63 Serum HSP60 levels are associated with the severity of heart failure in patients 64 |
N.D.?? |
| Transcription Factors regulating Chaperones | ||||
| 1. HSF1 | HSF1 binds the heat shock elements (HGAAN) of HSP72; HSP90 interacts to repress. | Increased per-load/mechanical stress increases HSF1 activity 71 | HSF1 levels increased, without an increase in activity in heart failure (8 weeks post-coronary artery ligation in rats) 7 | Rat cardiac ischemia-reperfusion results in increased HSF1 activity, but not HSF2 65. HSF1 induction in ischemia mediated by ROS and ATP levels 72, 73 |
| 2. HSF2 | HSF-2 binds the heat shock elements of HSP90, HSP27, c-Fos69; interacts with HSF1 and Nucleoporin62 70 | N.D. | HSF2 levels increased, without an increase in activity in heart failure (8 weeks post-coronary artery ligation in rats) 7 | |
| Co-Chaperones | ||||
| 1. DnaJ | DnaJ binds ribosome bound nascent polypeptides 74 | N.D. | N.D. | DnaJ-like pDJA1 increased 4 fold after reperfusion in a pig model of I/R82 |
| 2. BAG-1 | BAG-1 binds Bcl-2, Raf1 75 | N.D. | N.D. | BAG-1 protects against I/R injury 83, 84 |
| 3. Hop | Hop binds HSP70, HSP90 76 | N.D. | N.D. | N.D. |
| 4. carboxyl terminus of HSP70-interacting protein (CHIP) | CHIP binds HSP70, HSP90, HIF1-α77 | CHIP increases in response to high glucose and regulates pro-hypertrophic GATA4 in cardiomyocytes in vitro 81 | N.D.?? | CHIP protects against ischemia reperfusion injury 85 |
| 5. Immunophilins | FKBP38 binds mTOR complex 78 | N.D. | N.D. | N.D. |
| 6. Prefoldin | Prefoldin binds Nascent chain of actin and tubulin Chaperonin 79, 80 | N.D. | N.D. | N.D. |
In contrast to CryAB’s ability to attenutae cardiac hypertrophy, HSP70 is necessary for the induction of cardiac hypertrophy. One potential mechanism by which HSP70 may accomplish this is through its association with the activated form of histone deacetylase2 (HDAC2). Overexpressing a dominant negative form of HSP70 or decreasing HDAC2 with siRNA blunts the hypertrophic response in the heart. Furthermore, cardiac hypertrophy induced by isoproterenol infusion or aortic banding in mice lacking HSP70, results in a blunting of HDAC2 activity 57. This suggests a role for HSP70 in the induction of cardiac hypertrophy, possibly through its stabilization of HDAC2. This HSP70-dependence of HDAC2 activity is interesting because of the importance of HDAC2 in cardiac hypertrophy signaling. The HDACs are instrumental in regulating hypertrophic gene expression in pathological settings 49. Class II HDACs (HDAC 4, 6, 7, 9) negatively regulate hypertrophy by repressing MEF/GATA/NFAT-mediated gene transcription 87. Conversely, the Class I HDAC 2 has been implicated in having acetylase-dependent pro-hypertrophic activity, possibly by releasing repression of IGF-1 signaling 49, 57, 88.
Another cardiac chaperone, HSP22, has also been shown to increase during the development of cardiac hypertrophy. HSP22 has several names, the most common one used in the literature is H11 kinase, but alphaC-crystallin is sometimes used and indicates its relationship to other sHSPs (see recent review 89). Increasing HSP22 expression results in the activation of signaling pathways involved in survival and cell growth, including the PI3K/Akt pathway, AMPK, PKC epsilon, nitric oxide, and mTOR89. These signaling pathways induce pre-conditioning, growth, and protection against apoptosis among other cardioprotective pathways 89. Recently, studies have identified that HSP22 increases during the development of cardiac hypertrophy in a variety of animal models. Inducing a slowly progressive cardiac hypertrophy in puppies through use of aortic banding (that parallels the gradual progression of human disease to a much greater extent than acute aortic banding models), causes HSP22 to increase ~3 fold 59. Increasing HSP22 expression in cultured cardiomyocytes and the intact mouse heart (~ 7 fold) results in the development of a spontaneous hypertrophy, characterized by the re-expression of the fetal gene program 59, 90. This suggests that the many cardioprotective pathways HSP22 induces 89 may be detrimental if expressed chronically.
Physiologic versus Pathologic Hypertrophy
The differences in the underlying signaling between physiologic and pathologic cardiac hypertrophy is only superficially understood. Discovering these differences is critical to our understanding of what makes patients undergoing pathologic hypertrophy so susceptible to heart failure, whereas patients undergoing physiologic hypertrophy (for example through exercise) are not. Pathologic cardiac hypertrophy is induced by persistent pressure or volume overload as a result of hypertension or valvular heart disease. Physiologic hypertrophy, on the other hand, is induced by exercise. Both stimuli result in increases in cardiomyocyte size, however, pathologic hypertrophy is limited in its ability to maintain cardiac function, eventually resulting in heart failure. In contrast, physiologic cardiac hypertrophy maintains and improves function, as illustrated in athletes. The difference in cellular signaling between these two processes has been of great interest to researchers. A number of recent studies have lead to the hypothesis that HSF1 may regulate some of the differences in the development of physiologic and pathologic cardiac hypertrophy (recently reviewed by Toko et al.91). This is based largely on gene expression studies which have shown a differential expression of ~100 genes 92–95. Among these differentially expressed genes are a number of HSF1-regulated genes such as HSP70 and HSP27 as well as increases in HSF1 itself. HSF1 may be one of many differences regulating the differential signaling of physiologic and pathologic cardiac hypertrophy.
Heart Failure
In order to protect cardiomyocytes from injury, heat shock proteins within the cell increase in response to externally applied stressors, including oxidative stress and inflammation. Beginning in the late 1990s, researchers have been investigating the expression of heat shock proteins in the failing human heart (Table 1). To this end, investigators have examined the expression of HSP90, HSP72, HSC70, HSP27, and HSP60 from dilated cardiomyopathy (DCM) patients, ischemic cardiomyopathy patients, and normal controls 60. HSP72, HSC70, and HSP90 are not significantly changed between the 3 groups 60. In contrast, DCM patients exhibit a two-fold increase in HSP27 expression in the heart compared to healthy control patients. This is in addition to a doubling of the HSP60 level, which also occurs in the hearts from ischemic heart disease patients 60. The fact that HSP72 protein doesn’t increase in heart failure (even though it is cardioprotective 96), while levels of the HSP60 protein are doubled 60, suggests that there is differential regulation between HSP60 and either HSF1 or HSF-2-regulated heat shock proteins. Indeed, following induction of heart failure by placement of a permanent high left anterior descending coronary artery ligation in rats, no differences can be seen between HSF1 and HSF-2 activity as determined by EMSA 7. Additionally, HSP72 mRNA levels are not increased. In contrast, HSP60 mRNA increases and appears to be due to increased binding of NF-κB to both of the NF-κB binding elements in the HSP60 gene. Since HSP60 contains NF-κB binding elements, but HSP72 does not 7, this may explain why HSP60, but not other heat shock proteins, are increased during heart failure.
In more acute studies, where Wistar rats undergo a permanent left anterior descending coronary ligation to induce heart failure, acute increases in HSP72 and HSP27 are seen, whereas HSP60 expression remains unaffected 61. However, after 8 weeks, at which time the development of heart failure is significant, a decrease in HSP72 and HSP27 expression is observed, which appears to be somewhat contradictory when compared to the results seen in human heart failure patients (see above). Induction of heart failure in these rats also results in a parallel increase in cardiac HSP60 levels. Additional studies have determined that these increases in HSP60 correlate with a decrease in mitochondrial oxygen consumption rate as well as an increase in markers for reactive oxygen species (determined by thiobarbiturate reacting substance Toga, 2007 62). The differences in HSP regulation found in these Wistar rat studies compared to the studies in human disease may be due to the relatively acute nature of these studies compared to human studies, or possibly a result of species and even strain dependent effects.
HSP60 in heart failure
Although HSP60 is predominantly found in the mitochondria, approximately 15% of total cellular HSP60 normally resides within the cytoplasm 29. However, in both DMC and ischemic heart disease hearts the distribution of HSP60 changes, with cytosolic HSP60 translocating to the mitochondria 63. In other studies of heart failure using animal models and human explanted failing hearts, HSP60 has been found localized to the plasma membrane where it is detectable on the cell surface by both flow cytometry and confocal microscopy. Interestingly, localization of HSP60 to the plasma membrane of a cell correlates with an increase in apoptosis of the affected cell, possibly due to the fact that the cell surface HSP60 may be able to interact with other cells to trigger the innate immune response, resulting in the release of proinflammatory cytokines such as TNF-α. This would make HSP60 an early signal inducing myocyte loss and contributing to heart failure 56. These few studies indicate a number of potentially conflicting data that might be due to differences resulting from species or strain variations, or from experimental design. Considerably more work is needed to delineate the regulation of HSP60 in heart failure.
