Abstract
Methoxychlor (MXC) is an organochlorine pesticide that reduces fertility in female rodents by decreasing antral follicle numbers and increasing follicular death. MXC is metabolized in the body to mono-hydroxy MXC (mono-OH). Little is known about the effects of mono-OH on the ovary. Thus, this work tested the hypothesis that mono-OH exposure decreases production of 17β-estradiol (E2) by cultured mouse antral follicles. Antral follicles were isolated from CD-1 mice (age 35–39 days) and exposed to dimethylsulfoxide (DMSO), or mono-OH (0.1–10 μg/mL) for 96 hours. Media and follicles were collected for analysis of sex steroid levels and mRNA expression, respectively. Mono-OH treatment (10 μg/mL) decreased E2 (DMSO: 3009.72 ± 744.99 ng/mL; mono-OH 0.1 μg/mL: 1679.66 ± 461.99 ng/mL; 1 μg/mL: 1752.72 ± 532.41 ng/mL; 10 μg/mL: 45.89 ± 33.83 ng/mL), testosterone (DMSO: 15.43 ± 2.86 ng/mL; mono-OH 0.1 μg/mL: 17.17 ± 4.71 ng/mL; 1 μg/mL: 13.64 ± 3.53 ng/mL; 10 μg/mL: 1.29 ± 0.23 ng/mL), androstenedione (DMSO: 1.92 ± 0.34 ng/mL; mono-OH 0.1 μg/mL: 1.49 ± 0.43 ng/mL; 1 μg/mL: 0.64 ± 0.31 ng/mL; 10 μg/mL: 0.12 ± 0.06 ng/mL) and progesterone (DMSO: 24.11 ± 4.21 ng/mL; mono-OH 0.1 μg/mL: 26.77 ± 4.41 ng/mL; 1 μg/mL: 20.90 ± 3.75 ng/mL; 10 μg/mL: 9.44 ± 2.97 ng/mL) levels. Mono-OH did not alter expression of Star, Hsd3b1, Hsd17b1 and Cyp1b1, but it did reduce levels of Cyp11a1, Cyp17a1 and Cyp19a1 mRNA. Collectively, these data suggest that mono-OH significantly decreases levels of key sex steroid hormones and the expression of enzymes required for steroidogenesis.
Keywords: methoxychlor, metabolites, antral follicles, ovary, estradiol, steroidogenesis
INTRODUCTION
The functional unit of the mammalian ovary is the ovarian follicle. An ovary contains a non-renewable number of ovarian follicles in different stages of development. Ovarian follicles contain an oocyte (egg) surrounded by granulosa cells and theca cells. During the reproductive lifespan of a female, ovarian follicles mature, produce ovarian sex steroids, and may eject their oocytes during ovulation. Not all follicles become ovulated; instead, the majority undergoes a process of follicular death by apoptosis known as atresia (Hirshfield, 1991).
The major sex steroid-producing follicle type is the antral follicle. Antral follicles are responsible for producing 17β-estradiol (E2), which is necessary for normal ovarian function (Britt et al., 2004; Hirshfield, 1991). Damage to the ovarian follicle population may result in altered levels of E2 and therefore altered ovarian function. Alterations in ovarian function may lead to infertility (Levi and Widra, 1994). In addition, decreased E2 levels may increase a woman’s risk for disorders such as osteoporosis, cardiovascular disease and depression (Bagur and Mautzlen, 1992; Dennerstein et al., 1999; Hu et al., 1999).
Methoxychlor (MXC) is a widely used organochlorine pesticide that is a model endocrine disruptor and environmental estrogen (Cummings, 1997). In mice, MXC has been shown to reduce fertility, cause ovarian atrophy, decrease antral follicle numbers and increase antral follicle death or atresia (Borgeest et al., 2002; Cummings and Gray, 1989; Eroschenko et al., 1995; Martinez and Swartz, 1991; Swartz and Eroschenko, 1998; Swartz and Corkern, 1992). MXC has also been shown to inhibit mouse antral follicle growth and increase antral follicle atresia in vitro (Miller et al., 2005).
In rodents, MXC has been shown to be absorbed by the intestinal epithelium and then to become O-demethylated by hepatic cytochrome P450 enzymes to form 1,1,1-trichloro-2-(4-hydroxyphenyl)-2-(4-methoxyphenyl)ethane commonly referred to as mono-OH and 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane known as HPTE (Kapoor et al., 1970). Both metabolites have been shown to become glucuronidated and/or sulfonated following their formation and prior to excretion (Ohyama et al., 2005b; Ohyama et al., 2005a). Previous studies using isolated mouse antral follicles have demonstrated that mono-OH inhibits antral follicle growth and increases atresia at doses lower than those of MXC (Miller et al., 2006). Therefore, mono-OH is considered to be a more potent ovotoxicant than MXC.
Previous studies have evaluated the ability of MXC to disrupt ovarian steroidogenesis (Crellin et al., 2001; Tiemann et al., 1996), but no studies have evaluated the effect of mono-OH on levels of sex steroid hormones and expression of steroidogenic enzymes. Therefore, this study was designed to test the hypothesis that mono-OH alters E2 levels by altering ovarian steroidogenesis. To evaluate the effects of mono-OH treatment on steroidogenesis, we used an isolated follicle culture assay to expose mouse antral follicles to increasing concentrations of mono-OH. Following culture, we measured levels of sex steroid hormones in the media, and mRNA expression of key enzymes involved in the biosynthesis and catabolism of E2 within the follicles.
MATERIALS AND METHODS
Chemicals
Mono-OH (purity 99%) was synthesized by the laboratory of Dr. Vincent Njar (University of Maryland, Baltimore, MD; now at Thomas Jefferson University, Philadelphia, PA), dimethylsulfoxide (DMSO), ITS (insulin, transferrin, selenium), penicillin and streptomycin were obtained from Sigma-Aldrich (St. Louis, MO). Alpha-minimal essential media (α-MEM) was obtained from Invitrogen (Carlsbad, CA). Human recombinant follicle stimulating hormone (rFSH) was obtained from Dr. A.F. Parlow from the National Hormone and Peptide Program (Harbor-UCLA Medical Center, Torrance, CA). Fetal bovine serum (FBS) was obtained from Atlanta Biologicals (Lawrenceville, GA).
