Abstract
Pituitary adenylate cyclase activating peptide (PACAP), a neuroregulatory peptide, is found in germinative regions of the CNS, including the olfactory bulb, throughout adulthood. We show that 1) PACAP immunoreactivity is also present in the neonatal mouse and adult mouse and rat olfactory epithelium, 2) PACAP expression pattern differs between neonatal and adult mice, and 3) PACAP is produced by olfactory ensheathing cells. PACAP may thus be a key factor in the uniquely supportive role of olfactory ensheathing cells in regeneration of neurons from olfactory epithelium and lesioned spinal cord. Using calcium imaging, we demonstrated physiological responses to PACAP in both neonatal and adult olfactory receptor neurons (ORNs). We propose that PACAP plays an important role in normal turnover of ORNs by providing neurotrophic support during development and regeneration and neuroprotective support of mature neurons.
INTRODUCTION
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a somatic regulatory molecule whose pleiotropic effects include stimulation of cell proliferation, induction of differentiation, modulation of neurotransmitter release, vasodilation, bronchodilation, intestinal motility increase, suppression of inflammatory responses, and increases in hormone release (for review see Vaudry et al. 2000). Within the CNS, PACAP serves as a neurotransmitter, neurohormone, neuromodulator, secretagogue, neurotrophic factor, neuroprotectant, and glial effector (Arimura 1998).
The highest levels of PACAP and its G-protein–coupled PAC-1 receptors occur in germinative regions of the embryonic and postnatal nervous system, suggesting that PACAP plays an important role in both neuronal and glial development (Jaworski and Proctor 2000; Lindholm et al. 1998; Waschek et al. 1998). PACAP exerts an array of growth factor-like actions that depend on the source and developmental stage of the cells. PACAP promotes survival and induces neurite sprouting of cultured neuroblast cells (Gonzalez et al. 1997), and it prevents apoptosis in neurons grown under conditions that normally produce programmed cell death (Canonico 1996). PACAP also induces proliferation in cultured cerebellar granule cells and increases the volume of cerebellar cortex when injected into 8-day-old rats (Vaudry et al. 1999). Such neurotrophic effects are mediated through the adenylyl cyclase pathway through PKA, MAP kinases, ERK, or cyclic AMP-response element binding protein (CREB) phosphorylation and result in increased c-fos mediated gene expression (Vaudry et al. 1998). Activation of PAC-1 receptors can also lead to activation of phospholipase C (PLC), IP3 production, and mobilization of Ca2+ from intracellular stores (Beaudet et al. 2000; Kopp et al. 1999). The presence of other growth factors can markedly affect these actions of PACAP, for example, reversing PACAP’s effect from proliferative to antiproliferative (Lelievre et al. 2002). All of the pathways outlined above are present in the peripheral olfactory system and could provide the molecular substrates for mediating PACAP’s neurotrophic and neuroprotective effects.
Olfactory ensheathing cells (OECs) are specialized Schwann cells that wrap olfactory receptor neuron (ORN) axon bundles throughout the lamina propria and nerve fiber layer of the olfactory bulb. OECs are thought to play important roles in the unique regenerative capabilities of the olfactory system. OECs have also been used in transplant studies in the lesioned spinal cord and CNS, where they promote axonal regeneration (Ramón-Cueto et al. 1998; Li et al. 1997). Because OECs express many growth factors and adhesion molecules, they may influence olfactory neuronal axon outgrowth and targeting in the olfactory bulb (Kafitz and Greer 1999; Ramón-Cueto and Avila 1998; Ubink and Hökfelt 2000).
There is limited information available regarding the distribution and activity of PACAP and its receptors in the peripheral olfactory system (Hansel et al. 2001a,b). Thus in this study we examined the expression patterns of PACAP and the functionality of PACAP receptors in the olfactory system. We noted important species differences and found that PACAP is differentially expressed in the neonatal and adult olfactory epithelium (OE). Interestingly, OECs express PACAP in adults across the two species (rat and mouse). We found that PACAP elicits physiological responses in neonatal and adult mouse and in adult rat ORNs.
METHODS
Animals
All animal manipulations were performed in accordance with the requirements set by the National Institutes of Health Guide for Care and Use of Laboratory Animals. Swiss Webster mice and Simonsen Albino rats were used for all experiments conducted at the University of Utah, including all physiology and immunohistochemistry experiments. CD-1 mice were used for reverse transcriptase-polymerase chain reaction (RT-PCR) and immunocytochemistry conducted on OECs at the University of British Columbia.
Tissue preparation for immunohistochemistry
Postnatal day 0 (P0) through P5 Swiss Webster mice from three different litters were killed by decapitation and quickly dissected. Tissue was fixed by immersion in 4% paraformaldehyde (PF) in 0.1 M phosphate buffered saline (PBS) overnight at 4°C, rinsed for 20 min in PBS, and sequentially cryoprotected in cold 15% and 30% sucrose in PBS. Tissue was quick frozen in Tissue Freezing Medium, and 12-μm cryostat sections were collected on gelatin-coated glass slides. Adult Swiss Webster mice (approximately 40 g, n = 4) or adult Sprague-Dawley rats (approximately 200 g, n = 3) were deeply anesthetized and intracardial perfusion fixed with 4% PF for 15 min. Olfactory tissue was dissected and postfixed for 2 h in 4% PF. Tissue was rinsed in PBS and placed in a rapid decalcifier for 1 h (Apex Engineering, Plainfield, IL) before cryoprotection and sectioning as described above.
Staining and visualization
Primary antiserum was diluted in antibody dilution buffer consisting of 0.3% Triton X 100, 0.1 M PBS, and 0.02% sodium azide. Primary antibodies were applied to hydrated sections either as a mixture or on adjacent sections (depending on cross-reactivity of primary or secondary antibodies) at 4°C overnight. Primary and secondary antibodies and their dilutions are given in Table 1. Secondary antiserum was applied for 30 min. Control experiments included the omission of the primary antibody, omission of the secondary antibody, incubation with nonimmune serum in place of the primary antibody (when available), and preabsorption of primary antibody with antigen. Following three 10-min washes in PBS, sections were mounted in Vectashield mounting media and visualized on a Zeiss confocal LSM510 argon-krypton laser scanner attached to an upright Zeiss Axioskop 2FS microscope. Separate scans run sequentially using only one excitation/filter set eliminated the possibility of bleed through. FITC dye was excited at 488 nm and filtered at 505 nm, and tetramethyl rhodamine isothiocyanate (TRITC) dye was excited at 568 nm and filtered at 585 nm. Although the immunohistochemical data presented were from Swiss Webster mice, similar PACAP immunoreactivity patterns were obtained from limited studies independently performed in the Roskams’ laboratory on adult and P4 CD-1 mice.
TABLE 1.
