Abstract
Peroxisome proliferator-activated receptor (PPAR)-α, a member of a large nuclear receptor superfamily, plays a major role in the regulation of lipid metabolism. Recently, PPARα activation has been shown to confer additional benefits on endothelial function, kidney function, and anti-inflammation, suggesting that PPARα agonists may be good candidates for treating acute renal failure. In clinical application, PPAR-α activators, such as hypolipidemic drugs in fibric acid class, were proven to have therapeutic effects on metabolic syndrome and cardiovascular disease. This paper focuses on signaling pathways, ligand selectivity, and physio-pathological roles of PPARα in kidney diseases and the therapeutic utility of PPARα modulators in the treatment of diabetes and inflammation-induced nephropathy. Implication of new and more potent PPAR-α activators could provide important insights into the overall benefits of activating PPAR-α clinically for the treatment of dyslipidemia and the prevention of diabetic or inflammation-induced nephropathy in the future.
1. Peroxisome Proliferator-Activated Receptors
Peroxisome proliferator-activated receptors (PPARs) are nuclear hormone receptors, that is, ligand-dependent intracellular proteins that stimulate transcription of specific genes by binding to specific DNA sequences. When activated by appropriate ligand binding, their transcription factors affect development and metabolism. There are three PPAR subtypes, products of the distinct genes commonly designated as PPARα, PPARγ, and PPARβ/δ, or merely δ [1]. The PPARs usually heterodimerize with another nuclear receptor, the 9-cis-retinoic acid receptor (RXR), forming a complex that interacts with specific DNA-response elements within the promoter regions of the target genes. Ligand binding can activate this heterodimer complex which recruits transcription coactivators and regulates the transcription of genes involved in the regulation of lipid and carbohydrate metabolism [1]. Like several other nuclear hormone receptors, it heterodimerizes with RXR to form a transcriptionally competent complex [2].
2. Tissue Expression of PPARs and Their Role in Renal Injury
PPARα, PPARβ/δ, and PPARγ are differentially expressed in various tissues [3–5]. In general, PPARα is highly expressed in tissues that possess high mitochondrial and β-oxidation activity, including the liver, renal cortex, intestinal mucosa, and heart, with lower expression in several other tissues. PPARγ is highly enriched in adipose tissue, while lower expression levels are reported in the urinary bladder, intestine, kidney, spleen, adrenal, heart, liver, lung, brain, and vasculature. Unlike PPARα and PPARγ, low-level expressions of PPARβ/δ is ubiquitously found in almost every tissue examined. In the kidney, PPARα is abundantly expressed in the proximal tubules and the medullary thick ascending limbs with much lower expression in the glomerular mesangial cells [5, 6]. PPARγ is primarily expressed in the distal medullary collecting ducts, with lesser expression in the glomeruli and renal microvasculature [7]. In the kidney, PPARβ/δ is diffusely expressed in the renal cortex and medulla, including medullary interstitial and stromal cells [5]. This differential tissue distribution of the three PPAR isoforms may be related to their distinct roles in these tissues, including the kidney. Because the target genes of PPARα, -β/δ, and -γ in these tissues are mainly involved in adipogenesis, lipid metabolism, insulin sensitivity, glucose homeostasis, and cell growth and differentiation, PPARs could be the target candidates that modulate body metabolisms.
Prior studies in animal models had described the beneficial roles for PPARs in reducing renal injury and dysfunction. For instances, PPARβ/δ pretreatment could protect wild-type mice from renal I/R injury, with a reduction in medullary necrosis and inflammation [8]. PPARγ agonists rosiglitazone and pioglitazone had shown protective effects against renal ischemia/reperfusion (I/R), diabetic nephropathy, and various kidney injury [9, 10]. Although a role for PPARα in reducing renal injury and PPARα ligands could attenuate cisplatin-induced acute renal failure (ARF) was reported in animal models [11, 12], its exact mechanisms are still inconclusive. Therefore, this paper will focus on the role of PPARα and its agonist in renal diseases.