Animals
Cycling female CD-1 mice were purchased from Charles River Laboratories (Charles River, CA) and housed four animals per cage at the University of Illinois’ College of Veterinary Medicine Central Animal Facility. Food and water were ad libitum. Temperature was maintained at 22 ± 1°C and animals were subjected to 12L: 12D cycles. Animals were euthanized at 35–39 days old by carbon dioxide (CO2) inhalation followed by cervical dislocation. The ovaries were removed and antral follicles were isolated as described below. All experiments and methods involving animals were approved by the University of Illinois Institutional Animal Care and Use Committee (IACUC) and conformed to the Guide for the Care and Use of Experimental Animals (Institute of Laboratory Animal Resources, 1996).
Experimental dosages
A stock solution of mono-OH was prepared as previously described (Miller et al., 2006) using dimethylsulfoxide (DMSO) as a solvent. Various concentrations (13.3, 1.33, 0.133 mg/mL) were prepared to ensure that an equal volume would be added to culture wells for each treatment group to control for solvent concentration. Final concentrations of mono-OH in culture were 0.1, 1 and 10 μg/mL (0.30, 3.01 and 30.15 μM). These doses were selected for this study based on previous reports showing that these concentrations of mono-OH induce antral follicle toxicity and that mono-OH is more toxic to antral follicles than MXC (Miller et al., 2006). These in vitro mono-OH doses are comparable to in vivo and in vitro doses previously shown to result in MXC-induced decreased antral follicle growth and increased atresia (in vitro; 1–100 μg/mL), and increased number of atretic antral follicles in vivo (32 and 64 mg/kg; 20 days) (Borgeest et al., 2002; Borgeest et al., 2004; Miller et al., 2005). The selected doses are also environmentally relevant because environmental levels of MXC range from 40–160 ppm in waters downstream of areas exposed to MXC (Wallner et al., 1969) to 0.1–4.0 ppb/day in the human diet (Agency for Toxic Substances and Disease Registry, 2002).
Antral follicle culture
Cycling CD-1 mice were euthanized by CO2 inhalation followed by cervical dislocation. Ovaries were removed, trimmed of fat, and antral follicles were mechanically isolated based on relative size (diameter > 200 μm) using watchmaker forceps and placed in culture as previously described (Gupta et al., 2006; Miller et al., 2006; Miller et al., 2005). Follicles were isolated from 3–4 mice per culture with approximately 15–18 antral follicles obtained from each mouse. Each follicle culture experiment contained a minimum of 10–15 follicles per treatment. Following isolation, antral follicles were placed individually in 96-well culture plates with 75 μL of unsupplemented α-MEM prior to treatment.
Treatment groups included three doses of mono-OH (0.1, 1 and 10 μg/mL) and a vehicle control consisting of DMSO (≤ 0.075%). All dosing solutions were prepared individually in supplemented α-MEM with an equal volume of chemical added for each dose to control for the amount of vehicle in each preparation. Supplemented α-MEM contained 5% FBS, 1% ITS (10 ng/mL insulin, 5.5 ng/mL transferrin, 5.5 ng/mL selenium), 100 U/mL penicillin, 100 mg/mL streptomycin, and 5 IU/mL human rFSH. For treatment, unsupplemented media was replaced with 150 μL supplemented α-MEM containing vehicle or mono-OH at the various doses. Follicles were then incubated for 96 h at 37°C in 5% CO2.
Analysis of Follicular Growth
Antral follicles were cultured as described above for 96 h. Follicles were examined at 24 h intervals under an inverted microscope equipped with a calibrated ocular micrometer. Follicle growth was assessed by measuring the diameter of each follicle as described previously (Gupta et al., 2009; Miller et al., 2006). Antral follicles were considered as those having diameters of 200 μm or greater (Cortvrindt and Smitz, 2002), which correlates with the histological appearance of these follicles. At least three separate culture experiments were performed for each chemical treatment. Follicle diameter measurements were averaged among treatment groups and compared between the chemical treatments over time to confirm that inhibition of growth by mono-OH had occurred as reported previously (Miller et al., 2006).
Hormone Measurements
The medium from each culture well containing an individual follicle was collected and stored at −80°C. When at least 3 separate culture experiments were completed, media samples from all experiments were randomly selected and subjected to enzyme-linked immunosorbent assays (ELISA). Levels of E2, testosterone and androstenedione in media were measured using kits from DRG International (Mountainside, NJ) while progesterone was measured using kits from ALPCO (Salem, NH). The minimum detection limits for each kit were 9.71 pg/mL for E2, 0.083 ng/mL for testosterone, 0.019 ng/mL for androstenedione, and 0.1 ng/mL for progesterone. The intra-assay coefficients of variation (CVs) were 4.7% for E2, 7.1% for testosterone, 10.2% for androstenedione, and 11.4% for progesterone. The inter-assay CVs were 7.8% for E2, 3.6% for testosterone, 6.5% for androstenedione, and 10.4% for progesterone. These sex steroid hormones were selected because they are major intermediates in the synthesis of E2 (Jones and DeCherney, 2005). Sex steroid hormone data consisted of values from 12–18 individual follicles obtained from 3–6 separate culture experiments.
Quantitative Real-time Polymerase Chain Reaction (qPCR)
At the end of the 96 h culture period, follicles were immediately snap-frozen and stored at −80°C until qPCR analysis. Total RNA was extracted from pooled follicles (10–16 follicles per treatment) using a RNeasy Micro Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Additionally, samples were incubated (15 min) with DNAse (Qiagen) during the isolation to eliminate potential genomic DNA contamination. The RNA concentration of each sample was determined at 260 nm using a Nanodrop ND1000 UV-Vis spectrophotometer (Nanodrop Technologies, Wilmington, DE). RNA samples (200 ng) were reverse transcribed using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) according to manufacturer’s instructions. Each cDNA sample was diluted 1:4 with nuclease-free water.