HSP60’s involvement in heart failure is made even more complicated by the fact that it is also released from the cells and can be found circulating in plasma during early in heart failure 56. Circulating HSP60 has been hypothesized to play a role in atherosclerosis by inducing inflammation and autoimmune mechanisms (see recent reviews97, 98). The presence of HSP60 in the blood of normal patients was first described in 1999 99. Recent studies have investigated the relationship between chronic heart failure severity and serum HSP60 levels 64. In 112 patients with CHF and 62 control subjects, serum HSP60 levels were higher in patients with CHF compared to controls 64. CHF patients with advancing New York Heart Association functional classes had higher levels of HSP60 as well 64. Likewise, patients with cardiac events during the average 569 days of follow up had higher serum HSP60 levels compared to event-free patients 64. These findings demonstrate a relationship between serum HSP60 levels, the severity of CHF, and a high risk for adverse cardiac events in patients with heart failure. The role of circulating HSP60 in the underlying pathophysiology of heart failure has not been delineated.
Cardiac Ischemic Injury
Most studies investigating heat shock proteins in cardiac ischemia/reperfusion injury have reported on their cardioprotective effects. However, a few studies have concentrated on the regulation of chaperone protein expression and activity during the course of ischemia/reperfusion injury (Table 1). Reperfusion following 20 minutes of ischemia results in increases to both HSP70 and HSP90 mRNA levels, with the increase in HSP70 being much higher than that of HSP90 ( ~75 fold and HSP90 ~16 respectively) 65. This increase in HSP70 and HSP90 expression is most likely due to a concurrent increase in the transcription factor HSF1 (but not HSF2), which in turn appears to be driven by an accumulation of reactive oxygen species during ischemia/reperfusion injury 72. In addition though, other studies have identified that HSF1 activation can be modulated by ATP concentrations within the cell. Moderate decreases in intracellular ATP correlate with HSF1 activation, while severe ATP depletion results in an attenuated HSF1 response, which can subsequently be rescued upon ATP restoration 73. Studies investigating differential expression of genes in a pig model of ischemia/reperfusion injury reveal that HSP22 significantly increases ~ 3 fold after 1 hour of reperfusion 67. HSP22 is also significantly increased in cases of human hibernating myocardium and pig models of hibernating myocardium 68.
HSP70/HSP72
Both HSP70 and HSP72 have proven to be beneficial to the outcome of cardiac ischemia/reperfusion injury. Knocking-down HSP72 expression in isolated feline cardiomyocytes increases their susceptibility to cell death in response to hypoxia and reoxygenation 100. In addition, increasing HSP72 in adult male rats by successive bouts of endurance exercise improves the outcomes of ischemia/reperfusion injury, illustrated by a decrease in cardiac infarct size as well as the amount of cardiac apoptosis in endurance-trained rats compared to sedentary controls96. In the case of HSP70, adenovirus-mediated gene transfer into rabbit hearts results in a reduction in injury after ischemia/reperfusion injury 101. Furthermore, at least 4 studies have demonstrated that the transgenic overexpression of HSP70 in the heart of mice significantly protects against ischemia/reperfusion injury 102–105. Since all of these studies increase HSP70 prior to the ischemia/reperfusion insult, it is not clear what the clinical utility of increased HSP70 at therapeutically plausible time points (after ischemia/reperfusion injury) would be.
Small heat shock proteins: HSP20, HSP22, HSP27, alphaB-crystallin (CryAB) (Table 1)
Increasing the HSP20 expression in isolated cardiomyocytes improves their function 106 and protects against apoptosis induced by beta-agonist stimulation 107. Cardiac-specific overexpression of HSP20 in mouse models (~10 fold) protects against ischemia/reperfusion injury. When HSP20 transgenic hearts are challenged with ischemia/reperfusion injury ex vivo, they exhibit an improved contractile performance, a decrease in indices of myocyte cell death, and a significant decrease in infarct size compared to wild type hearts 108. This protective effect of HSP20 appears to be due to HSP20’s role in activating autophagy, a critical mechanism for dealing with ischemia/reperfusion injury 109. Transgenic mice in which serine 16 on HSP20 is mutated such that it is nonphosphorylatable are more susceptible to ischemia/reperfusion injury than wild-type mice, in part, due to the inability of the mutant HSP20 to activate autophagic pathways 109. HSP20 can protect against ischemia/reperfusion injury via other mechanisms also. HSP20 protects not only against oxidative stress due to ischemia/reperfusion injury, but recent studies have also found that it can protect against other injuries due to increased oxidative stress such as doxorubicin therapy 110. Recent studies have demonstrated that HSP20 expression is regulated, at least in part, by the mircoRNA miR-320. Down-regulation of miR-320 using an antagomir has been shown to be cardioprotective in ischemia reperfusion, in part, by its up-regulation of HSP2066. In the case of HSP22, transgenic mice that have increased expression of HSP22 are protected against ischemia/reperfusion injury. After 45 minutes of coronary artery occlusion and reperfusion, HSP22 transgenic mice have an 82% reduction in infarct size compared to controls 90, with HSP22 transgenic hearts exhibiting significant activation of a number of survival kinases, including Akt and AMPK to which HSP22 binds directly 90. The cardioprotective effect of HSP22 appears to be mediated specifically through BMP signaling via activation of the PI3K/Akt pathway111. The small heat shock protein HSP27 has also been shown to protect against ischemia/reperfusion injury using dog cardiomyocytes, with just minimal (2–3 fold) increases in expression 112.
Previously we described the protective nature of CryAB in inhibiting cardiac hypertrophy. However, this small heat shock protein is also cardioprotective against ischemia/reperfusion injury when its expression is increased prior to insult. Transgenic mice in which CryAB is overexpressed, suffer less cardiac oxidative stress, decreased extent of infarction, and attenuated apoptosis and necrosis when challenged with ischemia/reperfusion injury 113. Likewise, mice lacking CryAB and HSP27, both of which are highly expressed in the heart, subjected to ischemia/reperfusion challenge exhibit a nearly 2 fold decrease in contractility recovery, with parallel increases in necrosis and apoptosis measures compared to controls 114. These studies indicate that, while CryAB and HSP27) are not necessary for cardiac development (CryAB/HSP27 mice develop normally and have no discernable differences in heart structure from wild type), they do play a key role in anti-oxidative mechanisms during ischemia/reperfusion injury 114.
Heat shock factor proteins (HSF1)
As the heart senses stress, it induces heat shock proteins by a number of mechanisms. Studies have identified that many of the heat shock proteins are regulated by the HSF family of transcription factors (Table 1). In the context of cardiac ischemia/reperfusion, HSF1 expression can up-regulate heat shock protein expression to protect against subsequent ischemia/reperfusion injury. Mice with cardiac overexpression of HSF1 challenged with ischemia/reperfusion injury recover faster, have smaller infarct sizes and decreased cardiomyocyte cell death compared to wild-type mice 115. In addition, Akt is enhanced while Jun N-terminal kinase and caspase 3 (apoptotic mediators) are less activated than wild type mice 115.
The cardioprotective role of HSF1 has been studied by using experimental models known to induce HSF1. Specifcially, cardiac HSF1 has been induced by whole body hyperthermia (WBH) or by transgenic over-expression of CaMKII-delta B. Upon HSF1 induction, these models were then challenged with ischemia/reperfusion injury. Preconditioning mice with WBH for 48 hours, then subjecting the isolated hearts to 20 minutes of normothermic ischemia and 30 minutes of reperfusion results in an increase in both HSF1 mRNA and protein and overall cardioprotection 116 The increase in HSF1expression is directly related to the cardioprotection as this effect is abolished with siRNA HSF1 116. Inhibiting HSF1 with siRNA in the face of WBH results in an inhibition of HSP32, HSP47, and HSP60 and increased thermal intolerance, resulting in a higher mortality rate 116. The Ca2+/calmodulin-dependent kinase (CaMK)II is also instrumental in protecting the heart against ischemia/reperfusion injury. CaMKII is a multi-functional kinase that regulates Ca2+ handling and regulates cell death in response to ischemia/reperfusion injury. Increasing CaMKII-deltaB expression protects against oxidative stress, hypoxia and angiotensin II-induced apoptosis 117. Recent studies have determined that this cardioprotection is due, in part, to increasing inducible HSP70 through phosphorylation of HSF1 117. These studies suggest that HSF1 may be a common mechanism by which cardiomyocytes induce a number of heat shock proteins to protect against cardiac/ischemia reperfusion injury.
At least 13 chaperones and co-chaperones regulated by at leat 2 HSF transcription factors have been described identified in the heart (Table 1). Quite predictably, of the proteins identified, their expression increases in cardiac disease and is generally cardioprotective. While most studies have focused on the regulation of these chaperones, co-chaperones, and transcriptions factors in heart failure, a growing number of studies have demonstrated more broadly their regulation in cardiac hypertrophy and ischemia reperfusion injury (Table 1). This cardioprotection includes a host of mechanisms that regulate growth and inhibit apoptosis through a variety of systems including the PI3K/Akt pathway, AMPK, PKC epsilon, nitric oxide, and mTOR. Pharmacologic enhancement of these endogenous cardioprotective mechanisms may prove to be simple yet effective strategies for reducing the morbidity and mortality associated wth common cardiac diseases.