3. PPARα Ligands and Their Clinical Implications
Fibric acid derivatives or fibrates are PPARα ligands. Fibrates have been used in clinical practice for more than four decades to decrease triglyceride levels. Fibrates can also increase HDL cholesterol levels, with a limited but significant additional effect on decreasing low-density lipoprotein (LDL) cholesterol levels. In addition to its major effects on lipid profiles, mounting evidence shows that beneficial effects of fibrates may be due to their anti-inflammatory and antiatherosclerotic properties [13, 14]. The PPAR agonists can be synthetic molecules, such as fibrates used to treat hypertriglyceridemia or thiazolidinediones to treat insulin resistance, or natural ligands, such as fatty acids (FAs) and their derivatives (eicosanoids). Although fibrates are most efficient in patients with high TG and low HDL, marginal effects in the treatment of dyslipidemia were found in the recent ACCORD (Action to Control Cardiovascular Risk in Diabetes) trials to patients with type-2 diabetes [15]. Nevertheless, recent ACCORD studies demonstrated that fibrate therapy with intensive glycemia control could reduce renal microalbuminuria significantly [16]. Although microalbuminuria may rather be a marker for cardiovascular disease [17], its applications as a reversible marker of kidney and vascular damage were recently reported [18, 19].
4. PPARα and Diabetic Nephropathy
Although the abundance of PPARα in the kidney is well established, its role in renal physiology and diabetic nephropathy is just emerging. PPARα was implicated in the regulation of kidney metabolism and to maintain a sustained balance between energy production and expenditure [20], given its high level expression in the renal proximal tubules [5, 21, 22]. Clofibrate activates PPARα and induces expression of β-oxidation enzymes, long-chain and medium-chain acyl-CoA dehydrogenase, and acyl-CoA oxidase in the renal cortex [23]. It is suggested that renal PPARα might play a major role in triggering fatty acid utilization and adaptive response to dietary lipids. This idea is further supported by a recent study in which the beneficial effects of fasting-induced upregulation of pyruvate dehydrogenase kinases were blunted in PPARα-deficient mice, indicating that loss of PPARα can lead to abnormal renal regulation during starvation [24]. Although PPARα induction is beneficial in fasting and hyperlipidemia, effects of PPARα in diabetic nephropathy remain unclear. However, clinical evidence suggests a beneficial effect of fibrate treatment in patients with type-2 diabetes [25, 26], and data from the recent FIELD (Fenofibrate Intervention for Event Lowering in Diabetes) study also indicate promising effects with fenofibrate in preventing progression of diabetes-related microvascular complications [27]. In db/db type-2 diabetic mice, treatment with fenofibrate markedly lowers urinary albumin excretion and improves glomerular mesangial expansion [28, 29]. Therefore, both clinical observations and rodent experiments suggest that PPARα activation may play a beneficial role in diabetes induced nephropathy.
5. PPARα and Kidney Mesangial Cells
Clofibrate has been shown to inhibit oxidative stress-induced TGF-β expression in glomerular mesangial cells [30]. Expression of PPARα in glomerular mesangial cells has also been reported [31]; thus it is likely that PPARα activation in mesangial cells could block TGF-β signaling pathway and thereby attenuating glomerular matrix proliferation. Consistent with this suggestion, a recent study demonstrated that fenofibrate downregulates TGF-β1 and TGF-β signaling receptor II expression and decreases collagen IV deposition in the diabetic glomeruli [32]. Conversely, starved PPARα null mice would show increased albuminuria with albumin accumulation in the proximal tubules further confirming the beneficial role of PPAR-α [33]. Therefore, it is likely that PPARα activation may facilitate albumin reabsorption and degradation in the nephron segment [34, 35]. Taken together, fenofibrate treatment activated PPARα may reduce TGF-β-induced proliferation in mesangial cells, thus ameliorate kidney injury.