All qPCR experiments were carried out using a CFX96 Real-time System C1000 Thermal Cycler (Bio-Rad). All qPCR reactions were done in triplicate, and each contained 2 μL of diluted cDNA, 0.66 μL of gene-specific primers (Integrated DNA Technologies, Inc, Coralville, IA, Table 1), 2.34 μL of nuclease-free water and 5 μL of SsoFast EvaGreen Supermix (Bio-Rad) for a final volume of 10 μL. As suggested by the manufacturer’s instructions, the qPCR program consisted of: an enzyme activation step (95°C for 1 min), an amplification and quantification program (40 cycles of 95°C for 10 sec, 60°C for 10 sec, single fluorescence reading), a 72°C for 5 min step, a melt curve (65–95°C heating 0.5°C per sec with continuous fluorescence readings) and a final step at 72°C for 5 min.
Table 1.
| Accession No. | Gene name | Abbreviation | Forward | Reverse |
|---|---|---|---|---|
| NM_007393.3 | actin, beta | Actb | GGGCACAGTGTGGGTGAC | CTGGCACCACACCTTCTAC |
| NM_009994.1 | cytochrome P450, family 1, subfamily b, polypeptide 1 | Cyp1b1 | GCGACGATTCCTCCGGGCTG | TGCACGCGGGCCTGAACATC |
| NM_019779.3 | cytochrome P450, family 11, subfamily a, polypeptide 1 | Cyp11a1 | AGATCCCTTCCCCTGGTGACAATG | CGCATGAGAAGAGTATCGACGCATC |
| NM_007809.3 | cytochrome P450, family 17, subfamily a, polypeptide 1 | Cyp17a1 | CCAGGACCCAAGTGTGTTCT | CCTGATACGAAGCACTTCTCG |
| NM_007810.3 | cytochrome P450, family 19, subfamily a, polypeptide 1 | Cyp19a1 | CATGGTCCCGCAAACTGTGA | GTAGTAGTTGCAGGCACTTC |
| NM_008293.3 | hydroxy-delta-5-steriod dehydrogenase, 3 beta- and steroid delta-isomerase 1 | Hsd3b1 | CAGGAGAAAGAACTGCAGGAGGTC | GCACACTTGCTTGAACACAGGC |
| NH_010475.1 | hydroxysteriod (17 beta) dehydrogenase 1 | Hsd17b1 | ACTGTGCCAGCAAGTTTGCG | AAGCGGTTCGTGGAGAAGTAG |
| NM_011485.4 | steroidogenic acute regulatory protein | Star | CAGGGAGAGGTGGCTATGCA | CCGTGTCTTTTCCAATCCTCTG |
Primer sequences and information are listed in Table 1. Initially, primer specificity was assessed by matching primer sequences with that of the gene of interest using BLASTN 2.2.18+ (Altschul et al., 1990; Altschul et al., 1997) and by the presence of a single peak following melt curve analysis. Additionally, each primer pair was tested by agarose gel electrophoresis to confirm amplification of a single product of the correct size.
A standard curve was generated from five serial dilutions of one of the samples to calculate the amplification efficiencies of each primer and expression data were generated using a mathematical model for relative quantification of real-time PCR data developed by Pfaffl (Pfaffl, 2001). Briefly, the relative expression ratio of the target gene was calculated based on the amplification efficiency of each transcript and the ΔCt of the treated samples versus the vehicle control, and compared to the expression of a reference gene (β-actin, Actb). Reported data consist of mean relative mRNA expression ratios from 3–5 separate follicle culture experiments.
Statistical analysis
All data were analyzed using SPSS statistical software (SPSS Inc., Chicago, IL). For all comparisons, statistical significance was assigned at P<0.05. Comparisons between DMSO and the different doses of mono-OH were conducted on data obtained from 3–6 experiments using one-way analysis of variance (ANOVA) followed by Tukey’s or Dunnett’s post hoc tests when applicable.
RESULTS
Antral follicle growth
Both mono-OH and its parent compound, MXC, have been shown to inhibit mouse antral follicle growth in vitro (Miller et al., 2006; Miller et al., 2005). In agreement with past observations, mono-OH (10 μg/mL) inhibited follicle growth (0 h: 360.94 ± 16.1 μm vs. 96 h: 366.86 ± 14.4 μm, P<0.05) compared to DMSO (0 h: 336.68 ± 11.7 μm vs. 96 h: 448.50 ± 2.0 μm).
Effect of mono-OH MXC on levels of 17β-estradiol
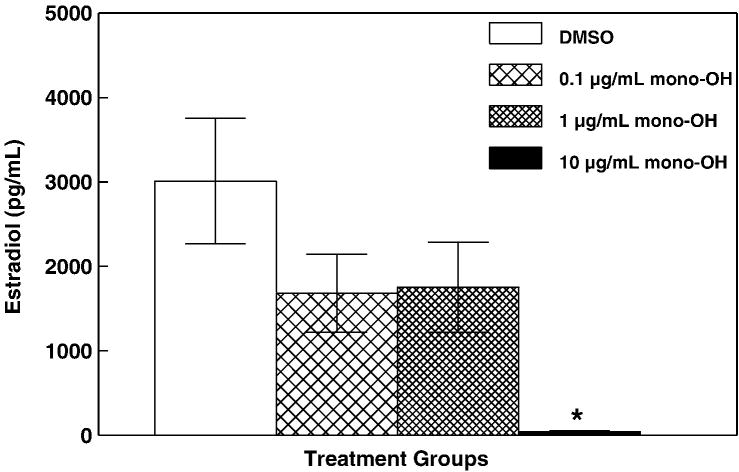
The purpose of this experiment was to determine whether exposure to mono-OH disrupts steroidogenesis by altering production of E2 by cultured mouse antral follicles. Isolated mouse antral follicles were cultured for 96 h in the presence of 0.1, 1 and 10 μg/mL mono-OH or DMSO as a vehicle control. Following culture, media samples from individual follicles were subjected to measurement of E2 (Figure 1). Compared to that from DMSO-treated follicles, media from follicles treated with the highest dose of mono-OH (10 μg/mL) contained decreased levels of E2 (DMSO: 3009.72 ± 744.99 pg/mL; mono-OH 0.1 μg/mL: 1679.66 ± 461.99 pg/mL; 1 μg/mL: 1752.72 ± 532.41 pg/mL; 10 μg/mL: 45.89 ± 33.83 pg/mL; P<0.05).