Antibodies and dyes used in these studies
| 1° Antiserum or Dye | Source | Host | Dilution | 2° Antiserum IgG | Conjugated Dye | Dilution |
|---|---|---|---|---|---|---|
| Anti-OMP | Gift from F. Margolis | Goat | 1:10,000 | Donkey anti-goat | TRITC | 1:100 |
| Anti-PACAP38 | Peninsula Labs | Rabbit | 1:250–1:500 | Donkey anti-rabbit | FITC | 1:100 |
| Anti-PACAP38 | Peninsula Labs | Guinea pig | 1:500 | Donkey anti-guinea pig | Cy2 | 1:100 |
| Anti-S-100β | Sigma | Mouse | 1:500 | Goat anti-mouse | Alexa 594 | 1:100 |
| Anti-S-100 | Neomarkers | Rabbit | 1:100 | Donkey anti-rabbit | FITC or TRITC | 1:100 |
| DAPI | Vector Labs | 1:10,000 |
TRITC, FITC, and Cy-2 conjugated dyes are from Jackson ImmunoResearch Labs, and Alexa conjugated dyes are from Molecular Probes.
Primary cultures and slices of olfactory epithelium
Adult Swiss Webster mouse and Simonsen albino rat ORNs were obtained using the same procedures as described in Vargas and Lucero (1999a). Briefly, tissue was placed in divalent cation-free Ringer containing 10 mg/ml bovine serum albumin, 1 mg/ml deoxyribonuclease II, and 44 U/ml dispase and incubated at 37°C for 45 min. The tissue was washed, triturated, and filtered through a 53-μm mesh, and 200 ml of cell suspension was plated onto concanavalin A-coated coverslips and allowed to settle for 20 min. An additional 1.5 ml of culture medium was added [DMEM supplemented with 100 μM ascorbic acid, 1× insulin-transferrin-selenium X (Gibco BRL), 2 mM glutamine, 100 U/ml penicillin G, and 100 μg/ml streptomycin]. The primary cultures from rat and mouse are very similar and contain a mix of ORNs, respiratory cells, immature neurons, fibroblasts, and OECs. ORNs were identified by their small size, round soma with bipolar processes, and calcium responses to elevated K+ Ringer. A single animal was used for each culture, and four coverslips were prepared from each mouse, whereas eight coverslips were prepared from each rat. In contrast to OEC cultures described below, we did not combine tissue from adult animals.
To prepare olfactory epithelium (OE) slices, neonatal mice (postnatal day 0–4) were quickly killed by decapitation, followed by removal of the skin and lower jaw. Tissue and supporting blocks of carrot were glued to a vibratome-cutting block with cyanoacrylate, and 250- to 300-μm slices were cut. Ice cold slices were transferred to oxygenated Ringer solution until ready to load with calcium indicator dye.
Solutions and odors
Standard Ringer solution contained (in mM) 140 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES, and 10 glucose. Ca2+-free Ringer was the same as standard Ringer with the omission of 2 mM CaCl2 and addition of 4 mM EGTA. Elevated K+ Ringer (High K+) substituted 90 mM KCl for 90 mM NaCl. In fura imaging of adult rat cultures, 25 μM n-amyl acetate was used as an odorant. In calcium imaging of adult mouse cultures and neonatal mouse slices, a mixture of 25 μM n-amyl acetate and 50 μM R-carvone was used. In these experiments, we included ATP as a positive control because we have recently identified purinergic receptors in rodent ORNs and found that ATP activates robust Ca2+ increases in a large percentage of cells (Hegg and Lucero 2001). Odors and ATP were made fresh daily. A 1:1,000 dilution of fluorescein in Ringer was used to determine the time course and access of test substances at the 100-μm recording depth in the slice. All chemicals were obtained from Sigma unless stated otherwise. PACAP is found in two forms: the 38 amino acid PACAP38 and the 27 amino acid PACAP27. The antibodies, PCR primers, and peptides used in these studies only tested the predominate form of the peptide, which is PACAP38.
Primary OEC cultures
Purified cultures of mouse OECs were prepared from the lamina propria of neonatal CD-1 mice exactly as previously described (Au and Roskams 2003). For each culture, the tissue was obtained from a litter of P4 or P5 mice. On average there were approximately 12 animals/litter.
RT-PCR of OEC cultures or OE
Total RNA was isolated from two separate purified mouse OEC cultures and whole OE from two adult mice using Trizol (Gibco BRL), followed by polyadenylation selection using a cellulose oligo(dT) matrix (QuickPrep Micro mRNA purification kit, Amersham Pharmacia Biotech). An aliquot of each was incubated with RNAse to use as a negative control. First-strand cDNA synthesis was carried out with 40 ng of mRNA in a random hexamer-primed 20-μl RT reaction using SuperScript II RNase H− RT and reagents (Gibco BRL) and incubated at 42°C for 50 min. A second control reaction, omitting the RT, was included to confirm absence of genomic contamination. A 2-μl sample of the first strand cDNA was used as a template for each 50 μl PCR and amplified using PlatinumTaq DNA polymerase (Gibco BRL). Primers for detection of PACAP transcripts (GeneBank accession no. AB0100149) were forward (5′-ATGTGTAGCGGAAGGCTGG-3′) and reverse (5′-CACTCGGACGGCATCTTCACAGATAG-3′), and primers for β-actin transcripts were 1038–1067 forward (5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′) and 1875–1905 reverse (5′-AGAAGCATTGCGGTGGACGATGGAGG-3′) oligonucleotides. Primers for the olfactory neuron specific transcription factor Olf-1/Early B-cell factor (O/E-1) were forward (5′-TGTCCACAATAACTCCAAGCACGG-3′) and reverse (5′-CAGAACTGCTTGGACTTGTACGAC-3′).
The initial PCR experiments used the PACAP primer pairs (100 μM) with a 35-cycle profile performed as follows: 95°C denaturation (5 min), 94°C denaturation (30 s), 55°C annealing (1 min), 72°C extension (3 min), and 4°C hold (Perkin-Elmer PCR thermal cycler). PCR products were separated and visualized using ethidium bromide–stained agarose gels (1%) and viewed under ultraviolet (UV) transillumination. To verify PCR products, resulting cDNA was excised from the gel and purified using the GlassMAX DNA Isolation Matrix System (Gibco BRL). Purified cDNA was ligated into the pCRII plasmid, transformed into one-shot competent cells (Invitrogen TA cloning), and then sequenced at the CMMT Sequencing Facility, University of British Columbia.