Co-chaperones in the heart: Chaperone assistants and Protein Triage
Co-chaperones have many functions in the heart including assisting chaperones with protein folding and/or assisting with other functions, including targeting damaged proteins for degradation by the ubiquitin proteasome system in a process called protein triage 118. Increased co-chaperone expression in the heart has been found to be cardioprotective in ischemia and necessary to regulate proteins involved in Long-QT syndrome. A number of co-chaperones have been identified that control the activity of chaperones, including DnaJ, BAGs, Hop, CHIP, Immunophilins, and Prefoldin (Figure 1). Of these 6 general types of co-chaperones, only DnaJ, BAG-1, Hop and CHIP have been described in the heart, and our understanding of their role is preliminary (Table 1).
Using a pig model of ischemia/reperfusion to identify genes participating in mechanisms of cell survival, the Dna J-like co-chaperone (pDJA1) was identified by microarray using subtractive hybridization 82. pDJA1 is restricted to cardiomyocytes and is not present in skeletal muscle, liver, lung, kidney, aorta, stomach, or spleen 82. pDJA1 increases somewhat during ischemia, but increases 4 fold following ischemia and is protective against staurosporine-induced apoptosis in isolated rat cardiomyocytes 82. Since the identification of pDJA1 in 2003, little more has been reported on it despite its potential role in limiting damage in the post-ischemic myocardium.
Studies on the role of BAG-1 in cardiac ischemia reperfusion injury have demonstrated the ability of BAG-1 to inhibit apoptosis and induce autophagy in order to protect cardiomyocytes. BAG-1 interacts with HSC70 and HSP70 and promotes cell survival by coordinating the function of these chaperones with the degradation of proteins by the proteasome. Both BAG-1 isoforms (BAG-1S and BAG-1L) are rapidly induced after ischemia challenge in rat cardiomyocytes, with the increase in BAG-1 being sustained after subsequent reperfusion 83. The interaction of BAG-1 with HSC70 increases after ischemia/reperfusion injury 83, and increasing BAG-1S and BAG-1L in cardiomyocytes reduces apoptosis after ischemia/reperfusion injury. When BAG-1S or BAG-1L are fused to a nuclear localization sequence to force their nuclear localization, they fail to protect cardiomyoctyes, similar to BAG-1 deletion mutants that are unable to bind HSC70/HSP70 83. BAG-1 deletion constructs missing the N-terminal ubiquitin-like domain, however, do not affect the proteins ability to protect against ischemia/reperfusion injury 83. These studies demonstrate a novel cardioprotective role for BAG-1, with a critical component related to its interaction with HSC70/HSP70 and cytoplasmic localization. In addition, subsequent studies have identified that autophagy plays an important role in the adaptation to ischemia-reperfusion injury in association with BAG-1 84. BAG-1 associates with the autophagosomal membrane protein LC3-II and may induce autophagy using HSC70 84, 119. Intracardial injection of BAG-1 siRNA attenuates the induction of LC3-II and abolishes the cardioprotection achieved by adaption 119. The BAG-3 isoform participates in the induction of macroautophagy in association with HSP22 84, demonstrating how BAG family members may shuttle damaged or oxidized proteins into the autophagy pathway to improve cell survival84.
The co-chaperone CHIP (carboxy terminus of HSP70 interacting protein) is one protein that plays a key role in both the folding system (as a co-chaperone regulating HSP70) and in the UPS as a ubiquitin ligase. CHIP directs the degradation of aggregate prone proteins120, 121, such as poly-glutamine proteins, which are prevalent in conformation diseases such as Alzheimer or Huntington’s disease122–124. Although CHIP binds HSP70, it can also target it for degradation in the absence of cargo, possibly as a feedback mechanism to adjust chaperone levels needed for the number of misfolded proteins (Figure 1B) 50. Recent studies have identified BAG-2, a specific inhibitor of CHIP-dependent ubiquitin ligase activity, as a common component of CHIP holocomplexes in vivo 125. CHIP plays an important role in the heart in response to ischemia/reperfusion injury. When CHIP −/− mice are challenged with ischemia/reperfusion injury in vivo, the ratio of the infarct area to the area of risk is 50% greater than that found in sibling wild type mice 85. CHIP −/− hearts are more prone to cell death indicating a critical role of CHIP in ischemia/reperfusion injury. These studies parallel the role of BAG proteins described above, indicating a critical role of CHIP in shuttling damaged and oxidized proteins into autophagic pathways after ischemia/reperfusion injury. The specific role of CHIP in autophagy has yet to be reported. Cardiomyocyte CHIP increases in response to high glucose and is responsible for the degradation of the pro-hypertrophic transcription factor GATA4 81. The significance of these findings in cardiac disease has yet to be reported.
The co-chaperone FKBP38 is an immunophilin-type small heat shock protein that has recently been implicated in the maturation of HERG (human Ether-à-go-go Related Gene, also known as KCNH2 in newer nomenclature)126. The HERG gene encodes the voltage-dependent delayed rectifier potassium channel (IKR) and mutations in HERG are among the most common underlying cause of hereditary Long QT syndrome. Recent studies have utilized proteomic screens to identify that HSC70, HSP90, HDJ2 (HSP-organizing protein) and BAG-2 are differentially expressed in models of Long QT syndrome caused by mutations in HERG 126. However, the most relevant findings of these studies are that the co-chaperone FKBP38 immunoprecipitates and co-localizes with HERG 126. Additionally, siRNA knock-down of FKBP38 causes a reduction in HERG trafficking and overexpression of wild type FKBP38 partially rescues HERG trafficking in the presence of F805C disease-causing KCNH2 mutation126. These studies suggest an important role for the co-chaperone FKBP38 in rescuing mutations in KCNH2 that lead to the Long QT syndrome.
A picture of the heat shock protein system as a mediator of protein quality control is emerging. Specific co-chaperones such as CHIP have the ability to ubiquitinate proteins that the chaperone/co-chaperone complex is unable to refold (Figure 1B). The ubiquitination of key structural proteins, such as sarcomere proteins and transcription factors are critical to the long term health of the heart (see recent reviews43, 51, 127, 128). These co-chaperones represent a system of triage whereby protein quality is maintained, and in the long run, the health of the cardiomyocyte is maintained.
Future directions: The role of drug therapies in cardiac health and pathophysiology
Drugs that induce heat shock proteins
A number of studies reviewed here have tested the hypothesis that increasing heat shock proteins improves the outcome of cardiac diseases experimentally, particularly in the context of ischemia/reperfusion injury. While these studies were primarily proof of concept that increasing heat shock proteins were cardioprotective, it is interesting to note that the fold increase in these proteins was as little as 2. From a clinical standpoint, there are several drugs and herbal products that increase heat shock proteins that may be beneficial in the treatment of cardiac diseases. However, their clinical utility has yet to be tested experimentally in the context of their regulation of heat shock proteins.
Geranylgeranylacetone (GGA)
GGA is a cyclic polyisoprenoid gastric ulcer drug that protects the gastric mucosa by inducing HSF1 and HSP70 mRNA 129. It has recently been shown experimentally to be cardioprotective by inducing HSP72 130, 131. It has also been shown experimentally to suppress poly-glutamine toxicity (see recent review 132).
Arimoclomol
Arimoclomol, developed by CytRx, is a small molecule that acts by inducing HSF1 resulting in downstream increases of HSP70 and HSP90 133. Experimentally, arimoclomol increases HSP70 and HSP90 approximately 5 fold in an experimental model of ALS 134. Arimoclomol is currently in phase II/II clinical trials as a treatment for ALS.
Celastrol
Celastrol is a triterpenoid compound with a retinoid skeleton extracted from Tripterygium wilfordii that is used in traditional Chinese medicine. It potently induces HSF1 and HSP70 expression, having both anti-oxidant and anti-inflammatory activities 135. Celastrol has been shown to ameliorate the neurodegeneration of SOD1 mutant mice; however, it has not been determined if this is through its anti-inflammatory effects and/or its affect on HSF1 and HSP70 induction 136.
Statins
In addition to their ability to decrease cholesterol synthesis via inhibition of HMG-CoA, statins have been shown to have many additional activities, including modulation of the immune system, reduction in apoptosis, and an affect on nitric oxide production 137–139. Both simvastatin and lovastatin induce HSP27, but not HSP70 and HSP90 in an osteoblast-like cell line 140. Simvastatin induces HSF1 in vascular endothelial cells, to induce nuclear translocation and the transcription of HSP70 and HSP90 141. Simvastatin induces HSP27 in axotomized retinal ganglion cells to enhance their survival after optic nerve transaction 142 Statins increase HSF1 and HSF-2 in retinal ganglion cells in vivo 143. Statins act to increase HSFs, HSP70, HSP90, and sHSPs, possibly in a cell dependent manner. Their effect on cardiac heat shock proteins has not been identified to date. With the discovery of statin’s ability to induce heat shock proteins, studies have identified a decreased prevalence of Alzheimer’s disease in patients taking statins 144, 145 and a decrease in neurofibrulary tangles 145. These studies suggest that a several drugs, including the widely prescribed statins, have the potential to be cardioprotective due to their ability to prime critical heat shock proteins in the heart. The use of most of these drugs has yet to be determined in human studies.