Figure 1. Mono-OH treatment decreases levels of 17β-estradiol in media from cultured mouse antral follicles.
Isolated mouse antral follicles were cultured for 96 h in the presence of 0.1, 1 and 10 μg/mL mono-OH or DMSO as a vehicle control. Following culture, media samples from individual follicles were subjected to measurement of 17β-estradiol by ELISA as described in Materials and Methods. Data are expressed as mean ± SEM calculated from follicles in 3 separate culture experiments. Asterisk (*) indicates P<0.05.
Effect of mono-OH MXC on levels of 17β-estradiol precursors
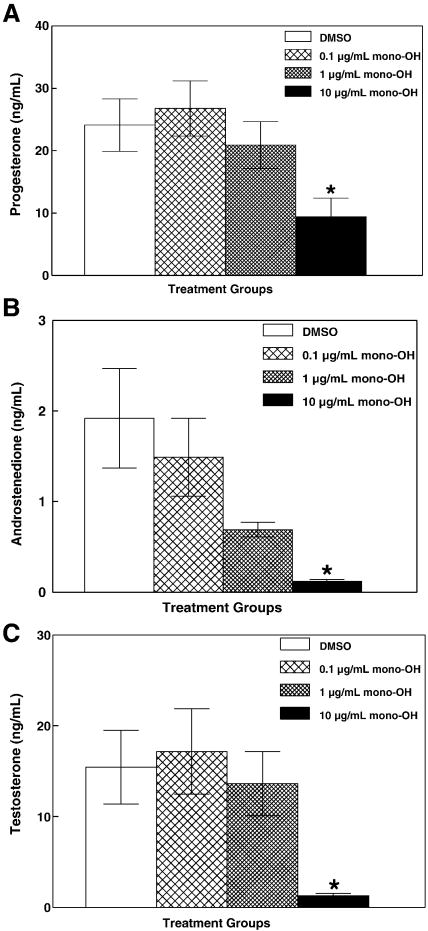
During ovarian steroidogenesis, several sex steroid precursors are produced in the steps preceding synthesis of E2 and, thus, are required to maintain physiological levels of E2. Levels of progesterone, androstenedione and testosterone were measured in media from mouse antral follicles treated with mono-OH for 96 h (Figure 2) to test whether levels of E2 were decreased due to a decrease in its precursor steroids. Levels of progesterone (Figure 2A; DMSO: 24.11 ± 4.21 ng/mL; mono-OH 0.1 μg/mL: 26.77 ± 4.41 ng/mL; 1 μg/mL: 20.90 ± 3.75 ng/mL; 10 μg/mL: 9.44 ± 2.97 ng/mL), androstenedione (Figure 2B; DMSO: 1.92 ± 0.34 ng/mL; mono-OH 0.1 μg/mL: 1.49 ± 0.43 ng/mL; 1 μg/mL: 0.64 ± 0.31 ng/mL; 10 μg/mL: 0.12 ± 0.06 ng/mL) and testosterone (Figure 2C; DMSO: 15.43 ± 2.86 ng/mL; mono-OH 0.1 μg/mL: 17.17 ± 4.71 ng/mL; 1 μg/mL: 13.64 ± 3.53 ng/mL; 10 μg/mL: 1.29 ± 0.23 ng/mL) were decreased (P<0.05) in media from antral follicles treated with 10 μg/mL mono-OH.
Figure 2. Mono-OH treatment decreases levels of precursors of 17β-estradiol in media from cultured mouse antral follicles.
Following culture, media samples from individual follicles were subjected to measurement of (A) progesterone, (B) androstenedione and (C) testosterone by ELISA as described in Materials and Methods. Data are expressed as mean ± SEM calculated from follicles in 3–6 separate culture experiments. Asterisk (*) indicates P<0.05.
Expression of enzymes that synthesize ovarian sex steroids
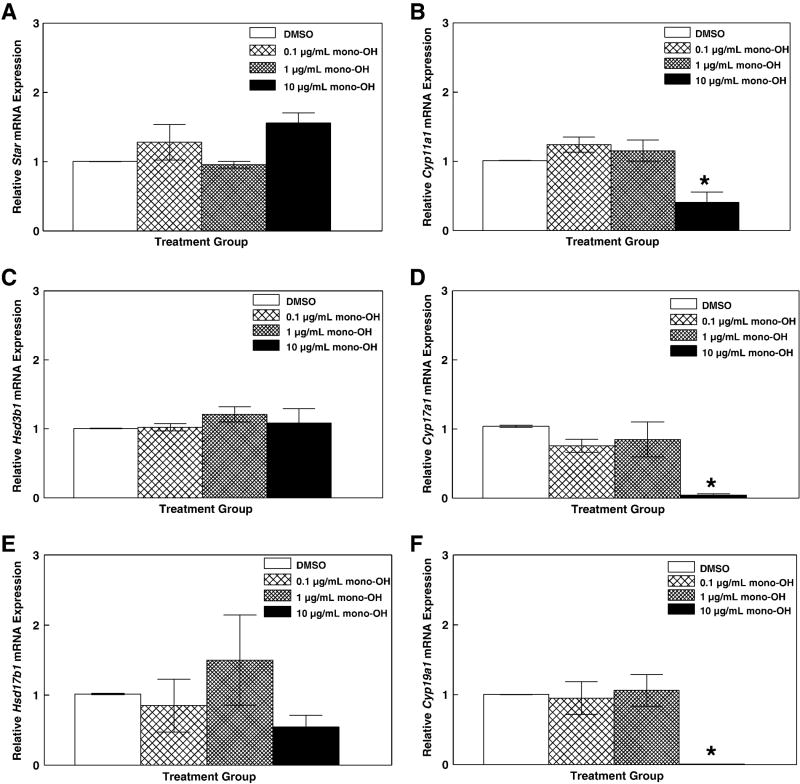
Following the 96 h culture period, antral follicles were removed from culture and processed for real-time quantitative polymerase chain reaction (qPCR). Because the activity of steroidogenic enzymes is transcriptionally regulated (Jones and DeCherney, 2005; Magoffin and Erickson, 1982; Miller, 1989; Wang et al., 1982), messenger RNA levels for the enzymes involved in ovarian synthesis of E2 were measured to test the hypothesis that decreased levels of sex steroids are accompanied by decreased expression of the enzymes responsible for their synthesis. Levels of steroidogenic acute regulatory protein (Star), cholesterol side chain cleavage enzyme (Cyp11a1), 3β-hydroxysteroid dehydrogenase (Hsd3b1), 17α-hydroxylase/17,20-lyase (Cyp17a1), 17β-hydroxysteroid dehydrogenase (Hsd17b1) and aromatase (Cyp19a1) mRNA were evaluated (Figure 3). There were no differences (P>0.05) in expression ratios of Star (Figure 2A; DMSO: 1.00 ± 0.002; mono-OH 0.1 μg/mL: 1.28 ± 0.25; 1 μg/mL: 0.96 ± 0.05; 10 μg/mL: 1.56 ± 0.15), Hsd3b1 (Figure 2C; DMSO: 1.01 ± 0.002; mono-OH 0.1 μg/mL: 1.02 ± 0.05; 1 μg/mL: 1.21 ± 0.11; 10 μg/mL: 1.08 ± 0.21) and Hsd17b1 (Figure 2E; DMSO: 1.02 ± 0.002; mono-OH 0.1 μg/mL: 0.85 ± 0.38; 1 μg/mL: 1.50 ± 0.64; 10 μg/mL: 0.54 ± 0.17) in mono-OH MXC-treated follicles relative to DMSO-treated follicles. However, decreased Cyp11a1 (Figure 2B; DMSO: 1.01 ± 0.003; 0.1 μg/mL mono-OH: 1.24 ± 0.11; 1 μg/mL: 1.15 ± 0.15 and 10 μg/ml: 0.41 ± 0.15; P<0.05); Cyp17a1 (Figure 2D; DMSO: 1.03 ± 0.17; 0.1 μg/mL: 0.76 ± 0.09; 1 μg/mL: 0.85 ± 0.25; 10 μg/mL: 0.04 ± 0.02; P<0.05) and Cyp19a1 mRNA (Figure 2E; DMSO: 1.00 ± 0.001; 0.1 μg/mL: 0.95 ± 0.23; 1 μg/mL: 1.06 ± 0.23; 10 μg/mL: 0.01 ± 0.001; P<0.05) was observed in follicles exposed to 10 μg/mL mono-OH MXC relative to DMSO-treated follicles.
Figure 3. Mono-OH treatment decreases expression of key steroidogenic enzymes in cultured mouse antral follicles.
Following 96 h, antral follicles were removed from culture and processed for real-time qPCR as described in Materials and Methods. Messenger RNA levels for (A) steroidogenic acute regulatory protein, Star; (B) cholesterol side chain cleavage enzyme, Cyp11a1; (C) 3β-hydroxysteroid dehydrogenase, Hsd3b1; (D) 17α-hydroxylase/17,20-lyase, Cyp17a1; (E) 17β-hydroxysteroid dehydrogenase, Hsd17b1; and (F) aromatase, Cyp19a1 were determined and normalized to β-actin (Actb). Data are expressed as mean relative expression ratios ± SEM calculated from 3–5 separate culture experiments. Asterisk (*) indicates P<0.05.
Expression of enzymes that breakdown 17β-estradiol
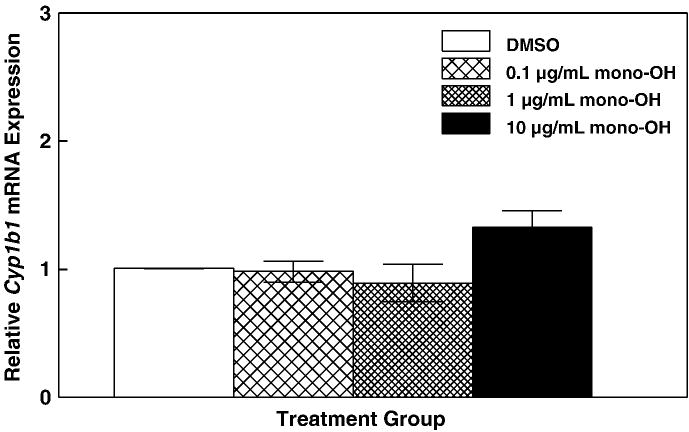
Because E2 can be metabolized by enzyme CYP1B1 into catechol estrogens (Hayes et al., 1996), decreased E2 levels can also result from increased metabolism by this enzyme. Therefore, mRNA levels for Cyp1b1 were determined in cultured mouse antral follicles following exposure to mono-OH (Figure 4). There were no differences (P>0.05) in Cyp1b1 mRNA levels between DMSO and mono-OH-treated follicles (DMSO: 1.01 ± 0.003; mono-OH 0.1 μg/mL: 0.98 ± 0.08; 1 μg/mL: 0.89 ± 0.14; 10 μg/mL: 1.34 ± 0.13).
Figure 4. Mono-OH treatment does not alter levels of an estrogen-metabolizing enzyme in cultured mouse antral follicles.
Messenger RNA levels for cytochrome P450, family 1, subfamily b, polypeptide 1 (Cyp1b1) were determined and normalized to β-actin (Actb). Data are expressed as mean relative expression ratios ± SEM calculated from 5 separate culture experiments.
DISCUSSION
Using an in vitro follicle culture assay, we exposed antral follicles to concentrations of mono-OH, evaluated growth, measured hormone levels in the media and determined mRNA expression of key enzymes involved in the synthesis and metabolism of E2. Significant inhibition of in vitro follicle growth at the 10 μg/mL dose was accompanied by reduced levels of E2, progesterone, androstenedione and testosterone in media, and decreased expression of Cyp11a1, Cyp17a1, Cyp19a1 in the follicles.