Intracellular calcium measurements
We measured intracellular calcium ([Ca2+]i) using either ratiometric digital imaging of fura-2 loaded cells or nonratiometric confocal imaging of fluo-4 loaded cells. Cells plated on concavalin A-coated coverslips or slices placed on 4% agarose-coated coverslips were placed in a laminar flow chamber (Warner Instruments) and perfused continuously with dye-free Ringer solution at a flow rate of 1.5–2.0 ml/min for 15 min prior to starting the experiment. Test solutions were applied using a small volume loop injector (200 μl) or bath exchange (0 calcium experiments). For desensitization experiments, we used a large volume loop injector (1–2 ml). There was a 15- to 20-s delay for test solutions to reach the slice following loop injection. We used our standard fura-2– based digital imaging technique as previously published (Kalyani et al. 1998; Mujtaba et al. 1999; Piper and Lucero 1999). For confocal calcium imaging, cells or slices were loaded with 45 μM fluo-4 AM (Molecular Probes, Eugene, OR) for 45 min at 25°C. A stock solution of 450 μM fluo-4 was prepared in 20% pluronic F-127 (in DMSO) and used at a final concentration of 45 μM fluo-4 AM, 0.02– 0.04% pluronic F-127 and 0.02– 0.04% DMSO. Tetrodotoxin (TTX; 400 nM), which blocks voltage-gated sodium currents, was added to the perfusion solution to avoid potential contribution of Ca2+ generated from spontaneous activity.
A Zeiss LSM 510 confocal laser scanning system attached to a Zeiss upright Axioskop 2 FS microscope was used, and data analysis was performed using Zeiss software. The laser intensity and scale were adjusted so that basal fluorescence intensity in arbitrary units was 35–50 on a scale (maximum = 255). Fluorescent emission was long-pass filtered at 510 nm. All scan head settings were kept the same for a series of experiments, with the exception of the gain, which was adjusted, if needed, to maintain a baseline fluorescence intensity of about 50 units. Data from time series experiments were collected as 1024 × 1024 pixel images at 0.5 Hz.
RESULTS
PACAP expression in the neonatal olfactory system differs from adult
We used immunohistochemistry and laser scanning confocal microscopy to examine the expression of PACAP in neonatal mice as well as in adult mice and rat olfactory systems. In neonatal mice, the distribution of PACAP immunoreactivity (IR) did not show obvious changes over the time frame investigated (P0–P5; n = 2–3 animals/time point). PACAP IR was present in the somas and processes of OECs located in the olfactory submucosa (Fig. 1, C and D), as indicated by the glial protein marker S100 that is present in OECs (Fig. 1, A and B). PACAP IR was also present at lower levels in the sustentacular cell layer of the olfactory epithelium (compare Fig. 1, B and D). In neonatal mice, PACAP IR was notably absent from the small population of mature ORNs, identified by an antibody to olfactory marker protein (OMP), a protein expressed in mature ORNs (Fig. 1, C and D). This staining pattern is different from that described for E13 rats, which showed PACAP expression throughout the developing OE (Hansel et al. 2001b). PACAP IR was also located in the germinative bone or cartilage cells of the septum and cribriform plate, according to PACAP IR described in developing cartilage and bone (Strange-Vognsen et al. 1997). Whereas we expected to see staining in the olfactory mucosa, we were surprised by its localization to OECs. This observation is very exciting because little is known about the unique mechanisms used by OECs to support and promote regeneration either within the olfactory epithelium or when transplanted (Ramón-Cueto et al. 1998).
FIG. 1.

Pituitary adenylate cyclase activating peptide (PACAP) immunoreactivity (IR) is present in olfactory ensheathing cells (OEC) in neonatal mouse olfactory epithelium (OE). A: OE from a representative neonatal mouse (P4) labeled with anti-OMP (1:10K) and anti-S-100 (1:100). OMP and S-100 were visualized with tetramethyl rhodamine isothiocyanate (TRITC)-(red) and FITC-(green) conjugated anti-immunoglobin secondary antibodies, respectively. B: magnified view of image outlined by white box in A shows mature olfactory receptor neurons (ORNs) labeled in red and OECs labeled in green. C: OE from the same mouse as in A labeled with anti-OMP (1:10,000, red) and anti-PACAP38 (1:500, green). D: magnified view of image outlined by white box in C. Note the S-100 and PACAP38-IR (B and D, respectively) in OEC processes in the lamina propria (LP) and faint PACAP38-IR in the sustentacular cell layer (SC) in D. NB, nerve bundle; C, cribriform plate; BCL, basal cell layer. All scale bars are 50 μm.
In adult OE, the PACAP staining pattern was strikingly different from that in neonatal mice (Fig. 2). In both mice (Fig. 2A) and rats (Fig. 2B), PACAP IR was found in the OMP-positive ORNs and the OMP-negative basal cells and could be blocked by preabsorption of the PACAP antibody with PACAP38 peptide (Fig. 2, G and H). In addition, PACAP IR was observed in the sustentacular cell layer in adult mice but not in adult rats (Fig. 2, A and B). A lack of PACAP expression in adult rat sustentacular cells has been previously reported (Hansel et al. 2001b). PACAP IR was also observed in the olfactory nerve and somas of cells in glomerular, mitral cell, and granule cell layers of adult mouse and rat olfactory bulbs, with faint or no staining in processes (Fig. 2, C and D), in a pattern similar to that described for the distribution of PAC-1 receptors in neonatal rats (Basille et al. 2000). Moreover, co-localization studies with PACAP and S100 demonstrated the presence of PACAP in OEC somas in adult mouse nerve bundles (Fig. 2E). In adult rat nerve bundles, we double-labeled for PACAP and OMP to show that the PACAP IR is present in the OEC soma and absent from the ORN nerve fibers (Fig. 2F). PACAP staining in the ORN and OEC somas was similar to the “Golgi-like distribution” of PACAP IR described in rat nodose ganglia (Reimer et al. 1999). The distribution of PACAP IR in neonatal mice and adult mice and rats demonstrates that PACAP is ideally situated to affect the development, maturation, and survival of olfactory neurons and glia.
FIG. 2.