6. Involvement of PPARα in Inflammation
PPARα plays a critical role as a primary sensor and regulator of lipid metabolism, and this role has increasingly been recognized to be important in inflammation-induced disorders including hypertension, metabolic disorders, cardiovascular disease, atherosclerosis, and inflammation-induced acute renal failure [36]. Fenofibrates, ligands for PPARα, are used clinically to treat patients with type-2 diabetes or coronary disease [37]. Fibrates can exert anti-inflammatory effects, by decreasing plasma levels of cytokines IL-6, TNFα, and IFNγ in patients with atherosclerosis [38] or level of CRP in patients with cardiovascular diseases [39]. In human endothelial cells, PPARα activators interfere with processes involved in leukocyte recruitment and cell adhesion by inhibiting the expression of VCAM-1. Since PPARα agonists (fenofibric acid and eicosapentaenoic acid) enhance e-NOS expression and NO release, this suggests a vaso-protective effect. In other studies, synthetic PPARα activators (fenofibric acid and WY14643) diminish thrombin-induced and oxidized LDL-induced expression of endothelin-1 [38]. PPARα activators can also modify inflammatory vascular smooth muscle cells (VSMC) activation by inhibiting IL-1-induced production of IL-6 and prostaglandins and by reducing the expression of cyclooxygenase-2 (COX-2). In addition, PPARα agonists reduce tissue factor and MMP expression in monocytes and macrophages. Moreover, PPARα activation, in the presence of TNFα and IFNγ, may promote macrophage apoptosis. Finally, activators of PPARα limit the production of proatherogenic Th1 cytokines such as IFNγ, TNFα, and IL-2 [38]. PPARα activators also inhibit the inflammatory response in hepatocytes by decreasing IL-1-induced CRP and IL-6-induced fibrinogen α, -β, and serum amyloid A expression [39]. PPARα thus acts as an antiatherogenic factor by modulating local and systemic inflammatory responses.
7. Involvement of PPARα in Ischemia-Reperfusion-Induced Kidney Injury
Although the causes of ARF are often multifactorial, they can be generally classified into three categories depending on the causes: (1) prerenal ARF, in which the kidney fails to receive an adequate blood supply, for example, due to a fall in systemic blood pressure subsequent to hemorrhage [40]; (2) intrinsic ARF, in which the failure originates within the kidney, for example, due to drug-induced nephrotoxicity like traditional cisplatin or gentamicin-induced nephrotoxicity; and (3) postrenal ARF, caused by impairment of urine flow from the kidney, for example, due to ureteral obstruction or bladder/prostate cancer. Increasing evidence supports a role for PPARα in the development of ARF. Several studies have demonstrated a reduction in PPARα expression, transcriptional activity, and inhibition of peroxisomal and mitochondrial fatty acid oxidation (FAO) enzymes in rodent renal tissue undergoing cisplatin- and I/R-induced ARF [41]. Activation of PPARα with ligands such as fibrate or WY14643 reduces cisplatin and I/R-induced acute kidney injury [42]. Importantly, these effects of fibrate and WY14643 are not observed in PPARα-null mice. These mice subjected to I/R injury by arterial ligation show enhanced cortical necrosis and impaired renal function [22]. However, such renal I/R injury could be rescued via induction of PPARα with recovery of normal kidney structure and function [22]. Recent investigations using kidney androgen-induced protein 2 (KAP2) promoter with tissue-restricted expression model further corroborate the essential role of PPARα in renal protection [43]. As KAP2 is exclusively expressed in the proximal tubules under the control of androgens, their studies delineated that the androgen-induced proximal tubules PPARα transgenic mice could afford protection against cisplatin- and I/R-induced inhibition of FAO and protected kidney function and morphology from these insults, in comparison with their effects on wild-type mice. In addition, the organ and tissue (proximal tubule-) restricted expression model in their studies further ruling out the potential PPARα-independent, renoprotective actions as well as excluding the potential PPARα-mediated, extrarenal effects in renal protection afforded by PPARα activators in the PPARα-null mouse [43]. We also demonstrated that prostacyclin may act as an inducer, which can enhance PPARα translocation into the nucleus and bind to inflammatory transcriptional factor NFκB thus inhibiting TNFα-induced apoptosis in renal epithelial cells. In addition, wild-type mice pretreated with a PPARα activator, docosahexaenoic acid (DHA), could significantly reduce I/R-induced renal dysfunction (lowered serum creatinine and urea nitrogen levels), apoptotic responses (decreased apoptotic cell number and caspase-3 and -8 activation), and NF-κB activation [33]. Altogether, these studies strongly endorse a critical role of PPARα in the preservation of renal morphology and function during cisplatin- or I/R-induced acute renal damage.