Our findings suggest that in vitro mono-OH treatment interferes with the synthesis of E2 in mouse antral follicles. We hypothesized that decreased synthesis of E2 may be the culprit behind decreased levels of E2 in media from follicles treated with mono-OH because progesterone, androstenedione and testosterone were also decreased by mono-OH treatment. Our hypothesis was further supported by decreased expression of Cyp11a1, Cyp17a1 and Cyp19a1 (decreased synthesis) observed in the absence of unaltered levels of Cyp1b1 (unaltered metabolism).
While no other studies have reported the effects of mono-OH treatment on sex steroid levels and expression of steroidogenic enzymes, some studies have reported altered sex steroid levels and steroidogenic enzyme expression following treatment with MXC and the bis-OH metabolite of MXC, 1,1,1-trichloro-2,2-bis(4-hydroxyphenyl)ethane (HPTE). In vivo exposure to MXC has been shown to reduce serum progesterone and in vitro production of E2 and testosterone by ovaries of pregnant rats dosed with MXC during gestation (Cummings and Laskey, 1993). Further, studies using stable porcine granulosa cells incubated with MXC for 48 h showed that MXC (1 μg/mL) decreased progesterone synthesis (Crellin et al., 2001). Another study using primary cultures of porcine granulosa cells showed that MXC (10 μM; 48 h) decreased both FSH and E2-stimulated progesterone synthesis without affecting levels of cAMP (Chedrese and Feyles, 2001). HPTE has also been shown to reduce FSH-stimulated synthesis of progesterone and E2 in cultured rat granulosa cells (10 μM; 48 h) (Zachow and Uzumcu, 2006). Our results indicate that mono-OH, like MXC and HPTE, also alters in vitro ovarian steroidogenesis resulting in decreased levels of sex steroids. Our study also shows that, in addition to decreasing levels of E2 and progesterone, mono-OH treatment decreases levels of testosterone and androstenedione. Therefore, we suggest that decreased levels of E2 following exposure to MXC and metabolites result from decreased availability of progesterone for conversion to androgens, which are then aromatized into E2.
Zachow and Uzumcu used their rat granulosa cells treated in vitro with HPTE (10 μM; 48 h) to determine mRNA expression of Star, Cyp11a1, Hsd3b1 and Cyp19a1 in the presence of FSH or the cAMP analog dibutyryl-cAMP (db-cAMP) (Zachow and Uzumcu, 2006). More recently, another study from the same group also used rat granulosa cells exposed to HPTE (1–10 μM; 48 h) to evaluate FSH-induced expression of Star, Cyp11a1, Hsd17b1, and Cyp19a1 (Harvey et al., 2009). Similar to our study with mono-OH, HPTE did not alter the expression of Star and Hsd17b1, but it decreased expression of Cyp11a1, and Cyp19a1. However, our findings differ from those obtained by Zachow and Uzumcu in that HPTE decreased FSH-stimulated expression of Hsd3b1 in rat granulosa cells while in mouse antral follicles treated with mono-OH, Hsd3b1 expression was not affected. Whether Hsd3b1 mRNA expression is affected differently by mono-OH and HPTE is not clear, but perhaps might suggest that the two metabolites may follow different mechanisms through which they can alter ovarian steroidogenesis.
The observation that mono-OH treatment does not alter levels of Hsd3b1 was unexpected since mono-OH decreased levels of progesterone in media obtained from these follicles. Perhaps decreased CYP11A1 enzyme is sufficient to lead to decreased progesterone production. Because Cyp11a1 is required for the synthesis of pregnenolone, which is converted into progesterone by Hsd3b1, decreased availability of pregnenolone may lead to decreased progesterone in media even in the absence of reduced Hsd3b1 expression. It is possible that the first step in mono-OH and HPTE-induced disruption of steroidogenesis involves decreasing the availability of pregnenolone. This idea is supported by the observation by Akgul et al. of decreased CYP11A1 activity in HPTE-treated theca interstitial cells obtained from pregnant mare serum gonadotropin primed immature rats (Akgul et al., 2008).
Alternatively, the tissue, species and system tested whether granulosa or theca cell only cultures, or whole mouse antral follicles containing intact oocyte, granulosa and theca cells, might also influence the effects observed following treatment with these metabolites. Perhaps susceptibility and response to insults by toxicants may differ when granulosa/theca/occyte interactions are intact from that when single cell types are exposed. This is evidenced by reports on the response of Cyp11a1 to treatment with MXC and HPTE that show different results depending on the system and conditions tested. For example, in contrast to their findings with FSH-stimulated Cyp11a1 expression, Zachow and Uzumcu observed that levels of Cyp11a1 were actually increased in response to HPTE when FSH receptor and adenylyl cyclase activation were bypassed by treating their rat granulosa cells with db-cAMP (Zachow and Uzumcu, 2006). Their findings agree with those from Crellin et al., who observed increased Cyp11a1 expression in response to MXC treatment when FSH receptor was bypassed and adenylyl cyclase constitutively activated by cholera toxin in porcine granulosa cells (Crellin et al., 2001). Further, despite showing decreased CYP11A1 activity in HPTE-treated theca interstitial cells, Akgul et al did not observe any alterations in expression of Star and Cyp11a1 mRNA (Akgul et al., 2008).
Changes in Cyp11a1, Cyp17a1 and Cyp19a1 mRNA as well as changes in levels of sex steroids in media observed here are thought to be the result of mono-OH toxicity rather than due to a generalized functional shut down caused by follicular death. This is supported by the observation that even though mono-OH causes atresia of antral follicles cultured for 96 hours (Miller et al., 2006), detectable levels of Actb, Star, Hsd3b1, Hsd17b1 and Cyp1b1 mRNAs were observed in mono-OH-treated follicles. Further, expression of these mRNAs was not different from that in viable vehicle-treated follicles in the present study. Although protein levels for these enzymes were not evaluated here, other studies, in which mRNA and protein for these enzymes have been measured, demonstrate agreement between their mRNA and protein expression (Conley et al., 1994; Havelock et al., 2006).
Together, the present findings provide evidence that the mono-OH metabolite of MXC may cause endocrine disruption in the mouse ovary through altered steroidogenesis and/or estrogen deficiency. Future studies should be aimed at understanding post-translational effects of mono-OH treatment on steroidogenesis such as alteration of catalytic activity by interactions of mono-OH with the enzymes themselves as well as alterations in function and/or availability of important co-factors (e.g. NADH, NADPH).