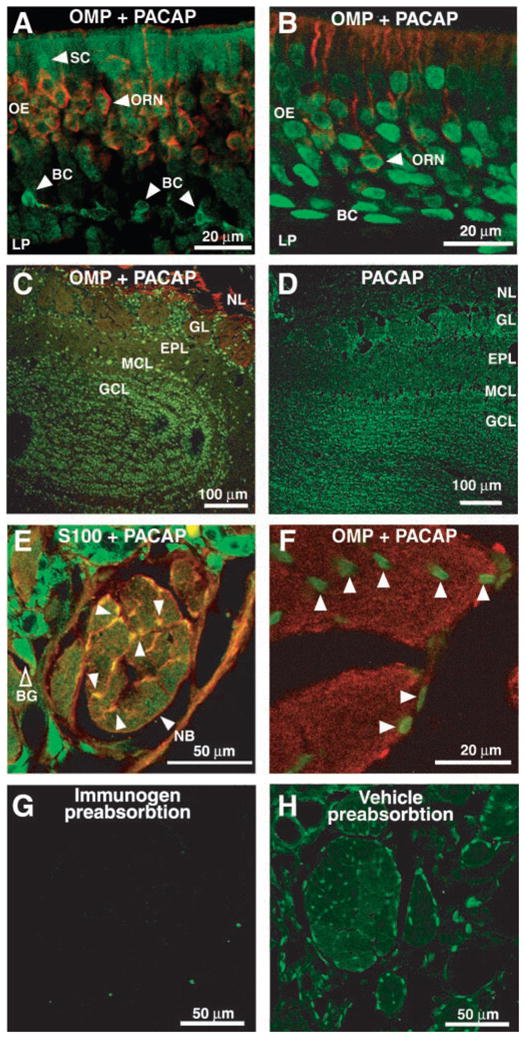
Identification of PACAP38 IR in adult OE and olfactory bulb (OB). Tissue was double labeled with either goat anti-OMP (1:10K) and rabbit anti-PACAP38 (1:500; A–C and F), rabbit anti-PACAP38 (1:500) alone (D and H), or rabbit anti-S100 (1:100) and guinea pig anti-PACAP (1:500) (E). OMP was visualized with TRITC-(red) goat anti-immunoglobin (IgG). PACAP was visualized with FITC-(green) donkey anti-rabbit (A–D and F–H) or Cy2-(green) anti-guinea pig (E) IgG. S100 was visualized with TRITC-(red) donkey anti-rabbit IgG. A: adult mouse OE showing PACAP38-IR in SCs, OMP+ ORNs, and in OMP− basal cells (BC). B: adult rat OE showing PACAP38 IR in OMP+ ORNs and in OMP− cells in BCs. C and D: views of mouse and rat olfactory bulbs, respectively, shows PACAP38-IR in mitral cell (MCL), granule cell (GCL), and glomerular layers (GLs). EPL, external plexiform layer, NL, nerve layer. E: olfactory nerve bundle (NB) from adult mouse showing PACAP38- and S100-IR in the same OECs identified by arrowheads. Preabsorption experiments similar to those shown in G revealed that the staining in Bowman’s gland cells (BG) was nonspecific. F: PACAP38-IR in rat nerve bundle is localized to OECs (arrowheads) and is absent from OMP-IR nerve fibers. G: preabsorption of anti-PACAP38 with PACAP38 peptide (immunogen preabsorption) completely eliminates staining in this section. H: adjacent section to that shown in G labeled with anti-PACAP38 preabsorbed with vehicle (1% acetic acid) shows PACAP38-IR in OECs of adult mouse nerve bundle.
PACAP is expressed in purified cultures of OECs and in adult OE
Because it is unusual to find PACAP IR in a glial cell (Figiel and Engele 2000), we used RT-PCR techniques to identify PACAP mRNA in cultured mouse OECs. RT-PCR was performed on homogenates of purified OEC cultures and adult OE (see METHODS). Figure 3A shows absence of bands from negative controls (−), which included RNAse-treated OECs before RT. Appropriate-sized bands for PACAP and β-actin were observed in lanes containing OEC and OE. Although not quantitative, the brighter signal in the OE may reflect PACAP production by both ORNs and OECs. The positive control utilized PACAP cDNA (+) (gift from Dr. N. Sherwood, University of Victoria) and showed that the PACAP primer pairs were amplifying a product of the same size. RT-PCR reactions using OE and OEC were also run using primers for the olfactory specific transcription factor Olf-1/Early B-cell factor (O/E-1; gift from R. Reed, Johns Hopkins University). The 300-kb band for O/E-1 was present in OE but absent in OEC, indicating that the purified OEC cultures do not have any ORN contamination and that the PACAP is indeed being produced by OECs. PACAP IR was also detected in cultured OECs using immunocytochemistry (Fig. 3B). We observed co-localization of S-100β and PACAP in the same cell. Note that all OECs express S-100β in vitro (Franceschini and Barnett 1996). Furthermore, our OEC cultures prepared from the lamina propria of neonatal mouse express the distinctive OEC combination of S-100β with glial fibrillary acidic protein (GFAP) and low affinity nerve growth factor receptor (lNGFR or p75) (Au and Roskams 2003; Pixley 1992; Gong et al. 1994).
FIG. 3.
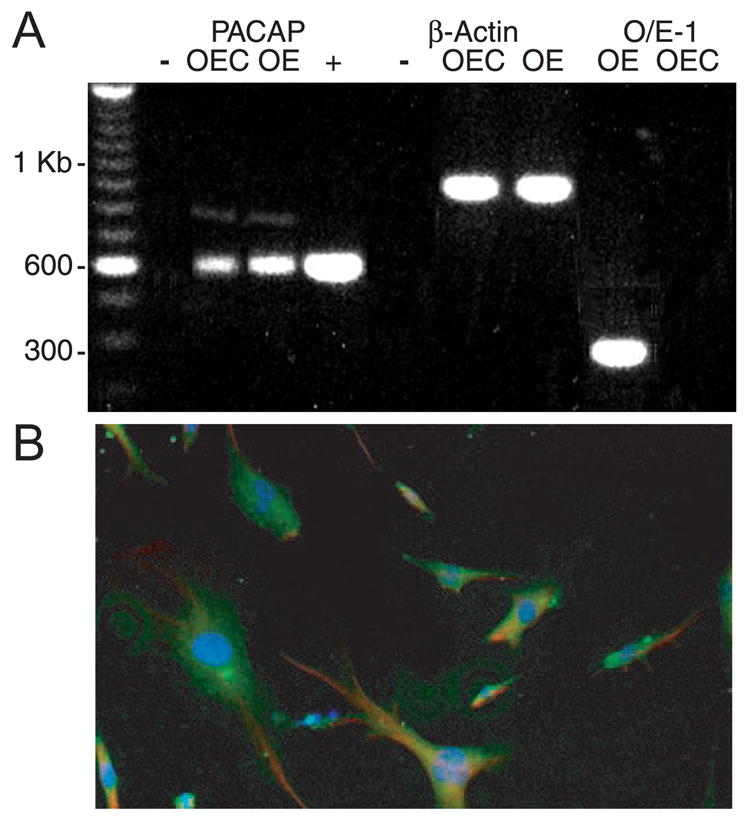
Cultured olfactory ensheathing cells express PACAP. A: reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of PACAP mRNA expression in OEC cultures and adult OEs. The 600-bp product represents the presence of PACAP in both OECs and OEs. Control β-actin and olfactory specific transcription factor Olf-1 (O/E-1) RT-PCR reactions are shown. + indicates PACAP cDNA positive control; − indicates treatment with RNAse. B: olfactory ensheathing glia isolated from postnatal day 5 mice were cultured for 12 days and immunostained with mouse anti-S100β (1:500) and rabbit anti-PACAP38 (1:250). PACAP was visualized with Alexa 488 goat anti-rabbit IgG (green), and S100β was visualized with Alexa 594 goat anti-mouse IgG (red). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, 1:10,000; blue). Scale bar, 50 μm. Note that all OECs in vitro express S-100β (Franceschini and Barnett 1996).