8. Regulation of PPARα
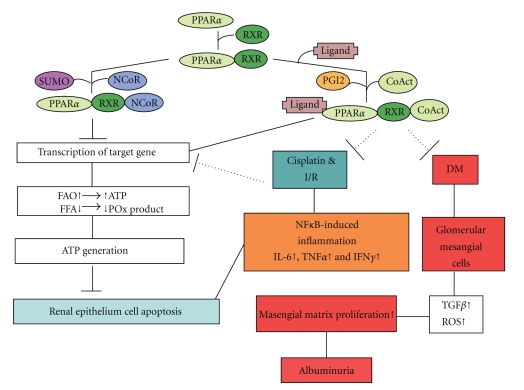
Ligands binding to PPARα unmask an interaction area (of PPARα) for coactivators such as cAMP response element-binding protein (CREB-) binding protein (CBP)/p300. The latter possesses histone acetyl transferase (HAT) activity resulting in chromatin decondensation and PPARα heterodimerization with RXR. The binding of this heterodimer to PPRE on PPARα promoter then regulates target genes expression. In addition, PPARα(s) are substrates for several kinases activated by a variety of endogenous or exogenous signals. These kinase include: extracellular receptor kinase-mitogen-activated protein kinase (ERK-MAPK), JNK and p38 MAPK, Protein kinase A, Protein kinase C (PKC), 5′-AMP-activated protein kinase (AMPK), and glycogen synthase kinase 3 (GSK3). Recently, SUMOylation of PPARα has reported that SUMOylated hPPARα on lysine 185 resulted in down-regulation of its transcriptional activity by promoting its interaction with the corepressor NCoR [44]. Therefore, it is interesting to investigate whether PPARα modification, including phosporylation, SUMOylation, and ubiquitination, is involved in inflammation-induced renal failure. Recently, we also demonstrated that adiponectin exerts protective effect against renal ischemic-reperfusion injury via prostacyclin- PPARα-heme oxygenase-1 signaling pathway (unpublished data). A schematic diagram presenting the regulation of PPARα in renal disease is depicted in Figure 1.
Figure 1.
Schematic diagram presenting the signaling pathways of PPARα involved in the mechanisms of ischemic/reperfusion-, drugs-, or diabetic-induced renal damage. PPARα forms heterodimer with RXR. In the absence of ligands, the dimer may recruit a corepressor, inhibiting PPARα-mediated transcription of target genes. The presence of an agonist, or an activator such as PGI2, triggers the recruitment of a coactivator complex which induces transcriptional activity of PPARα onto its target genes. This leads to an increase in fatty acid catabolism and adenosine triphosphate (ATP) production, also to decrease the levels of cytotoxic fatty acid peroxidation (POx) products, and, consequently, to promote cell viability and inhibit renal epithelium cell death. In addition, PPARα complex can attenuate NFκB-induced inflammatory factors (IL-6, INFγ, or TNFα) induced by ischemic/reperfusion injury (I/R) or drugs. Furthermore, PPARα complex can inhibit masengial matrix proliferation induced by TGFβ or reactive oxidative stress (ROS) which then resulted in albuminuria. After SUMOylation of PPARα, SUMOylated PPARα resulted in downregulation of its transcriptional activity by promoting its interaction with the corepressor NCoR, which will compromise cell viability and activate cell death processes. CoAct, coactivator; DM, diabetes mellitus; FAO, fatty acid oxidation; FFA, free fatty acid; IFNγ, interferon γ; IL-6, interleukine-6; I/R, ischemia/reperfusion; NCoR, nuclear corepressor; NF-κB, nuclear factor-κB; PGI2, prostacyclin; POx, peroxidation; PPARα, peroxisome proliferator-activated receptor-α; RXR, retinoid X receptor; TGFβ, tumor growth factor β; TNFα, tumor necrosis factor α.