Finally, because ovarian physiology in the mouse holds key similarities to that in women, understanding the mechanisms by which this endocrine disruption occurs may provide essential information to aid in the treatment of infertility and prevention of debilitating conditions such as osteoporosis, cardiovascular disease and depression that are caused by estrogen deficiency (Bagur and Mautzlen, 1992; Dennerstein et al., 1999; Hu et al., 1999).
Acknowledgments
The authors thank Bethany Karman and Tessie Paulose for technical help, Lalji Gedia (Dr. Vincent Njar laboratory) for the synthesis of mono-OH methoxychlor, and grant support provided by the National Institute of Environmental Health Sciences (NIEHS) grants R01ES012893 (JAF), R01ES019178 (JAF), T32ES07326 (RKG), a Colgate-Palmolive Postdoctoral Fellowship in In Vitro Toxicology (KPH), and the Billie A. Field Fellowship in Reproductive Biology (ZRC).
Grant Support: This research was supported by National Institute on Environmental Health grants R01ES012893 (JAF), R01ES019178 (JAF) and T32ES07326 (RKG), a Colgate-Palmolive Postdoctoral Fellowship in In Vitro Toxicology (KPH), and the Billie A. Field Fellowship in Reproductive Biology (ZRC).
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Zelieann R. Craig, Email: zelieann@gmail.com.
Traci C. Leslie, Email: traci.leslie@gmail.com.
Kimberly P. Hatfield, Email: kpm9786@yahoo.com.
Rupesh K. Gupta, Email: drrupesh@illinois.edu.
Jodi A. Flaws, Email: jflaws@illinois.edu.
References
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Methoxychlor. U.S. Department of Health and Human Services Public Health Service; Atlanta, GA: 2002. [PubMed] [Google Scholar]
- Akgul Y, Derk R, Meighan T, Rao K, Murono E. The methoxychlor metabolite, HPTE, directly inhibits the catalytic activity of cholesterol side-chain cleavage (P450scc) in cultured rat ovarian cells. Reprod Toxicol. 2008;25:67–75. doi: 10.1016/j.reprotox.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers E, Lipman D. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden T, Schäffer A, Zhang J, Zhang J, Zhang Z, Miller W, Lipman D. Gapped BLAST and PSI-BLAST: a new generation of protein database searcg programs. Nucleic Acid Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagur AC, Mautzlen CA. Risk for developing osteoporosis in untreated premature menopause. Calcif Tiss Int. 1992;51:4–7. doi: 10.1007/BF00296207. Ref Type: Journal (Full) [DOI] [PubMed] [Google Scholar]
- Borgeest C, Symonds D, Mayer LP, Hoyer PB, Flaws JA. Methoxychlor may cause ovarian follicular atresia and proliferation of the ovarian epithelium in the mouse. Toxicol Sci. 2002;68:473–478. doi: 10.1093/toxsci/68.2.473. [DOI] [PubMed] [Google Scholar]
- Borgeest C, Miller K, Gupta R, Greenfeld C, Hruska K, Hoyer P, Flaws J. Methoxychlor-induced atresia in the mouse involved Bcl-2 family member, but not gonadotropins or estradiol. Biol Reprod. 2004;70:1828–1835. doi: 10.1095/biolreprod.103.022889. [DOI] [PubMed] [Google Scholar]
- Britt K, Saunders PK, McPherson SJ, Misso ML, Simpson ER, Findlay JK. Estrogen actions on follicle formation and early follicle development. Biol Reprod. 2004;71:1712–1723. doi: 10.1095/biolreprod.104.028175. [DOI] [PubMed] [Google Scholar]
- Chedrese P, Feyles F. The diverse mechanism of action of dichlorodiphenyldichloroethylene (DDE) and methoxychlor in ovarian cells in vitro. Reprod Toxicol. 2001;15:693–698. doi: 10.1016/s0890-6238(01)00172-1. [DOI] [PubMed] [Google Scholar]
- Conley A, Howard H, Slanger W, Ford J. Steroidogenesis in the preovulatory porcine follicle. Biol Reprod. 1994;51:655–661. doi: 10.1095/biolreprod51.4.655. [DOI] [PubMed] [Google Scholar]
- Cortvrindt RG, Smitz J. Follicle culture in reproductive toxicology: a tool for in-vitro testing of ovarian function? Hum Reprod Update. 2002;8:243–254. doi: 10.1093/humupd/8.3.243. [DOI] [PubMed] [Google Scholar]
- Crellin NK, Kang HG, Swan CL, Chedrese PJ. Inhibition of basal and stimulated progesterone synthesis by dichlorodiphenyldichloroethylene and methoxychlor in a stable pig granulosa cell line. Reproduction. 2001;121:485–492. [PubMed] [Google Scholar]
- Cummings A. Methoxychlor as a model for environmental estrogens. Crit Rev Toxicol. 1997;27:367–379. doi: 10.3109/10408449709089899. [DOI] [PubMed] [Google Scholar]
- Cummings A, Gray LJ. Antifertility effect of methoxychlor in female rats: Dose- and time-dependent blockade of pregnancy. Toxicol Appl Pharmacol. 1989;97:454–462. doi: 10.1016/0041-008x(89)90250-0. [DOI] [PubMed] [Google Scholar]
- Cummings A, Laskey J. Effect of methoxychlor on ovarian steroidogenesis: role in early pregnancy loss. Reprod Toxicol. 1993;7:17–23. doi: 10.1016/0890-6238(93)90005-r. [DOI] [PubMed] [Google Scholar]
- Dennerstein L, Lehert P, Burger H, Dudley E. Mood and the menopausal transition. J Nerv Ment Dis. 1999;187(11):685–691. doi: 10.1097/00005053-199911000-00006. [DOI] [PubMed] [Google Scholar]
- Eroschenko VP, Abuel-Atta AA, Grober MS. Neonatal exposures to technical methoxychlor alters ovaries in adult mice. Reprod Toxicol. 1995;9:379–387. doi: 10.1016/0890-6238(95)00025-6. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Meachum S, Hernández-Ochoa I, Peretz J, Yao HH, Flaws JA. Methoxychlor inhibits growth of antral follicles by altering cell cycle regulators. Toxicol Appl Pharmacol. 2009;240:1–7. doi: 10.1016/j.taap.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Miller KP, Babus JK, Flaws JA. Methoxychlor inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. Toxicol Sci. 2006;93:382–389. doi: 10.1093/toxsci/kfl052. [DOI] [PubMed] [Google Scholar]