PACAP elicits increases in [Ca2+]i in isolated adult and in situ neonatal ORNs
Our initial studies of PACAP were performed using cultured adult rat ORNs loaded with the Ca2+ indicator dye fura-2 AM. Responses to odor (25 μM n-amyl acetate) and elevated K+ Ringer were used to identify cells as ORNs. Criteria for positive responses were that the latency was at least as long as the elevated K+ response and amplitude greater that 10% of the baseline noise. In these experiments, we grouped the data obtained from 27 coverslips containing cells isolated from 11 different rats. PACAP elicited relatively rapid and transient increases in intracellular Ca2+ (Fig. 4A). Of 86 cells that responded to elevated K+ with increases in [Ca2+]i, 14% responded to PACAP and 8% responded to odor. Thus a one-to-one correspondence between cells that responded to PACAP and the odor n-amyl acetate was not evident. Although a few cells in primary cultures of rat ORNs were capable of responding to repeated application of PACAP (Fig. 4B), the responses did tend to run down following more than two PACAP applications, suggesting some level of desensitization. To further investigate desensitization, we applied 100 nM PACAP continuously for 120 s. The transient nature of the PACAP response was not solely due to short agonist applications; PACAP responses desensitized while still in the presence of PACAP (Fig. 4C). Because PACAP can activate both the cAMP and IP3 pathways, we tested whether the increase in [Ca2+]i was due to Ca2+ influx or release from intracellular stores. We examined the responses to PACAP in Ca2+-free solutions in 11 coverslips of ORNs isolated from seven rats. We found that 5/12 ORNs that responded to PACAP in normal Ringer also responded in the absence of extracellular Ca2+ (Fig. 4D; 3 different cells). Due to the desensitization described above, we were unable to obtain a recovery response to PACAP on return to normal Ca2+. Thus we cannot make conclusions regarding the cells that failed to respond in Ca2+-free Ringer. Either they required extracellular Ca2+, their stores were depleted, or they had desensitized. For the cells that did respond in zero Ca2+, we can conclude that Ca2+ was released from intracellular stores. Our cultured cells did not have caffeine-sensitive stores (data not shown), suggesting that the intracellular Ca2+ mobilization was due to IP3-sensitive stores.
FIG. 4.
PACAP-elicited responses in fura-2 AM loaded cultured rat ORNs. A: ratiometric fluorescence increases from an ORN in response to 100 nM PACAP, 25 μM n-amyl acetate + 50 μM R-carvone (odor), and high K+ Ringer solution. Note the transient increase in Ca2+ elicited by PACAP. Breaks in the traces correspond to 15 min when images were not collected. B: representative trace from a rat ORN that responded to multiple applications of PACAP (100 nM). C: PACAP-elicited intracellular Ca2+ transient returns to basal levels in the maintained presence of PACAP. Data are from 2 different ORNs. D: fluorescence increases from 3 rat ORNs that responded to 100 nM PACAP in 0 Ca2+ + EGTA.
We also utilized primary cultures of isolated adult mouse ORNs to test for functional PAC-1 receptors in adult mice. Fura imaging experiments were performed on six coverslips containing primary OE cultures from three mice. As with cultured adult rat ORNs, mouse neurons were sensitive to 100 nM PACAP, elevated K+, the odor mixture of 25 μM n-amyl acetate and 50 μM R-carvone, and 10 μM ATP (Fig. 5A).
FIG. 5.
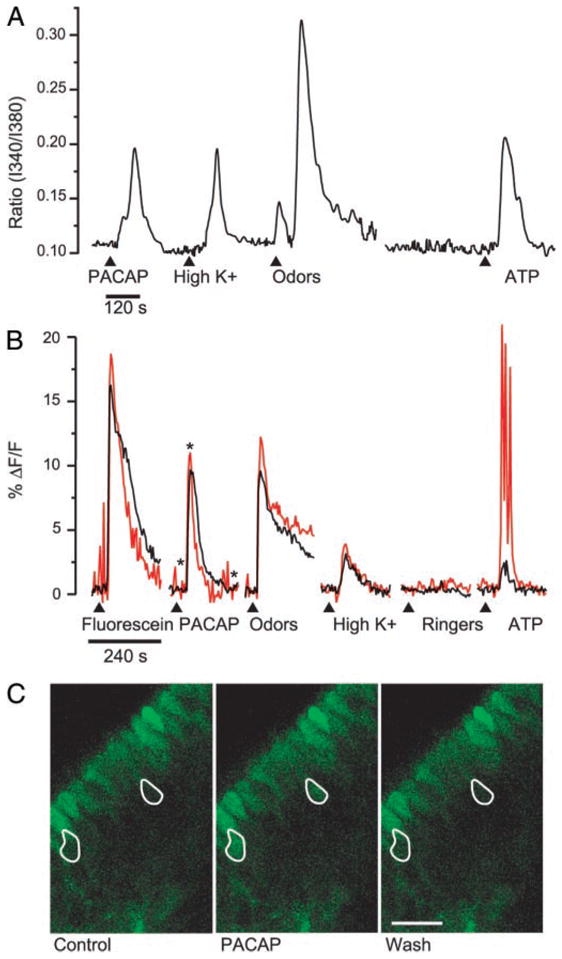
PACAP-elicited calcium responses in adult cultures and neonatal slices of mouse olfactory epithelium. A: ratiometric fura imaging experiments were performed on adult mouse ORN cultures using 6 coverslips obtained from 3 different mice. A representative trace shows increases in intracellular Ca2+ in response to 100 nM PACAP, high K+ Ringer, 25 μM n-amyl acetate + 50 μM R-carvone (odors), and 10 μM ATP. B: 2 traces obtained from a slice of P4 mouse OE loaded with fluo-4 AM are shown. Test solutions (1:1,000 fluorescein; 100 nM PACAP; 25 μM n-amyl acetate + 50 μM R-carvone (odors); high K+ Ringer; Ringer control; 10 μM ATP) were loop injected into bath flow (15- to 20-s delay) at the arrowheads. There was a 4-min wash between test substance application (data not shown). The data are expressed as a percent change in fluorescence over the baseline (%ΔF/F). Traces were obtained from the ORNs outlined in C. C: pseudocolor confocal images taken at the times indicated by the asterisks on the PACAP trace in B. Control (left) was before PACAP application, PACAP (middle) was during the peak of the response, and Wash (right) was following superfusion of PACAP onto the slice. Fluorescent cells along the surface are sustentacular cells that have loaded with fluo-4. Scale bar, 50 μm.