Conclusion
During the development of cardiac hypertrophy, heart failure, and ischemia/reperfusion injury, there is a general increase in a number of chaperones, co-chaperones, and the transcription factors that regulate them. In this review, we discuss the their uniformly protective mechanisms and the possibility that therapeutic regulation may enhance both acute and chronic health of the heart. With the recent discovery that increases in soluble pre-amyloid oligomers play a significant role in cardiac disease and are able to induce cardiomyopathy experimentally5, there is a need for a rational way to increase chaperone/co-chaperone function to combat the accumulation of misfolded proteins. A number of drugs with the potential to increase heat shock proteins are being developed for neurodegenerative diseases caused by misfolded proteins (i.e. poly-glutamine diseases such as Huntington’s disease). Given the parallel mechanisms found in cardiac diseases and the overwhelming evidence that increasing chaperones/co-chaperones is cardioprotective against the most common cardiac diseases, there may be future clinical applicability in cardiology of these drugs.
Acknowledgments
Funding: The authors are supported by the American Heart Associations (Scientist Development Grant to M.S.W.) and the National Heart, Lung, and Blood Institute (R01HL065619 to C.P).
Non-standard abbreviations
- BAG
Bcl2-associated athanogene
- CHIP
carboxy terminus of HSP70-binding protein
- CryAB
alphaB-crystallin
- ERAD
endoplasmic reticulum-associated degradation
- HDAC
histone de-acetylase
- HSC
cognate of heat shock protein
- HSF
heat shock factor
- HSP
heat shock protein
- PAO
preamyloid oligomers
- PolyQ
poly-glutamine
- TRiC
(TCP1-ring complex, or chaperonin containing TCP1 (CCT)
- UPS
Ubiquitin proteasome system
Footnotes
Disclosures: The authors do not have any potential conflicts of interest relevant to this article to report.
References
- 1.Sanbe A, Osinska H, Saffitz JE, Glabe CG, Kayed R, Maloyan A, Robbins J. Desmin-related cardiomyopathy in transgenic mice: a cardiac amyloidosis. Proc Natl Acad Sci U S A. 2004;101:10132–10136. doi: 10.1073/pnas.0401900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tannous P, Zhu H, Nemchenko A, Berry JM, Johnstone JL, Shelton JM, Miller FJ, Jr, Rothermel BA, Hill JA. Intracellular Protein Aggregation Is a Proximal Trigger of Cardiomyocyte Autophagy. Circulation. 2008 doi: 10.1161/CIRCULATIONAHA.107.763870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothermel BA, Hill JA. The heart of autophagy: Deconstructing cardiac proteotoxicity. Autophagy. 2008:4. doi: 10.4161/auto.6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothermel BA, Hill JA. Autophagy in load-induced heart disease. Circ Res. 2008;103:1363–1369. doi: 10.1161/CIRCRESAHA.108.186551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pattison JS, Sanbe A, Maloyan A, Osinska H, Klevitsky R, Robbins J. Cardiomyocyte expression of a polyglutamine preamyloid oligomer causes heart failure. Circulation. 2008;117:2743–2751. doi: 10.1161/CIRCULATIONAHA.107.750232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kubota H. Quality control against misfolded proteins in the cytosol: a network for cell survival. J Biochem. 2009;146:609–616. doi: 10.1093/jb/mvp139. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Chen L, Hagiwara N, Knowlton AA. Regulation of heat shock protein 60 and 72 expression in the failing heart. J Mol Cell Cardiol. 2010;48:360–366. doi: 10.1016/j.yjmcc.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wegele H, Muller L, Buchner J. Hsp70 and Hsp90--a relay team for protein folding. Rev Physiol Biochem Pharmacol. 2004;151:1–44. doi: 10.1007/s10254-003-0021-1. [DOI] [PubMed] [Google Scholar]
- 9.Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–294. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- 10.Zhao R, Davey M, Hsu YC, Kaplanek P, Tong A, Parsons AB, Krogan N, Cagney G, Mai D, Greenblatt J, Boone C, Emili A, Houry WA. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell. 2005;120:715–727. doi: 10.1016/j.cell.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 11.Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- 12.Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 13.Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young JC. Mechanisms of the Hsp70 chaperone system. Biochem Cell Biol. 2010;88:291–300. doi: 10.1139/o09-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young JC, Agashe VR, Siegers K, Hartl FU. Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol. 2004;5:781–791. doi: 10.1038/nrm1492. [DOI] [PubMed] [Google Scholar]
- 16.Young JC, Barral JM, Ulrich Hartl F. More than folding: localized functions of cytosolic chaperones. Trends Biochem Sci. 2003;28:541–547. doi: 10.1016/j.tibs.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Kubota H. Function and regulation of cytosolic molecular chaperone CCT. Vitam Horm. 2002;65:313–331. doi: 10.1016/s0083-6729(02)65069-1. [DOI] [PubMed] [Google Scholar]
- 18.Spiess C, Meyer AS, Reissmann S, Frydman J. Mechanism of the eukaryotic chaperonin: protein folding in the chamber of secrets. Trends Cell Biol. 2004;14:598–604. doi: 10.1016/j.tcb.2004.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ecroyd H, Carver JA. Crystallin proteins and amyloid fibrils. Cell Mol Life Sci. 2009;66:62–81. doi: 10.1007/s00018-008-8327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haslbeck M, Franzmann T, Weinfurtner D, Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol. 2005;12:842–846. doi: 10.1038/nsmb993. [DOI] [PubMed] [Google Scholar]
- 21.Arrigo AP, Simon S, Gibert B, Kretz-Remy C, Nivon M, Czekalla A, Guillet D, Moulin M, Diaz-Latoud C, Vicart P. Hsp27 (HspB1) and alphaB-crystallin (HspB5) as therapeutic targets. FEBS Lett. 2007;581:3665–3674. doi: 10.1016/j.febslet.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 22.Efthymiou CA, Mocanu MM, de Belleroche J, Wells DJ, Latchmann DS, Yellon DM. Heat shock protein 27 protects the heart against myocardial infarction. Basic Res Cardiol. 2004;99:392–394. doi: 10.1007/s00395-004-0483-6. [DOI] [PubMed] [Google Scholar]
- 23.Brown DD, Christine KS, Showell C, Conlon FL. Small heat shock protein Hsp27 is required for proper heart tube formation. Genesis. 2007;45:667–678. doi: 10.1002/dvg.20340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennardini F, Wrzosek A, Chiesi M. Alpha B-crystallin in cardiac tissue. Association with actin and desmin filaments. Circ Res. 1992;71:288–294. doi: 10.1161/01.res.71.2.288. [DOI] [PubMed] [Google Scholar]
- 25.Djabali K, de Nechaud B, Landon F, Portier MM. AlphaB-crystallin interacts with intermediate filaments in response to stress. J Cell Sci. 1997;110 ( Pt 21):2759–2769. doi: 10.1242/jcs.110.21.2759. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Klevitsky R, Huang W, Glasford J, Li F, Robbins J. AlphaB-crystallin modulates protein aggregation of abnormal desmin. Circ Res. 2003;93:998–1005. doi: 10.1161/01.RES.0000102401.77712.ED. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Osinska H, Gerdes AM, Robbins J. Desmin filaments and cardiac disease: establishing causality. J Card Fail. 2002;8:S287–292. doi: 10.1054/jcaf.2002.129279. [DOI] [PubMed] [Google Scholar]
- 28.Gupta RS. Evolution of the chaperonin families (Hsp60, Hsp10 and Tcp-1) of proteins and the origin of eukaryotic cells. Mol Microbiol. 1995;15:1–11. doi: 10.1111/j.1365-2958.1995.tb02216.x. [DOI] [PubMed] [Google Scholar]
- 29.Soltys BJ, Gupta RS. Immunoelectron microscopic localization of the 60-kDa heat shock chaperonin protein (Hsp60) in mammalian cells. Exp Cell Res. 1996;222:16–27. doi: 10.1006/excr.1996.0003. [DOI] [PubMed] [Google Scholar]
- 30.Knowlton AA, Srivatsa U. Heat-shock protein 60 and cardiovascular disease: a paradoxical role. Future Cardiol. 2008;4:151–161. doi: 10.2217/14796678.4.2.151. [DOI] [PubMed] [Google Scholar]