- Harvey C, Esmail M, Wang Q, Brooks AI, Zachow R, Uzumcu M. Effect of the Methoxychlor Metabolite HPTE on the Rat Ovarian Granulosa Cell Transcriptome in Vitro. Toxicological Sciences. 2009:kfp089. doi: 10.1093/toxsci/kfp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelock J, Rainey W, Bradshaw K, Carr B. The post-menopausal ovary displays a unique pattern of steroidogenic enzyme expression. Human Reprod. 2006;21:317. doi: 10.1093/humrep/dei373. [DOI] [PubMed] [Google Scholar]
- Hayes C, Spink D, Spink B, Cao J, Walker N. 17β-estradiol hydroxylation catalyzed by human cytochrome P450 1B1. Proc Natl Acad Sci. 1996;93:9776–9781. doi: 10.1073/pnas.93.18.9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield A. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Hu FB, Grodstein F, Hennekens CH, Colditz GA, Johnson M, Manson JE, et al. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med. 1999;159(10):1061–1066. doi: 10.1001/archinte.159.10.1061. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. Guide for the care and use of laboratory animals. National Academies Press; Washington, DC: 1996. [Google Scholar]
- Jones E, DeCherney A. The female reproductive system. In: Boron W, Boulpaep E, editors. Medical physiology: a cellular and molecular approach. Elsevier Saunders; Philadelphia, PA: 2005. [Google Scholar]
- Kapoor IP, Metcalf RL, Nystrom RF, Sangha GK. Comparative metabolism of methoxychlor, methiochlor, and DDT in mouse, insects, and in a model ecosystem. J Agric Food Chem. 1970;18:1145–1152. doi: 10.1021/jf60172a017. [DOI] [PubMed] [Google Scholar]
- Levi AJ, Widra EA. Basic infertility: Etiology and Therapy. In: Seifer DB, Samuels P, Kniss DA, editors. The physiologic basis of gynecology and obstetrics. Lippincott Williams & Wilkins; Philadelphia, PA: 1994. pp. 245–263. [Google Scholar]
- Magoffin D, Erickson G. Primary culture of differentiating ovarian androgen-producing cells in defined medium. J Biol Chem. 1982;25:4507–4513. [PubMed] [Google Scholar]
- Martinez EM, Swartz WJ. Effects of methoxychlor on the reproductive system of the adult female mouse. 1 Gross and histologic observations. Reprod Toxicol. 1991;5:139–147. doi: 10.1016/0890-6238(91)90042-e. [DOI] [PubMed] [Google Scholar]
- Miller KP, Gupta RK, Flaws JA. Methoxychlor metabolites may cause ovarian toxicity through estrogen-regulated pathways. Toxicol Sci. 2006;93:180–188. doi: 10.1093/toxsci/kfl034. [DOI] [PubMed] [Google Scholar]
- Miller KP, Gupta RK, greenfeld CR, Babus JK, Flaws JA. Methoxychlor directly affects ovarian antral follicle growth and atresia through Bcl-2- and Bax-mediated pathways. Toxicol Sci. 2005;88:213–221. doi: 10.1093/toxsci/kfi276. [DOI] [PubMed] [Google Scholar]
- Miller W. Regulation of mRNAs for human steroidogenic enzymes. Endocr Res. 1989;15:1–16. doi: 10.1080/07435808909039085. [DOI] [PubMed] [Google Scholar]
- Ohyama K, Maki S, Sato K, Kato Y. Comparative in vitro metabolism of methoxychlor in male and female rats: Metabolism of demethylated methoxychlor metabolites by precision-cut rat liver slices. Xenobiotica. 2005a;35:683–695. doi: 10.1080/00498250500230693. [DOI] [PubMed] [Google Scholar]
- Ohyama K, Maki S, Sato K, Kato Y. Comparative in vitro metabolism of the suspected pro-estrogenic compound, methoxychlor in precision-cut liver slices from male and female rats. Xenobiotica. 2005b;35:331–342. doi: 10.1080/00498250500087309. [DOI] [PubMed] [Google Scholar]
- Pfaffl M. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acid Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz WJ, Eroschenko VP. Neonatal exposure to technical methoxychlor alters pregnancy outcome in female mice. Reprod Toxicol. 1998;12:565–573. doi: 10.1016/s0890-6238(98)00041-0. [DOI] [PubMed] [Google Scholar]
- Swartz W, Corkern M. Effects of methoxychlor treatment of pregnant mice on female offspring of the treated and subsequent pregnancies. Reprod Toxicol. 1992;6:431–437. doi: 10.1016/0890-6238(92)90006-f. [DOI] [PubMed] [Google Scholar]
- Tiemann U, Pöhland R, Schneider F. Influence of organochlorine pesticides on physiological potency of cultured granulosa cells from bovine preovulatory follicles. Theriogenology. 1996;46:253–265. doi: 10.1016/0093-691x(96)00182-3. [DOI] [PubMed] [Google Scholar]
- Wallner WE, Leeling NC, Zabik MJ. The fate of methoxychlor applied by helicopter for smaller European elm bark beetle control. J Econ Entomol. 1969;62:1039–1042. [Google Scholar]
- Wang C, Hsueh A, Erickson G. The role of cyclic AMP in the induction of estrogen and progestin synthesis in cultured granulosa cells. Mol Cell Endocrinol. 1982;25:73–83. doi: 10.1016/0303-7207(82)90170-8. [DOI] [PubMed] [Google Scholar]
- Zachow R, Uzumcu M. The methoxychlor metabolite, 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane, inhibits steroidogenesis in rat ovarian granulosa cells in vitro. Reproductive Toxicology. 2006;22:659–665. doi: 10.1016/j.reprotox.2006.04.018. [DOI] [PubMed] [Google Scholar]