Our immunocytochemical studies indicated that PACAP was present in the OE of adult animals and in the lamina propria and sustentacular cell layer of neonatal mice. To determine if functional PAC-1 receptors were present in ORNs of neonatal mice, we tested the effects of PACAP using a neonatal mouse OE slice preparation. Confocal data from a slice of P4 mouse OE that was loaded for 60 min with the membrane permeant Ca2+ indicator dye fluo-4 AM are shown in Fig. 5, B and C. All solutions contained 400 nM TTX to eliminate spontaneous activity, and test solutions were applied using the small volume (200 μl) loop injector. The ratio of the percentange change in fluorescence intensity is plotted over time, and the control application of a 1:1,000 dilution of fluorescein shows the 240-s time course of test solution application as it passed over two ORNs. In this particular region of the slice, two ORNs responded to the following test solutions: 100 nM PACAP, an odor mixture (25 μM n-amyl acetate and 50 μM R-carvone), elevated K+ Ringer, and 10 μM ATP; there was no response to the Ringer control (Fig. 5B). The tracings in Fig. 5B were obtained from the ORNs outlined in white in Fig. 5C, which shows images taken before (Control), during the peak response (PACAP), and after recovery (Wash) from application of 100 nM PACAP. We observed similar effects of PACAP on cells identified as ORNs by their responses to odors and elevated K+ in 17 cells from nine slices obtained from six neonatal mice. As with the primary cultures, every cell that responded to either odors or PACAP responded to elevated K+, and most cells that responded to either odors or PACAP responded to both. Thus our calcium imaging data demonstrate that neonatal and adult mouse and adult rat ORNs respond to PACAP with increases in intracellular calcium. These observations are consistent with the idea that functional PAC-1 receptors are expressed in ORNs.
DISCUSSION
A distinctive attribute of olfactory receptor neurons in the mature neuroepithelium is that they are continuously renewed from a population of basal (progenitor) cells. Hence, at any given time, subpopulations of cells in the olfactory epithelium are turning over (Graziadei and Monti-Graziadei 1978). Numerous studies have investigated the roles of growth factors and signaling pathways that determine whether a neuron will be born, extend processes, express a particular odorant receptor, or die (Buckland and Cunningham 1999; Mahanthappa and Schwarting 1993). The olfactory bulb and mucosa are rich sources of these regulatory factors, and the process is not only complex but also under exquisite control.
We became interested in olfactory neuron differentiation during an investigation of dopaminergic modulation of ORNs (Vargas and Lucero 1999b). Previous studies showed that dopamine plays an important role in ORN differentiation and apoptosis (Coronas et al. 1997; Féron et al. 1999; Mehlen et al. 1999; Murrell and Hunter 1999). Because dopamine alone could not cause olfactory cell lines to differentiate fully (Alatorre and Lucero 2000), we sought to identify other factors that had not been previously tested in the peripheral olfactory system and found that PACAP induced functional maturation of cells from an olfactory cell line (Illing et al. 2002). Recently, others have shown that PACAP and its receptor PAC-1 are present in the olfactory system and that PACAP can have both neurotrophic and neuroprotective effects on ORNs (Au and Roskams 2003; Hansel et al. 2001b).
This study shows that one source of PACAP in olfactory mucosa differs between the mouse and rat. The main difference between species is the expression of PACAP in the sustentacular cells of the adult mouse but not in the adult rat. The broad apical portion of sustentacular cells is situated in the upper portion of the OE, and a narrow cytoplasmic process extends to the base of the epithelium. Thus in the adult mouse, PACAP in sustentacular cells provides an additional source of PACAP, whereas in the rat, PACAP IR is only found in ORNs, OECs, and basal cells.
We also observed notable differences in PACAP distribution between neonatal mice and adult mice and rats. The neonates expressed PACAP mainly in the OECs, whereas in the adults, PACAP was present in ORNs and basal cells as well as OECs. In a previous study, PACAP IR was observed throughout the OE and underlying lamina propria of the E13 rat (Hansel et al. 2001b). PACAP was not specifically localized to the OECs, although this may be because connections between axons and olfactory ensheathing glia are just forming around E15. It is known that OECs express many growth factors and adhesion molecules that could influence olfactory neuron axon outgrowth and targeting to the olfactory bulb (for review, see Chuah and West 2002). Thus we propose that PACAP may facilitate activity-dependent mechanisms in developing ORNs in neonates and in regenerating ORNs in adults. In contrast, PACAP expressed in basal cells could mediate cell proliferation and induction of differentiation in the normal turnover or during regeneration of adult OE.
PACAP has previously been identified in GFAP+ glial cells in neonatal rat primary cultures (Hansel et al. 2001b). In this study, we conclusively show that olfactory ensheathing cells in neonatal and adult rat and mice express PACAP both in vivo and in vitro. To our knowledge, ours is the first report of PACAP in adult glial cells, albeit a highly plastic glial cell population. PACAP has been described in neonatal OEC (Au and Roskams 2003) but was absent from cortical glia (Figiel and Engele 2000). Given the unique capabilities of OEC for promoting axonal regeneration, our finding suggests that PACAP may be an important peptide to consider when teasing out the regeneration-promoting mechanisms of OEC.
PACAP receptors are G-protein– coupled 7-transmembrane-spanning receptors that fall into two groups: type I receptors (PAC-1) that have a high affinity for PACAP (Kd approximately 0.5 nM) and a much lower affinity for VIP (Kd > 500 nM) (Pisegna 1993) and type II receptors (VPAC-1 and VPAC-2) of which VPAC-1 has equal affinity for PACAP and VIP (Kd approximately 1 nM), while VPAC-2 has a higher affinity for helodermin > PACAP ~ VIP (Lutz et al. 1993). Within the PAC-1 receptor subtype, ≥5 splice variants have been identified that not only show a slightly different affinity for PACAP38 compared with PACAP27 but also couple differently to cAMP and IP3 second messenger systems (Spengler et al. 1993). Both the PAC-1 short and very short (containing 1 and 0 cassettes, respectively) splice variants have been identified in rat OE (Hansel et al. 2001b). Although the cassette type (HIP or HOP) was not reported, binding of PACAP38 to either the very short or the HOP splice variants can activate both the cAMP and the IP3 pathways, while activation of the HIP variant only stimulates the adenylyl cyclase pathway (Spengler et al. 1993). Our data provide the first recordings of physiological responses to PACAP in the mammalian olfactory system. Using two different calcium imaging techniques, we found that PACAP activates receptors present on ORNs from adult rat and neonatal and adult mice. While it is possible that PACAP is acting on odorant receptors or on closely related VIP receptors, this seems unlikely because the concentration of PACAP used (100 nM) is much lower than concentrations used for odor responses and because PAC-1 receptors are expressed in ORNs (Hansel et al. 2001b).
In a subset of cells, we observed PACAP-induced calcium responses in the absence of extracellular calcium, suggesting release from intracellular stores. These data are consistent with the idea that PACAP is activating PAC-1 receptors on ORNs. The observation that a subset of cells responded to PACAP with mobilization of intracellular stores suggests that, at least in these cells, the PLC/IP3 pathway is being activated. Because of desensitization, we were unable to conclusively determine whether extracellular Ca2+ entry also occurred. However, the potential exists that, as in olfactory bulb neurons (Spengler et al. 1993), different splice variants of the PAC-1 receptor may be expressed in different ORNs. This differential control of receptors and transduction pathways may be important for determining whether PACAP has a neuroprotective or neurotrophic effect on an ORN at a given stage of its development. Given that PACAP has multiple effects in other systems, it is possible that PACAP also has multiple as yet unidentified functions in OE. In addition, not every ORN responded to PACAP. Both in primary cultures and acutely prepared slices, only a subpopulation of ORNs showed Ca2+ increases in the presence of PACAP. We cannot determine whether lack of Ca2+ responses reflects absence of receptor, lack of transduction machinery, state of Ca2+ stores, differences in developmental stages of the cells, or Ca2+-independant PACAP responses. However, we can conclude that, physiologically, ORNs are not a homogenous population.