- 31.Kirchhoff SR, Gupta S, Knowlton AA. Cytosolic heat shock protein 60, apoptosis, and myocardial injury. Circulation. 2002;105:2899–2904. doi: 10.1161/01.cir.0000019403.35847.23. [DOI] [PubMed] [Google Scholar]
- 32.Shan YX, Liu TJ, Su HF, Samsamshariat A, Mestril R, Wang PH. Hsp10 and Hsp60 modulate Bcl-2 family and mitochondria apoptosis signaling induced by doxorubicin in cardiac muscle cells. J Mol Cell Cardiol. 2003;35:1135–1143. doi: 10.1016/s0022-2828(03)00229-3. [DOI] [PubMed] [Google Scholar]
- 33.Richarme G, Kohiyama M. Amino acid specificity of the Escherichia coli chaperone GroEL (heat shock protein 60) J Biol Chem. 1994;269:7095–7098. [PubMed] [Google Scholar]
- 34.Summers DW, Douglas PM, Ramos CH, Cyr DM. Polypeptide transfer from Hsp40 to Hsp70 molecular chaperones. Trends Biochem Sci. 2009;34:230–233. doi: 10.1016/j.tibs.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gassler CS, Wiederkehr T, Brehmer D, Bukau B, Mayer MP. Bag-1M accelerates nucleotide release for human Hsc70 and Hsp70 and can act concentration-dependent as positive and negative cofactor. J Biol Chem. 2001;276:32538–32544. doi: 10.1074/jbc.M105328200. [DOI] [PubMed] [Google Scholar]
- 36.Jiang J, Ballinger CA, Wu Y, Dai Q, Cyr DM, Hohfeld J, Patterson C. CHIP is a U-box-dependent E3 ubiquitin ligase: identification of Hsc70 as a target for ubiquitylation. J Biol Chem. 2001;276:42938–42944. doi: 10.1074/jbc.M101968200. [DOI] [PubMed] [Google Scholar]
- 37.Demand J, Alberti S, Patterson C, Hohfeld J. Cooperation of a ubiquitin domain protein and an E3 ubiquitin ligase during chaperone/proteasome coupling. Curr Biol. 2001;11:1569–1577. doi: 10.1016/s0960-9822(01)00487-0. [DOI] [PubMed] [Google Scholar]
- 38.Lundin VF, Stirling PC, Gomez-Reino J, Mwenifumbo JC, Obst JM, Valpuesta JM, Leroux MR. Molecular clamp mechanism of substrate binding by hydrophobic coiled-coil residues of the archaeal chaperone prefoldin. Proc Natl Acad Sci U S A. 2004;101:4367–4372. doi: 10.1073/pnas.0306276101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohtaki A, Kida H, Miyata Y, Ide N, Yonezawa A, Arakawa T, Iizuka R, Noguchi K, Kita A, Odaka M, Miki K, Yohda M. Structure and molecular dynamics simulation of archaeal prefoldin: the molecular mechanism for binding and recognition of nonnative substrate proteins. J Mol Biol. 2008;376:1130–1141. doi: 10.1016/j.jmb.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 40.Conde R, Belak ZR, Nair M, O’Carroll RF, Ovsenek N. Modulation of Hsf1 activity by novobiocin and geldanamycin. Biochem Cell Biol. 2009;87:845–851. doi: 10.1139/o09-049. [DOI] [PubMed] [Google Scholar]
- 41.Anckar J, Sistonen L. Heat shock factor 1 as a coordinator of stress and developmental pathways. Adv Exp Med Biol. 2007;594:78–88. doi: 10.1007/978-0-387-39975-1_8. [DOI] [PubMed] [Google Scholar]
- 42.Shi Y, Mosser DD, Morimoto RI. Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev. 1998;12:654–666. doi: 10.1101/gad.12.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willis MS, Townley-Tilson WH, Kang EY, Homeister JW, Patterson C. Sent to destroy: the ubiquitin proteasome system regulates cell signaling and protein quality control in cardiovascular development and disease. Circ Res. 2010;106:463–478. doi: 10.1161/CIRCRESAHA.109.208801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ostling P, Bjork JK, Roos-Mattjus P, Mezger V, Sistonen L. Heat shock factor 2 (HSF2) contributes to inducible expression of hsp genes through interplay with HSF1. J Biol Chem. 2007;282:7077–7086. doi: 10.1074/jbc.M607556200. [DOI] [PubMed] [Google Scholar]
- 45.Fujimoto M, Hayashida N, Katoh T, Oshima K, Shinkawa T, Prakasam R, Tan K, Inouye S, Takii R, Nakai A. A novel mouse HSF3 has the potential to activate nonclassical heat-shock genes during heat shock. Mol Biol Cell. 2010;21:106–116. doi: 10.1091/mbc.E09-07-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujimoto M, Izu H, Seki K, Fukuda K, Nishida T, Yamada S, Kato K, Yonemura S, Inouye S, Nakai A. HSF4 is required for normal cell growth and differentiation during mouse lens development. EMBO J. 2004;23:4297–4306. doi: 10.1038/sj.emboj.7600435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu J, Bao E, Yan J, Lei L. Expression and localization of Hsps in the heart and blood vessel of heat-stressed broilers. Cell Stress Chaperones. 2008;13:327–335. doi: 10.1007/s12192-008-0031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li R, Soosairajah J, Harari D, Citri A, Price J, Ng HL, Morton CJ, Parker MW, Yarden Y, Bernard O. Hsp90 increases LIM kinase activity by promoting its homo-dimerization. FASEB J. 2006;20:1218–1220. doi: 10.1096/fj.05-5258fje. [DOI] [PubMed] [Google Scholar]
- 49.Vondriska TM, Wang Y. A new (heat) shocking player in cardiac hypertrophy. Circ Res. 2008;103:1194–1196. doi: 10.1161/CIRCRESAHA.108.189118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qian SB, McDonough H, Boellmann F, Cyr DM, Patterson C. CHIP-mediated stress recovery by sequential ubiquitination of substrates and Hsp70. Nature. 2006;440:551–555. doi: 10.1038/nature04600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Willis MS, Schisler JC, Portbury AL, Patterson C. Build it up-Tear it down: protein quality control in the cardiac sarcomere. Cardiovasc Res. 2009;81:439–448. doi: 10.1093/cvr/cvn289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldfarb LG, Dalakas MC. Tragedy in a heartbeat: malfunctioning desmin causes skeletal and cardiac muscle disease. J Clin Invest. 2009;119:1806–1813. doi: 10.1172/JCI38027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tessier DJ, Komalavilas P, Panitch A, Joshi L, Brophy CM. The small heat shock protein (HSP) 20 is dynamically associated with the actin cross-linking protein actinin. J Surg Res. 2003;111:152–157. doi: 10.1016/s0022-4804(03)00113-6. [DOI] [PubMed] [Google Scholar]
- 54.Chowdary TK, Raman B, Ramakrishna T, Rao Ch M. Interaction of mammalian Hsp22 with lipid membranes. Biochem J. 2007;401:437–445. doi: 10.1042/BJ20061046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parcellier A, Schmitt E, Gurbuxani S, Seigneurin-Berny D, Pance A, Chantome A, Plenchette S, Khochbin S, Solary E, Garrido C. HSP27 is a ubiquitin-binding protein involved in I-kappaBalpha proteasomal degradation. Mol Cell Biol. 2003;23:5790–5802. doi: 10.1128/MCB.23.16.5790-5802.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin L, Kim SC, Wang Y, Gupta S, Davis B, Simon SI, Torre-Amione G, Knowlton AA. HSP60 in heart failure: abnormal distribution and role in cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol. 2007;293:H2238–2247. doi: 10.1152/ajpheart.00740.2007. [DOI] [PubMed] [Google Scholar]
- 57.Kee HJ, Eom GH, Joung H, Shin S, Kim JR, Cho YK, Choe N, Sim BW, Jo D, Jeong MH, Kim KK, Seo JS, Kook H. Activation of histone deacetylase 2 by inducible heat shock protein 70 in cardiac hypertrophy. Circ Res. 2008;103:1259–1269. doi: 10.1161/01.RES.0000338570.27156.84. [DOI] [PubMed] [Google Scholar]
- 58.Agnetti G, Bezstarosti K, Dekkers DH, Verhoeven AJ, Giordano E, Guarnieri C, Caldarera CM, Van Eyk JE, Lamers JM. Proteomic profiling of endothelin-1-stimulated hypertrophic cardiomyocytes reveals the increase of four different desmin species and alpha-B-crystallin. Biochim Biophys Acta. 2008;1784:1068–1076. doi: 10.1016/j.bbapap.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Depre C, Hase M, Gaussin V, Zajac A, Wang L, Hittinger L, Ghaleh B, Yu X, Kudej RK, Wagner T, Sadoshima J, Vatner SF. H11 kinase is a novel mediator of myocardial hypertrophy in vivo. Circ Res. 2002;91:1007–1014. doi: 10.1161/01.res.0000044380.54893.4b. [DOI] [PubMed] [Google Scholar]