9. Conclusion and Perspectives
PPARα, in the last few years, has emerged as the key regulator of lipid homeostasis in in vitro experiments and clinical medicine. In addition, PPARα negatively regulates inflammation-mediated phenomenon like atherosclerosis and ARF. PPARα ligand and fibrates are pharmacologic agents with pleiotropic effects. Fibrates have beneficial effects in alleviating cardiovascular abnormalities, ARF-, diabetic- or drug-induced nephropathy, in both animal models and clinical trials [45, 46]. Although the effects of PPARα have not been fully investigated, they are shown to be protective in chronic kidney diseases.
Acknowledgments
This work was supported by Grants from the Tzu Chi University (nos. TCIRP 95007-01 and -02) to C.-F. Cheng and H. Lin, respectively, and Tzu Chi Hospital (nos. TCRD-TPE-95-15 and TCRD-I 9801-01) to C.-F. Cheng. There were no conflict of interests for any of the authors.
References
- 1.Berger JP, Akiyama TE, Meinke PT. PPARs: therapeutic targets for metabolic disease. Trends in Pharmacological Sciences. 2005;26(5):244–251. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Fruchart JC, Staels B, Duriez P. The role of fibric acids in atherosclerosis. Current Atherosclerosis Reports. 2001;3(1):83–92. doi: 10.1007/s11883-001-0015-x. [DOI] [PubMed] [Google Scholar]
- 3.Fajas L, Debril M-B, Auwerx J. Peroxisome proliferator-activated receptor-γ: from adipogenesis to carcinogenesis. Journal of Molecular Endocrinology. 2001;27(1):1–9. doi: 10.1677/jme.0.0270001. [DOI] [PubMed] [Google Scholar]
- 4.Mukherjee R, Jow L, Noonan D, McDonnell DP. Human and rat peroxisome proliferator activated receptors (PPARs) demonstrate similar tissue distribution but different responsiveness to PPAR activators. Journal of Steroid Biochemistry and Molecular Biology. 1994;51(3-4):157–166. doi: 10.1016/0960-0760(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 5.Guan Y, Zhang Y, Davis L, Breyer MD. Expression of peroxisome proliferator-activated receptors in urinary tract of rabbits and humans. American Journal of Physiology. 1997;273(6, part 2):F1013–F1022. doi: 10.1152/ajprenal.1997.273.6.F1013. [DOI] [PubMed] [Google Scholar]
- 6.Ruan XZ, Moorhead JF, Fernando R, Wheeler DC, Powis SH, Varghese Z. PPAR agonists protect mesangial cells from interleukin 1β-induced intracellular lipid accumulation by activating the ABCA1 cholesterol efflux pathway. Journal of the American Society of Nephrology. 2003;14(3):593–600. doi: 10.1097/01.asn.0000050414.52908.da. [DOI] [PubMed] [Google Scholar]
- 7.Guan Y, Zhang Y, Schneider A, Davis L, Breyer RM, Breyer MD. Peroxisome proliferator-activated receptor-γ activity is associated with renal microvasculature. American Journal of Physiology. 2001;281(6):F1036–F1046. doi: 10.1152/ajprenal.0025.2001. [DOI] [PubMed] [Google Scholar]
- 8.Letavernier E, Perez J, Joye E, et al. Peroxisome proliferator-activated receptor β/δ exerts a strong protection from ischemic acute renal failure. Journal of the American Society of Nephrology. 2005;16(8):2395–2402. doi: 10.1681/ASN.2004090802. [DOI] [PubMed] [Google Scholar]