Collectively, we show that PACAP and PACAP responsive neurons are present in neonatal mouse and adult rat and mouse OE. Interestingly, PACAP IR is present in sustentacular cells of mice but absent from these cells in rats. In adults from both species, PACAP is present in OECs (Fig. 2) and absent in other types of glia (Figiel and Engele 2000). We hypothesize that PACAP could be an important component of neuronal-glial intercommunication and the regulatory mechanisms that underlie the unique regenerative properties of the olfactory system.
Acknowledgments
The authors thank Drs. Nancy Sherwood for the gift of PACAP cDNA, Frank Margolis for the gift of anti-OMP antibody, and Randy Reed for the gift of primers for the olfactory specific transcription factor O/E-1. We thank T. Leinders-Zufall for helpful suggestions in using fluo-4 and Dr. Larry Stensaas for helpful suggestions on the manuscript.
Footnotes
DISCLOSURES
This research is supported by National Institute of Deafness and Other Communication Disorders Grants DC002994 to M. T. Lucero, DC-04953 to C. C. Hegg, and DC-04579 and International Spinal Research Trust Grant STR 052 to A. J. Roskams.
References
- Alatorre R, Lucero MT. Odora cells are not electrically excitable. Chem Senses. 2000;25:630. [Google Scholar]
- Arimura A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol. 1998;48:301–331. doi: 10.2170/jjphysiol.48.301. [DOI] [PubMed] [Google Scholar]
- Au E, Roskams AJ. Olfactory ensheathing cells of the lamina propria in vivo and in vitro. Glia. 2003;41:224–236. doi: 10.1002/glia.10160. [DOI] [PubMed] [Google Scholar]
- Basille M, Vaudry D, Coulouarn Y, Jegou S, Lihrmann I, Fournier A, Vaudry H, Gonzalez B. Comparative distribution of pituitary adenylate cyclase-activating polypeptide (PACAP) binding sites and PACAP receptor mRNAs in the rat brain during development. J Comp Neurol. 2000;425:495–509. [PubMed] [Google Scholar]
- Beaudet MM, Parsons RL, Braas KM, May V. Mechanisms mediating pituitary adenylate cyclase-activating polypeptide depolarization of rat sympathetic neurons. J Neurosci. 2000;20:7353–7361. doi: 10.1523/JNEUROSCI.20-19-07353.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckland ME, Cunningham AM. Alterations in expression of the neurotrophic factors glial cell line-derived neurotrophic factor, ciliary neurotrophic factor and brain-derived neurotrophic factor, in the target-deprived olfactory neuroepithelium. Neuroscience. 1999;90:333–347. doi: 10.1016/s0306-4522(98)00270-x. [DOI] [PubMed] [Google Scholar]
- Canonico PL. Activation of pituitary adenylate cyclase-activating polypeptide receptors prevents apoptotic cell death in cultured cerebellar granule cells. Ann NY Acad Sci. 1996;805:470–472. doi: 10.1111/j.1749-6632.1996.tb17505.x. [DOI] [PubMed] [Google Scholar]
- Chuah MI, West AK. Cellular and molecular biology of ensheathing cells. Microsc Res Tech. 2002;58:216–227. doi: 10.1002/jemt.10151. [DOI] [PubMed] [Google Scholar]
- Coronas V, Feron F, Hen R, Sicard G, Jourdan F, Moyse E. In vitro induction of apoptosis or differentiation by dopamine in an immortalized olfactory neuronal cell line. J Neurochem. 1997;69:1870–1881. doi: 10.1046/j.1471-4159.1997.69051870.x. [DOI] [PubMed] [Google Scholar]
- Féron F, Vincent A, Mackay-Sim A. Dopamine promotes differentiation of olfactory neuron in vitro. Brain Res. 1999;845:252–259. doi: 10.1016/s0006-8993(99)01959-9. [DOI] [PubMed] [Google Scholar]
- Figiel M, Engele J. Pituitary adenylate cyclase-activating polypeptide (PACAP), a neuron-derived peptide regulating glial glutamate transport and metabolism. J Neurosci. 2000;20:3596–3605. doi: 10.1523/JNEUROSCI.20-10-03596.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini IA, Barnett SC. Low-affinity NGF-Receptor and E-N-CAM expression define two types of olfactory nerve ensheathing cells that share a common lineage. Dev Biol. 1996;173:327–343. doi: 10.1006/dbio.1996.0027. [DOI] [PubMed] [Google Scholar]
- Gong Q, Bailey MS, Pixley SK, Ennis M, Liu W, Shipley MT. Localization and regulation of low affinity nerve growth factor receptor expression in the rat olfactory system during development and regeneration. J Comp Neurol. 1994;344:336–348. doi: 10.1002/cne.903440303. [DOI] [PubMed] [Google Scholar]
- Gonzalez BJ, Basille M, Vaudry D, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide promotes cell survival and neurite outgrowth in rat cerebellar neuroblasts. Neuroscience. 1997;78:419–430. doi: 10.1016/s0306-4522(96)00617-3. [DOI] [PubMed] [Google Scholar]
- Graziadei PPC, Monti-Graziadei GA. Continuous nerve cell renewal in the olfactory system. In: Jacobson M, editor. Handbook of Sensory Physiology. Vol. 9. New York: Springer; 1978. pp. 55–83. [Google Scholar]
- Hansel DE, Eipper BA, Ronnett GV. Regulation of olfactory neurogenesis by amidated neuropeptides. J Neurosci Res. 2001a;66:1–7. doi: 10.1002/jnr.1191. [DOI] [PubMed] [Google Scholar]
- Hansel DE, May V, Eipper BA, Ronnett GV. Pituitary adenylyl cyclase-activating peptides and alpha-amidation in olfactory neurogenesis and neuronal survival in vitro. J Neurosci. 2001b;21:4625–4636. doi: 10.1523/JNEUROSCI.21-13-04625.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegg CC, Lucero MT. ATP evokes Ca2+ increases and inward currents in mouse olfactory receptor neurons. Chem Senses. 2001;26:1073. [Google Scholar]
- Illing N, Boolay S, Siwoski JS, Casper D, Lucero MT, Roskams AJ. Conditionally immortalized clonal cell lines from the mouse olfactory placode differentiate into olfactory receptor neurons. Mol Cell Neurosci. 2002;20:225–243. doi: 10.1006/mcne.2002.1106. [DOI] [PubMed] [Google Scholar]
- Jaworski DM, Proctor MD. Developmental regulation of pituitary adenylate cyclase-activating polypeptide and PAC(1) receptor mRNA expression in the rat central nervous system. Brain Res Dev Brain Res. 2000;120:27–39. doi: 10.1016/s0165-3806(99)00192-3. [DOI] [PubMed] [Google Scholar]