- 60.Knowlton AA, Kapadia S, Torre-Amione G, Durand JB, Bies R, Young J, Mann DL. Differential expression of heat shock proteins in normal and failing human hearts. J Mol Cell Cardiol. 1998;30:811–818. doi: 10.1006/jmcc.1998.0646. [DOI] [PubMed] [Google Scholar]
- 61.Tanonaka K, Toga W, Yoshida H, Takeo S. Myocardial heat shock protein changes in the failing heart following coronary artery ligation. Heart Lung Circ. 2003;12:60–65. doi: 10.1046/j.1444-2892.2003.00139.x. [DOI] [PubMed] [Google Scholar]
- 62.Toga W, Tanonaka K, Takeo S. Changes in Hsp60 level of the failing heart following acute myocardial infarction and the effect of long-term treatment with trandolapril. Biol Pharm Bull. 2007;30:105–110. doi: 10.1248/bpb.30.105. [DOI] [PubMed] [Google Scholar]
- 63.Sidorik L, Kyyamova R, Bobyk V, Kapustian L, Rozhko O, Vigontina O, Ryabenko D, Danko I, Maksymchuk O, Kovalenko VN, Filonenko VV, Chaschin NA. Molecular chaperone, HSP60, and cytochrome P450 2E1 co-expression in dilated cardiomyopathy. Cell Biol Int. 2005;29:51–55. doi: 10.1016/j.cellbi.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 64.Niizeki T, Takeishi Y, Watanabe T, Nitobe J, Miyashita T, Miyamoto T, Kitahara T, Suzuki S, Sasaki T, Bilim O, Ishino M, Kubota I. Relation of serum heat shock protein 60 level to severity and prognosis in chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2008;102:606–610. doi: 10.1016/j.amjcard.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 65.Nishizawa J, Nakai A, Higashi T, Tanabe M, Nomoto S, Matsuda K, Ban T, Nagata K. Reperfusion causes significant activation of heat shock transcription factor 1 in ischemic rat heart. Circulation. 1996;94:2185–2192. doi: 10.1161/01.cir.94.9.2185. [DOI] [PubMed] [Google Scholar]
- 66.Ren XP, Wu J, Wang X, Sartor MA, Qian J, Jones K, Nicolaou P, Pritchard TJ, Fan GC. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation. 2009;119:2357–2366. doi: 10.1161/CIRCULATIONAHA.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Depre C, Tomlinson JE, Kudej RK, Gaussin V, Thompson E, Kim SJ, Vatner DE, Topper JN, Vatner SF. Gene program for cardiac cell survival induced by transient ischemia in conscious pigs. Proc Natl Acad Sci U S A. 2001;98:9336–9341. doi: 10.1073/pnas.171297498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Depre C, Kim SJ, John AS, Huang Y, Rimoldi OE, Pepper JR, Dreyfus GD, Gaussin V, Pennell DJ, Vatner DE, Camici PG, Vatner SF. Program of cell survival underlying human and experimental hibernating myocardium. Circ Res. 2004;95:433–440. doi: 10.1161/01.RES.0000138301.42713.18. [DOI] [PubMed] [Google Scholar]
- 69.Wilkerson DC, Skaggs HS, Sarge KD. HSF2 binds to the Hsp90, Hsp27, and c-Fos promoters constitutively and modulates their expression. Cell Stress Chaperones. 2007;12:283–290. doi: 10.1379/CSC-250.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoshima T, Yura T, Yanagi H. The trimerization domain of human heat shock factor 2 is able to interact with nucleoporin p62. Biochem Biophys Res Commun. 1997;240:228–233. doi: 10.1006/bbrc.1997.7662. [DOI] [PubMed] [Google Scholar]
- 71.Nishizawa J, Nakai A, Komeda M, Ban T, Nagata K. Increased preload directly induces the activation of heat shock transcription factor 1 in the left ventricular overloaded heart. Cardiovasc Res. 2002;55:341–348. doi: 10.1016/s0008-6363(02)00404-2. [DOI] [PubMed] [Google Scholar]
- 72.Nishizawa J, Nakai A, Matsuda K, Komeda M, Ban T, Nagata K. Reactive oxygen species play an important role in the activation of heat shock factor 1 in ischemic-reperfused heart. Circulation. 1999;99:934–941. doi: 10.1161/01.cir.99.7.934. [DOI] [PubMed] [Google Scholar]
- 73.Chang J, Knowlton AA, Xu F, Wasser JS. Activation of the heat shock response: relationship to energy metabolites. A (31)P NMR study in rat hearts. Am J Physiol Heart Circ Physiol. 2001;280:H426–433. doi: 10.1152/ajpheart.2001.280.1.H426. [DOI] [PubMed] [Google Scholar]
- 74.Hendrick JP, Langer T, Davis TA, Hartl FU, Wiedmann M. Control of folding and membrane translocation by binding of the chaperone DnaJ to nascent polypeptides. Proc Natl Acad Sci U S A. 1993;90:10216–10220. doi: 10.1073/pnas.90.21.10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang HG, Takayama S, Rapp UR, Reed JC. Bcl-2 interacting protein, BAG-1, binds to and activates the kinase Raf-1. Proc Natl Acad Sci U S A. 1996;93:7063–7068. doi: 10.1073/pnas.93.14.7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carrigan PE, Nelson GM, Roberts PJ, Stoffer J, Riggs DL, Smith DF. Multiple domains of the co-chaperone Hop are important for Hsp70 binding. J Biol Chem. 2004;279:16185–16193. doi: 10.1074/jbc.M314130200. [DOI] [PubMed] [Google Scholar]
- 77.Liu YV, Baek JH, Zhang H, Diez R, Cole RN, Semenza GL. RACK1 competes with HSP90 for binding to HIF-1alpha and is required for O(2)-independent and HSP90 inhibitor-induced degradation of HIF-1alpha. Mol Cell. 2007;25:207–217. doi: 10.1016/j.molcel.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bai X, Ma D, Liu A, Shen X, Wang QJ, Liu Y, Jiang Y. Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38. Science. 2007;318:977–980. doi: 10.1126/science.1147379. [DOI] [PubMed] [Google Scholar]
- 79.Rommelaere H, De Neve M, Neirynck K, Peelaers D, Waterschoot D, Goethals M, Fraeyman N, Vandekerckhove J, Ampe C. Prefoldin recognition motifs in the nonhomologous proteins of the actin and tubulin families. J Biol Chem. 2001;276:41023–41028. doi: 10.1074/jbc.M106591200. [DOI] [PubMed] [Google Scholar]
- 80.Hansen WJ, Cowan NJ, Welch WJ. Prefoldin-nascent chain complexes in the folding of cytoskeletal proteins. J Cell Biol. 1999;145:265–277. doi: 10.1083/jcb.145.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kobayashi S, Mao K, Zheng H, Wang X, Patterson C, O’Connell TD, Liang Q. Diminished GATA4 protein levels contribute to hyperglycemia-induced cardiomyocyte injury. J Biol Chem. 2007;282:21945–21952. doi: 10.1074/jbc.M703048200. [DOI] [PubMed] [Google Scholar]
- 82.Depre C, Wang L, Tomlinson JE, Gaussin V, Abdellatif M, Topper JN, Vatner SF. Characterization of pDJA1, a cardiac-specific chaperone found by genomic profiling of the post-ischemic swine heart. Cardiovasc Res. 2003;58:126–135. doi: 10.1016/s0008-6363(02)00845-3. [DOI] [PubMed] [Google Scholar]
- 83.Townsend PA, Cutress RI, Carroll CJ, Lawrence KM, Scarabelli TM, Packham G, Stephanou A, Latchman DS. BAG-1 proteins protect cardiac myocytes from simulated ischemia/reperfusion-induced apoptosis via an alternate mechanism of cell survival independent of the proteasome. J Biol Chem. 2004;279:20723–20728. doi: 10.1074/jbc.M400399200. [DOI] [PubMed] [Google Scholar]
- 84.Gurusamy N, Lekli I, Gherghiceanu M, Popescu LM, Das DK. BAG-1 induces autophagy for cardiac cell survival. Autophagy. 2009;5:120–121. doi: 10.4161/auto.5.1.7303. [DOI] [PubMed] [Google Scholar]
- 85.Zhang C, Xu Z, He XR, Michael LH, Patterson C. CHIP, a cochaperone/ubiquitin ligase that regulates protein quality control, is required for maximal cardioprotection after myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2005;288:H2836–2842. doi: 10.1152/ajpheart.01122.2004. [DOI] [PubMed] [Google Scholar]
- 86.Kumarapeli AR, Su H, Huang W, Tang M, Zheng H, Horak KM, Li M, Wang X. Alpha B-crystallin suppresses pressure overload cardiac hypertrophy. Circ Res. 2008;103:1473–1482. doi: 10.1161/CIRCRESAHA.108.180117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang CL, McKinsey TA, Chang S, Antos CL, Hill JA, Olson EN. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell. 2002;110:479–488. doi: 10.1016/s0092-8674(02)00861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hamamori Y, Schneider MD. HATs off to Hop: recruitment of a class I histone deacetylase incriminates a novel transcriptional pathway that opposes cardiac hypertrophy. J Clin Invest. 2003;112:824–826. doi: 10.1172/JCI19834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Danan IJ, Rashed ER, Depre C. Therapeutic potential of H11 kinase for the ischemic heart. Cardiovasc Drug Rev. 2007;25:14–29. doi: 10.1111/j.1527-3466.2007.00002.x. [DOI] [PubMed] [Google Scholar]