- 9.Chung BH, Lim SW, Ahn KO, et al. Protective effect of peroxisome proliferator activated receptor gamma agonists on diabetic and non-diabetic renal diseases. Nephrology. 2005;10(supplement 2):S40–S43. doi: 10.1111/j.1440-1797.2005.00456.x. [DOI] [PubMed] [Google Scholar]
- 10.Cuzzocrea S. Peroxisome proliferator-activated receptors gamma ligands and ischemia and reperfusion injury. Vascular Pharmacology. 2004;41(6):187–195. doi: 10.1016/j.vph.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Portilla D, Dai G, Peters JM, Gonzalez FJ, Crew MD, Proia AD. Etomoxir-induced PPARα-modulated enzymes protect during acute renal failure. American Journal of Physiology. 2000;278(4):F667–F675. doi: 10.1152/ajprenal.2000.278.4.F667. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Bhatt R, Megyesi J, Gokden N, Shah SV, Portilla D. PPAR-α ligand ameliorates acute renal failure by reducing cisplatin-induced increased expression of renal endonuclease G. American Journal of Physiology. 2004;287(5):F990–F998. doi: 10.1152/ajprenal.00206.2004. [DOI] [PubMed] [Google Scholar]
- 13.Staels B, Fruchart J-C. Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes. 2005;54(8):2460–2470. doi: 10.2337/diabetes.54.8.2460. [DOI] [PubMed] [Google Scholar]
- 14.Israelian-Konaraki Z, Reaven PD. Peroxisome proliferator-activated receptor-alpha and atherosclerosis: from basic mechanisms to clinical implications. Cardiology. 2005;103(1):1–9. doi: 10.1159/000081845. [DOI] [PubMed] [Google Scholar]
- 15.Wierzbicki AS. Fibrates: no ACCORD on their use in the treatment of dyslipidaemia. Current Opinion in Lipidology. 2010;21(4):352–358. doi: 10.1097/MOL.0b013e32833c1e74. [DOI] [PubMed] [Google Scholar]
- 16.Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. The Lancet. 2010;376(9739):419–430. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Zeeuw D, Parving H-H, Henning RH. Microalbuminuria as an early marker for cardiovascular disease. Journal of the American Society of Nephrology. 2006;17(8):2100–2105. doi: 10.1681/ASN.2006050517. [DOI] [PubMed] [Google Scholar]
- 18.Czekalski S. Microalbuminuria as a reversible marker of kidney and vascular damage. Nefrologia i Dializoterapia Polska. 2006;10(4):166–168. [Google Scholar]
- 19.Cerasola G, Cottone S, Mulè G. The progressive pathway of microalbuminuria: from early marker of renal damage to strong cardiovascular risk predictor. doi: 10.1097/HJH.0b013e32833ec377. Journal of Hypertension. In press. [DOI] [PubMed] [Google Scholar]
- 20.Portilla D. Energy metabolism and cytotoxicity. Seminars in Nephrology. 2003;23(5):432–438. doi: 10.1016/s0270-9295(03)00088-3. [DOI] [PubMed] [Google Scholar]
- 21.Beck F, Plummer S, Senior PV, Byrne S, Green S, Brammar WJ. The ontogeny of peroxisome-proliferator-activated receptor gene expression in the mouse and rat. Proceedings of the Royal Society B. 1992;247(1319):83–87. doi: 10.1098/rspb.1992.0012. [DOI] [PubMed] [Google Scholar]
- 22.Portilla D, Dai G, Peters JM, Gonzalez FJ, Crew MD, Proia AD. Etomoxir-induced PPARα-modulated enzymes protect during acute renal failure. American Journal of Physiology. 2000;278(4):F667–F675. doi: 10.1152/ajprenal.2000.278.4.F667. [DOI] [PubMed] [Google Scholar]