- Kafitz KW, Greer CA. Olfactory ensheathing cells promote neurite extension from embryonic olfactory receptor cells in vitro. Glia. 1999;25:99–110. [PubMed] [Google Scholar]
- Kalyani AJ, Piper D, Mujtaba T, Lucero MT, Rao MS. Spinal cord neuronal precursors generate multiple neuronal phenotypes in culture. J Neurosci. 1998;18:7856–7868. doi: 10.1523/JNEUROSCI.18-19-07856.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp MD, Schomerus C, Dehghani F, Korf HW, Meissl H. Pituitary adenylate cyclase-activating polypeptide and melatonin in the suprachiasmatic nucleus: effects on the calcium signal transduction cascade. J Neurosci. 1999;19:206–219. doi: 10.1523/JNEUROSCI.19-01-00206.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelievre V, Hu Z, Byun JY, Ioffe Y, Waschek JA. Fibroblast growth factor-2 converts PACAP growth action on embryonic hindbrain precursors from stimulation to inhibition. J Neurosci Res. 2002;67:566–573. doi: 10.1002/jnr.10153. [DOI] [PubMed] [Google Scholar]
- Li Y, Field PM, Raisman G. Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science. 1997;277:2000–2002. doi: 10.1126/science.277.5334.2000. [DOI] [PubMed] [Google Scholar]
- Lindholm D, Skoglosa Y, Takei N. Developmental regulation of pituitary adenylate cyclase activating polypeptide (PACAP) and its receptor 1 in rat brain: function of PACAP as a neurotrophic factor. Ann NY Acad Sci. 1998;865:189–196. doi: 10.1111/j.1749-6632.1998.tb11178.x. [DOI] [PubMed] [Google Scholar]
- Lutz EM, Sheward WJ, West KM, Morrow JA, Fink G, Harmar AJ. The VIP2 receptor: molecular characterisation of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS Lett. 1993;334:3–8. doi: 10.1016/0014-5793(93)81668-p. [DOI] [PubMed] [Google Scholar]
- Mahanthappa NK, Schwarting GA. Peptide growth factor control of olfactory neurogenesis and neuron survival in vitro: Roles of EGF and TGF-βs. Neuron. 1993;10:293–305. doi: 10.1016/0896-6273(93)90319-m. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Coronas V, Ljubic-Thibal V, Ducasse C, Granger L, Jourdan F, Arrigo AP. Small stress protein Hsp27 accumulation during dopamine-mediated differentiation of rat olfactory neurons counteracts apoptosis. Cell Death Differ. 1999;6:227–233. doi: 10.1038/sj.cdd.4400483. [DOI] [PubMed] [Google Scholar]
- Mujtaba T, Piper DR, Kalyani A, Groves AK, Lucero MT, Rao MS. Lineage-restricted neural precursors can be isolated from both the mouse neural tube and cultured ES cells. Dev Biol. 1999;214:113–127. doi: 10.1006/dbio.1999.9418. [DOI] [PubMed] [Google Scholar]
- Murrell JR, Hunter DD. An olfactory sensory neuron line, Odora, properly targets olfactory proteins and responds to odorants. J Neurosci. 1999;19:8260–8270. doi: 10.1523/JNEUROSCI.19-19-08260.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper DR, Lucero MT. Calcium signalling in squid olfactory receptor neurons. Biol Signals. 1999;8:329–337. doi: 10.1159/000014606. [DOI] [PubMed] [Google Scholar]
- Pisegna JR. Molecular cloning and functional expression of the pituitary adenylate cyclase-activating polypeptide type I receptor. Proc Natl Acad Sci USA. 1993;90:6345–6349. doi: 10.1073/pnas.90.13.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pixley SK. The olfactory nerve contains two populations of glia, identified both in vivo and in vitro. Glia. 1992;5:269–284. doi: 10.1002/glia.440050405. [DOI] [PubMed] [Google Scholar]
- Ramón-Cueto A, Avila J. Olfactory ensheathing glia: Properties and function. Brain Res Bull. 1998;46:175–187. doi: 10.1016/s0361-9230(97)00463-2. [DOI] [PubMed] [Google Scholar]
- Ramón-Cueto A, Plant GW, Avila J, Bunge MB. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J Neurosci. 1998;18:3803–3815. doi: 10.1523/JNEUROSCI.18-10-03803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer M, Moller K, Sundler F, Hannibal J, Fahrenkrug J, Kanje M. Increased expression, axonal transport and release of pituitary adenylate cyclase-activating polypeptide in the cultured rat vagus nerve. Neuroscience. 1999;88:213–222. doi: 10.1016/s0306-4522(98)00240-1. [DOI] [PubMed] [Google Scholar]
- Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- Strange-Vognsen HH, Arnbjerg J, Hannibal J. Immunocytochemical demonstration of pituitary adenylate cyclase activating polypeptide (PACAP) in the porcine epiphyseal cartilage canals. Neuropeptides. 1997;31:137–141. doi: 10.1016/s0143-4179(97)90082-2. [DOI] [PubMed] [Google Scholar]
- Ubink R, Hökfelt T. Expression of neuropeptide Y in olfactory ensheathing cells during prenatal development. J Comp Neurol. 2000;423:13–25. doi: 10.1002/1096-9861(20000717)423:1<13::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Vargas G, Lucero MT. A method for maintaining odor-responsive adult rat olfactory receptor neurons in short-term culture. Chem Senses. 1999a;24:211–216. doi: 10.1093/chemse/24.2.211. [DOI] [PubMed] [Google Scholar]
- Vargas G, Lucero MT. Dopamine modulates inwardly rectifying hyper-polarization-activated current (Ih) in cultured rat olfactory receptor neurons. J Neurophysiol. 1999b;81:149–158. doi: 10.1152/jn.1999.81.1.149. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Basille M, Anouar Y, Fournier A, Vaudry H, Gonzalez BJ. The neurotrophic activity of PACAP on rat cerebellar granule cells is associated with activation of the protein kinase A pathway and c-fos gene expression. Ann NY Acad Sci. 1998;865:92–99. doi: 10.1111/j.1749-6632.1998.tb11167.x. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Fournier A, Vaudry H. Neurotrophic activity of pituitary adenylate cyclase-activating polypeptide on rat cerebellar cortex during development. Proc Natl Acad Sci USA. 1999;96:9415–9420. doi: 10.1073/pnas.96.16.9415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- Waschek JA, Casillas RA, Nguyen TB, DiCicco-Bloom EM, Carpenter EM, Rodriguez WI. Neural tube expression of pituitary adenylate cyclase-activationg peptide (PACAP) and receptor: potential role in patterning and neurogenesis. Proc Natl Acad Sci USA. 1998;95:9602–9607. doi: 10.1073/pnas.95.16.9602. [DOI] [PMC free article] [PubMed] [Google Scholar]