- 90.Depre C, Wang L, Sui X, Qiu H, Hong C, Hedhli N, Ginion A, Shah A, Pelat M, Bertrand L, Wagner T, Gaussin V, Vatner SF. H11 kinase prevents myocardial infarction by preemptive preconditioning of the heart. Circ Res. 2006;98:280–288. doi: 10.1161/01.RES.0000201284.45482.e8. [DOI] [PubMed] [Google Scholar]
- 91.Toko H, Minamino T, Komuro I. Role of heat shock transcriptional factor 1 and heat shock proteins in cardiac hypertrophy. Trends Cardiovasc Med. 2008;18:88–93. doi: 10.1016/j.tcm.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 92.Sakamoto M, Minamino T, Toko H, Kayama Y, Zou Y, Sano M, Takaki E, Aoyagi T, Tojo K, Tajima N, Nakai A, Aburatani H, Komuro I. Upregulation of heat shock transcription factor 1 plays a critical role in adaptive cardiac hypertrophy. Circ Res. 2006;99:1411–1418. doi: 10.1161/01.RES.0000252345.80198.97. [DOI] [PubMed] [Google Scholar]
- 93.Richey PA, Brown SP. Pathological versus physiological left ventricular hypertrophy: a review. J Sports Sci. 1998;16:129–141. doi: 10.1080/026404198366849. [DOI] [PubMed] [Google Scholar]
- 94.Iemitsu M, Miyauchi T, Maeda S, Sakai S, Kobayashi T, Fujii N, Miyazaki H, Matsuda M, Yamaguchi I. Physiological and pathological cardiac hypertrophy induce different molecular phenotypes in the rat. Am J Physiol Regul Integr Comp Physiol. 2001;281:R2029–2036. doi: 10.1152/ajpregu.2001.281.6.R2029. [DOI] [PubMed] [Google Scholar]
- 95.McMullen JR, Jennings GL. Differences between pathological and physiological cardiac hypertrophy: novel therapeutic strategies to treat heart failure. Clin Exp Pharmacol Physiol. 2007;34:255–262. doi: 10.1111/j.1440-1681.2007.04585.x. [DOI] [PubMed] [Google Scholar]
- 96.Quindry JC, Hamilton KL, French JP, Lee Y, Murlasits Z, Tumer N, Powers SK. Exercise-induced HSP-72 elevation and cardioprotection against infarct and apoptosis. J Appl Physiol. 2007;103:1056–1062. doi: 10.1152/japplphysiol.00263.2007. [DOI] [PubMed] [Google Scholar]
- 97.Wick G, Knoflach M, Xu Q. Autoimmune and inflammatory mechanisms in atherosclerosis. Annu Rev Immunol. 2004;22:361–403. doi: 10.1146/annurev.immunol.22.012703.104644. [DOI] [PubMed] [Google Scholar]
- 98.Henderson B. Integrating the cell stress response: a new view of molecular chaperones as immunological and physiological homeostatic regulators. Cell Biochem Funct. 28:1–14. doi: 10.1002/cbf.1609. [DOI] [PubMed] [Google Scholar]
- 99.Pockley AG, Bulmer J, Hanks BM, Wright BH. Identification of human heat shock protein 60 (Hsp60) and anti-Hsp60 antibodies in the peripheral circulation of normal individuals. Cell Stress Chaperones. 1999;4:29–35. doi: 10.1054/csac.1998.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nakano M, Mann DL, Knowlton AA. Blocking the endogenous increase in HSP72 increases susceptibility to hypoxia and reoxygenation in isolated adult feline cardiocytes. Circulation. 1997;95:1523–1531. doi: 10.1161/01.cir.95.6.1523. [DOI] [PubMed] [Google Scholar]
- 101.Okubo S, Wildner O, Shah MR, Chelliah JC, Hess ML, Kukreja RC. Gene transfer of heat-shock protein 70 reduces infarct size in vivo after ischemia/reperfusion in the rabbit heart. Circulation. 2001;103:877–881. doi: 10.1161/01.cir.103.6.877. [DOI] [PubMed] [Google Scholar]
- 102.Marber MS, Mestril R, Chi SH, Sayen MR, Yellon DM, Dillmann WH. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest. 1995;95:1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Plumier JC, Ross BM, Currie RW, Angelidis CE, Kazlaris H, Kollias G, Pagoulatos GN. Transgenic mice expressing the human heat shock protein 70 have improved post-ischemic myocardial recovery. J Clin Invest. 1995;95:1854–1860. doi: 10.1172/JCI117865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Radford NB, Fina M, Benjamin IJ, Moreadith RW, Graves KH, Zhao P, Gavva S, Wiethoff A, Sherry AD, Malloy CR, Williams RS. Cardioprotective effects of 70-kDa heat shock protein in transgenic mice. Proc Natl Acad Sci U S A. 1996;93:2339–2342. doi: 10.1073/pnas.93.6.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Trost SU, Omens JH, Karlon WJ, Meyer M, Mestril R, Covell JW, Dillmann WH. Protection against myocardial dysfunction after a brief ischemic period in transgenic mice expressing inducible heat shock protein 70. J Clin Invest. 1998;101:855–862. doi: 10.1172/JCI265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chu G, Egnaczyk GF, Zhao W, Jo SH, Fan GC, Maggio JE, Xiao RP, Kranias EG. Phosphoproteome analysis of cardiomyocytes subjected to beta-adrenergic stimulation: identification and characterization of a cardiac heat shock protein p20. Circ Res. 2004;94:184–193. doi: 10.1161/01.RES.0000107198.90218.21. [DOI] [PubMed] [Google Scholar]
- 107.Fan GC, Chu G, Mitton B, Song Q, Yuan Q, Kranias EG. Small heat-shock protein Hsp20 phosphorylation inhibits beta-agonist-induced cardiac apoptosis. Circ Res. 2004;94:1474–1482. doi: 10.1161/01.RES.0000129179.66631.00. [DOI] [PubMed] [Google Scholar]
- 108.Fan GC, Ren X, Qian J, Yuan Q, Nicolaou P, Wang Y, Jones WK, Chu G, Kranias EG. Novel cardioprotective role of a small heat-shock protein, Hsp20, against ischemia/reperfusion injury. Circulation. 2005;111:1792–1799. doi: 10.1161/01.CIR.0000160851.41872.C6. [DOI] [PubMed] [Google Scholar]
- 109.Qian J, Ren X, Wang X, Zhang P, Jones WK, Molkentin JD, Fan GC, Kranias EG. Blockade of Hsp20 phosphorylation exacerbates cardiac ischemia/reperfusion injury by suppressed autophagy and increased cell death. Circ Res. 2009;105:1223–1231. doi: 10.1161/CIRCRESAHA.109.200378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fan GC, Zhou X, Wang X, Song G, Qian J, Nicolaou P, Chen G, Ren X, Kranias EG. Heat shock protein 20 interacting with phosphorylated Akt reduces doxorubicin-triggered oxidative stress and cardiotoxicity. Circ Res. 2008;103:1270–1279. doi: 10.1161/CIRCRESAHA.108.182832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sui X, Li D, Qiu H, Gaussin V, Depre C. Activation of the bone morphogenetic protein receptor by H11kinase/Hsp22 promotes cardiac cell growth and survival. Circ Res. 2009;104:887–895. doi: 10.1161/CIRCRESAHA.108.192328. [DOI] [PubMed] [Google Scholar]
- 112.Vander Heide RS. Increased expression of HSP27 protects canine myocytes from simulated ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2002;282:H935–941. doi: 10.1152/ajpheart.00660.2001. [DOI] [PubMed] [Google Scholar]
- 113.Ray PS, Martin JL, Swanson EA, Otani H, Dillmann WH, Das DK. Transgene overexpression of alphaB crystallin confers simultaneous protection against cardiomyocyte apoptosis and necrosis during myocardial ischemia and reperfusion. FASEB J. 2001;15:393–402. doi: 10.1096/fj.00-0199com. [DOI] [PubMed] [Google Scholar]
- 114.Morrison LE, Whittaker RJ, Klepper RE, Wawrousek EF, Glembotski CC. Roles for alphaB-crystallin and HSPB2 in protecting the myocardium from ischemia-reperfusion-induced damage in a KO mouse model. Am J Physiol Heart Circ Physiol. 2004;286:H847–855. doi: 10.1152/ajpheart.00715.2003. [DOI] [PubMed] [Google Scholar]
- 115.Zou Y, Zhu W, Sakamoto M, Qin Y, Akazawa H, Toko H, Mizukami M, Takeda N, Minamino T, Takano H, Nagai T, Nakai A, Komuro I. Heat shock transcription factor 1 protects cardiomyocytes from ischemia/reperfusion injury. Circulation. 2003;108:3024–3030. doi: 10.1161/01.CIR.0000101923.54751.77. [DOI] [PubMed] [Google Scholar]