- 23.Ouali F, Djouadi F, Merlet-Bénichou C, Bastin J. Dietary lipids regulate β-oxidation enzyme gene expression in the developing rat kidney. American Journal of Physiology. 1998;275(5):F777–F784. doi: 10.1152/ajprenal.1998.275.5.F777. [DOI] [PubMed] [Google Scholar]
- 24.Sugden MC, Bulmer K, Gibbons GF, Holness MJ. Role of peroxisome proliferator-activated receptor-α in the mechanism underlying changes in renal pyruvate dehydrogenase kinase isoform 4 protein expression in starvation and after refeeding. Archives of Biochemistry and Biophysics. 2001;395(2):246–252. doi: 10.1006/abbi.2001.2586. [DOI] [PubMed] [Google Scholar]
- 25.Fried LF, Orchard TJ, Kasiske BL. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney International. 2001;59(1):260–269. doi: 10.1046/j.1523-1755.2001.00487.x. [DOI] [PubMed] [Google Scholar]
- 26.Smolders YM, van Eeden AE, Stehouwer CDA, Weijers RNM, Slaats EH, Silberbusch J. Can reduction in hypertriglyceridaemia slow progression of microalbuminuria in patients with non-insulin-dependent diabetes mellitus? European Journal of Clinical Investigation. 1997;27(12):997–1002. doi: 10.1046/j.1365-2362.1997.2330779.x. [DOI] [PubMed] [Google Scholar]
- 27.Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. The Lancet. 2005;366(9500):1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 28.Park CW, Zhang Y, Fan XF, et al. A PPARα agonist improves diabetic nephropathy in db/db mice. Journal of the American Society of Nephrology. 2003;14:p. 393A. [Google Scholar]
- 29.Park CW, Zhang Y, Zhang X, et al. PPARα agonist fenofibrate improves diabetic nephropathy in db/db mic. Kidney International. 2006;69(9):1511–1517. doi: 10.1038/sj.ki.5000209. [DOI] [PubMed] [Google Scholar]
- 30.Wilmer WA, Dixon CL, Hebert C, Lu L, Rovin BH. PPAR-α ligands inhibit H2O2-mediated activation of transforming growth factor-β1 in human mesangial cells. Antioxidants & Redox Signaling. 2002;4(6):877–884. doi: 10.1089/152308602762197416. [DOI] [PubMed] [Google Scholar]
- 31.Hou X, Shen YH, Li C, et al. PPARα agonist fenofibrate protects the kidney from hypertensive injury in spontaneously hypertensive rats via inhibition of oxidative stress and MAPK activity. Biochemical and Biophysical Research Communications. 2010;394(3):653–659. doi: 10.1016/j.bbrc.2010.03.043. [DOI] [PubMed] [Google Scholar]
- 32.Li L, Emmett N, Mann D, Zhao X. Fenofibrate attenuates tubulointerstitial fibrosis and inflammation through suppression of nuclear factor-κB and transforming growth factor-β1/Smad3 in diabetic nephropathy. Experimental Biology and Medicine. 2010;235(3):383–391. doi: 10.1258/ebm.2009.009218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen H-H, Chen T-W, Lin H. Prostacyclin-induced peroxisome proliferator-activated receptor-α translocation attenuates NF-κB and TNF-α activation after renal ischemia-reperfusion injury. American Journal of Physiology. 2009;297(4):F1109–F1118. doi: 10.1152/ajprenal.00057.2009. [DOI] [PubMed] [Google Scholar]
- 34.Liao J, Soltani Z, Ebenezer P, et al. Tesaglitazar, a dual peroxisome proliferator-activated receptor agonist (PPARα/γ), improves metabolic abnormalities and reduces renal injury in obese Zucker rats. Nephron Experimental Nephrology. 2009;114(2):e61–e68. doi: 10.1159/000254567. [DOI] [PubMed] [Google Scholar]