- 116.Yin C, Xi L, Wang X, Eapen M, Kukreja RC. Silencing heat shock factor 1 by small interfering RNA abrogates heat shock-induced cardioprotection against ischemia-reperfusion injury in mice. J Mol Cell Cardiol. 2005;39:681–689. doi: 10.1016/j.yjmcc.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 117.Peng W, Zhang Y, Zheng M, Cheng H, Zhu W, Cao CM, Xiao RP. Cardioprotection by CaMKII-deltaB is mediated by phosphorylation of heat shock factor 1 and subsequent expression of inducible heat shock protein 70. Circ Res. 2010;106:102–110. doi: 10.1161/CIRCRESAHA.109.210914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Arndt V, Rogon C, Hohfeld J. To be, or not to be--molecular chaperones in protein degradation. Cell Mol Life Sci. 2007;64:2525–2541. doi: 10.1007/s00018-007-7188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gurusamy N, Lekli I, Gorbunov NV, Gherghiceanu M, Popescu LM, Das DK. Cardioprotection by adaptation to ischaemia augments autophagy in association with BAG-1 protein. J Cell Mol Med. 2009;13:373–387. doi: 10.1111/j.1582-4934.2008.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miller VM, Nelson RF, Gouvion CM, Williams A, Rodriguez-Lebron E, Harper SQ, Davidson BL, Rebagliati MR, Paulson HL. CHIP suppresses polyglutamine aggregation and toxicity in vitro and in vivo. J Neurosci. 2005;25:9152–9161. doi: 10.1523/JNEUROSCI.3001-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jana NR, Dikshit P, Goswami A, Kotliarova S, Murata S, Tanaka K, Nukina N. Co-chaperone CHIP associates with expanded polyglutamine protein and promotes their degradation by proteasomes. J Biol Chem. 2005;280:11635–11640. doi: 10.1074/jbc.M412042200. [DOI] [PubMed] [Google Scholar]
- 122.Reid SJ, van Roon-Mom WM, Wood PC, Rees MI, Owen MJ, Faull RL, Dragunow M, Snell RG. TBP, a polyglutamine tract containing protein, accumulates in Alzheimer’s disease. Brain Res Mol Brain Res. 2004;125:120–128. doi: 10.1016/j.molbrainres.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 123.Reid SJ, Rees MI, van Roon-Mom WM, Jones AL, MacDonald ME, Sutherland G, During MJ, Faull RL, Owen MJ, Dragunow M, Snell RG. Molecular investigation of TBP allele length: a SCA17 cellular model and population study. Neurobiol Dis. 2003;13:37–45. doi: 10.1016/s0969-9961(03)00014-7. [DOI] [PubMed] [Google Scholar]
- 124.McGowan DP, van Roon-Mom W, Holloway H, Bates GP, Mangiarini L, Cooper GJ, Faull RL, Snell RG. Amyloid-like inclusions in Huntington’s disease. Neuroscience. 2000;100:677–680. doi: 10.1016/s0306-4522(00)00391-2. [DOI] [PubMed] [Google Scholar]
- 125.Dai Q, Qian SB, Li HH, McDonough H, Borchers C, Huang D, Takayama S, Younger JM, Ren HY, Cyr DM, Patterson C. Regulation of the cytoplasmic quality control protein degradation pathway by BAG2. J Biol Chem. 2005;280:38673–38681. doi: 10.1074/jbc.M507986200. [DOI] [PubMed] [Google Scholar]
- 126.Walker VE, Atanasiu R, Lam H, Shrier A. Co-chaperone FKBP38 promotes HERG trafficking. J Biol Chem. 2007;282:23509–23516. doi: 10.1074/jbc.M701006200. [DOI] [PubMed] [Google Scholar]
- 127.Willis MS, Patterson C. Into the heart: the emerging role of the ubiquitin-proteasome system. J Mol Cell Cardiol. 2006;41:567–579. doi: 10.1016/j.yjmcc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 128.Willis MS, Schisler JC, Patterson C. Appetite for destruction: E3 ubiquitin-ligase protection in cardiac disease. Future Cardiol. 2008;4:65–75. doi: 10.2217/14796678.4.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bruening W, Roy J, Giasson B, Figlewicz DA, Mushynski WE, Durham HD. Up-regulation of protein chaperones preserves viability of cells expressing toxic Cu/Zn-superoxide dismutase mutants associated with amyotrophic lateral sclerosis. J Neurochem. 1999;72:693–699. doi: 10.1046/j.1471-4159.1999.0720693.x. [DOI] [PubMed] [Google Scholar]
- 130.Ooie T, Takahashi N, Saikawa T, Nawata T, Arikawa M, Yamanaka K, Hara M, Shimada T, Sakata T. Single oral dose of geranylgeranylacetone induces heat-shock protein 72 and renders protection against ischemia/reperfusion injury in rat heart. Circulation. 2001;104:1837–1843. doi: 10.1161/hc3901.095771. [DOI] [PubMed] [Google Scholar]
- 131.Yamagami K, Yamamoto Y, Ishikawa Y, Yonezawa K, Toyokuni S, Yamaoka Y. Effects of geranyl-geranyl-acetone administration before heat shock preconditioning for conferring tolerance against ischemia-reperfusion injury in rat livers. J Lab Clin Med. 2000;135:465–475. doi: 10.1067/mlc.2000.106806. [DOI] [PubMed] [Google Scholar]
- 132.Sajjad MU, Samson B, Wyttenbach A. Heat Shock Proteins: Therapeutic Drug Targets for Chronic Neurodegeneration? Curr Pharm Biotechnol. 2010;11:198–215. doi: 10.2174/138920110790909641. [DOI] [PubMed] [Google Scholar]
- 133.Vigh L, Literati PN, Horvath I, Torok Z, Balogh G, Glatz A, Kovacs E, Boros I, Ferdinandy P, Farkas B, Jaszlits L, Jednakovits A, Koranyi L, Maresca B. Bimoclomol: a nontoxic, hydroxylamine derivative with stress protein-inducing activity and cytoprotective effects. Nat Med. 1997;3:1150–1154. doi: 10.1038/nm1097-1150. [DOI] [PubMed] [Google Scholar]
- 134.Kieran D, Kalmar B, Dick JR, Riddoch-Contreras J, Burnstock G, Greensmith L. Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat Med. 2004;10:402–405. doi: 10.1038/nm1021. [DOI] [PubMed] [Google Scholar]
- 135.Allison AC, Cacabelos R, Lombardi VR, Alvarez XA, Vigo C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:1341–1357. doi: 10.1016/s0278-5846(01)00192-0. [DOI] [PubMed] [Google Scholar]
- 136.Kiaei M, Kipiani K, Petri S, Chen J, Calingasan NY, Beal MF. Celastrol blocks neuronal cell death and extends life in transgenic mouse model of amyotrophic lateral sclerosis. Neurodegener Dis. 2005;2:246–254. doi: 10.1159/000090364. [DOI] [PubMed] [Google Scholar]
- 137.Palinski W. Immunomodulation: a new role for statins? Nat Med. 2000;6:1311–1312. doi: 10.1038/82107. [DOI] [PubMed] [Google Scholar]
- 138.Erl W. Statin-induced vascular smooth muscle cell apoptosis: a possible role in the prevention of restenosis? Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:135–144. doi: 10.2174/1568006043586134. [DOI] [PubMed] [Google Scholar]
- 139.Laufs U, Endres M, Custodis F, Gertz K, Nickenig G, Liao JK, Bohm M. Suppression of endothelial nitric oxide production after withdrawal of statin treatment is mediated by negative feedback regulation of rho GTPase gene transcription. Circulation. 2000;102:3104–3110. doi: 10.1161/01.cir.102.25.3104. [DOI] [PubMed] [Google Scholar]
- 140.Wang X, Tokuda H, Hatakeyama D, Hirade K, Niwa M, Ito H, Kato K, Kozawa O. Mechanism of simvastatin on induction of heat shock protein in osteoblasts. Arch Biochem Biophys. 2003;415:6–13. doi: 10.1016/s0003-9861(03)00213-3. [DOI] [PubMed] [Google Scholar]
- 141.Uchiyama T, Atsuta H, Utsugi T, Oguri M, Hasegawa A, Nakamura T, Nakai A, Nakata M, Maruyama I, Tomura H, Okajima F, Tomono S, Kawazu S, Nagai R, Kurabayashi M. HSF1 and constitutively active HSF1 improve vascular endothelial function (heat shock proteins improve vascular endothelial function) Atherosclerosis. 2007;190:321–329. doi: 10.1016/j.atherosclerosis.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 142.Kretz A, Schmeer C, Tausch S, Isenmann S. Simvastatin promotes heat shock protein 27 expression and Akt activation in the rat retina and protects axotomized retinal ganglion cells in vivo. Neurobiol Dis. 2006;21:421–430. doi: 10.1016/j.nbd.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 143.Schmeer C, Gamez A, Tausch S, Witte OW, Isenmann S. Statins modulate heat shock protein expression and enhance retinal ganglion cell survival after transient retinal ischemia/reperfusion in vivo. Invest Ophthalmol Vis Sci. 2008;49:4971–4981. doi: 10.1167/iovs.07-1597. [DOI] [PubMed] [Google Scholar]
- 144.Ojo MA, Akpata O. Case report of dentigerous cyst of lower incisor. Afr Dent J. 1992;6:34–37. [PubMed] [Google Scholar]
- 145.Li G, Higdon R, Kukull WA, Peskind E, Van Valen Moore K, Tsuang D, van Belle G, McCormick W, Bowen JD, Teri L, Schellenberg GD, Larson EB. Statin therapy and risk of dementia in the elderly: a community-based prospective cohort study. Neurology. 2004;63:1624–1628. doi: 10.1212/01.wnl.0000142963.90204.58. [DOI] [PubMed] [Google Scholar]