- 35.Calkin AC, Giunti S, Jandeleit-Dahm KA, Allen TJ, Cooper ME, Thomas MC. PPAR-α and -γ agonists attenuate diabetic kidney disease in the apolipoprotein E knockout mouse. Nephrology Dialysis Transplantation. 2006;21(9):2399–2405. doi: 10.1093/ndt/gfl212. [DOI] [PubMed] [Google Scholar]
- 36.Robinson E, Grieve DJ. Significance of peroxisome proliferator-activated receptors in the cardiovascular system in health and disease. Pharmacology & Therapeutics. 2009;122(3):246–263. doi: 10.1016/j.pharmthera.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Seiler C, Suter TM, Hess OM. Exercise-induced vasomotion of angiographically normal and stenotic coronary arteries improves after cholesterol-lowering drug therapy with bezafibrate. Journal of the American College of Cardiology. 1995;26(7):1615–1622. doi: 10.1016/0735-1097(95)00379-7. [DOI] [PubMed] [Google Scholar]
- 38.Gilde AJ, van der Lee KAJM, Willemsen PHM, et al. Peroxisome proliferator-activated receptor (PPAR) α and PPARβ/δ, but not PPARγ, modulate the expression of genes involved in cardiac lipid metabolism. Circulation Research. 2003;92(5):518–524. doi: 10.1161/01.RES.0000060700.55247.7C. [DOI] [PubMed] [Google Scholar]
- 39.Zambon A, Gervois P, Pauletto P, Fruchart J-C, Staels B. Modulation of hepatic inflammatory risk markers of cardiovascular diseases by PPAR-α activators: clinical and experimental evidence. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26(5):977–986. doi: 10.1161/01.ATV.0000204327.96431.9a. [DOI] [PubMed] [Google Scholar]
- 40.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. The New England Journal of Medicine. 1996;334(22):1448–1460. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 41.Lopez-Hernandez FJ, Lopez-Novoa JM. Potential utility of PPARα activation in the prevention of ischemic and drug-induced acute renal damage. Kidney International. 2009;76(10):1022–1024. doi: 10.1038/ki.2009.229. [DOI] [PubMed] [Google Scholar]
- 42.Sivarajah A, Chatterjee PK, Hattori Y, et al. Agonists of peroxisome-proliferator activated receptor-α (clofibrate and WY14643) reduce renal ischemia/reperfusion injury in the rat. Medical Science Monitor. 2002;8(12):BR532–BR539. [PubMed] [Google Scholar]
- 43.Li S, Nagothu KK, Desai V, et al. Transgenic expression of proximal tubule peroxisome proliferator-activated receptor-α in mice confers protection during acute kidney injury. Kidney International. 2009;76(10):1049–1062. doi: 10.1038/ki.2009.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pourcet B, Pineda-Torra I, Derudas B, Staels B, Glineur C. SUMOylation of human peroxisome proliferator-activated receptor α inhibits its trans-activity through the recruitment of the nuclear corepressor NCoR. Journal of Biological Chemistry. 2010;285(9):5983–5992. doi: 10.1074/jbc.M109.078311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dogra G, Irish A, Chan D, Watts G. A randomized trial of the effect of statin and fibrate therapy on arterial function in CKD. American Journal of Kidney Diseases. 2007;49(6):776–785. doi: 10.1053/j.ajkd.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Sica DA. Fibrate therapy and renal function. Current Atherosclerosis Reports. 2009;11(5):338–342. doi: 10.1007/s11883-009-0051-5. [DOI] [PubMed] [Google Scholar]