The cortical hypoperfusion following unclamping has been considered to play a critical role in the course of postischemic acute tubular necrosis of preserved kidneys.1,2 In this study, the mechanism of protection of University of Wisconsin (UW) solution was investigated in canine renal autografts with special attention to renal hemodynamics and microvasculatures.
MATERIALS AND METHODS
Animals and Operative Procedures
Fifty mongrel dogs of either sex were used. Anesthesia was induced with IV sodium pentobarbital, ketamine, and pancuronium and maintained under mechanical ventilation. The left kidney was harvested and flushed out immediately in an ice-saline with heparinized lactate Ringer’s solution followed by re-flushing with Euro-Collins (EC) or University of Wisconsin (UW) solution. After 72 or 120 hours of ice-cold storage, the renal grafts were autotransplanted into the right iliac fossa.
Graft Function
Graft function was evaluated by serial measurements of serum creatinine levels and by survival of the animals. All the dogs were killed at the 15th postoperative day.
Renal Blood Flow
An Ultrasonic Blood Flowmeter (Model T-201, Transonic Systems Inc., Ithaca, New York) was used for measurement of total renal blood flow. Blood flow was measured before nephrectomy, at 0.5, 1.0, 2.0, and 3.0 hours after graft revascularization, respectively, and was expressed as mL/min/g wet kidney weight.
Renal Microvasculatures
The silicon rubber compound Microfil (Canton Biological Medical Company, Denver, CO) was infused via the renal artery under 100 cm hydrostatic pressure, at the end of cold storage or 1 hour after graft reflow. Tissue slices were examined by stereoscopic dissecting microscope. Filling of renal microvasculatures with silicon rubber compound was graded from 0 (severe defect) to 4 (excellent filling) without knowledge of experimental groups.
Histology
Renal tissues were taken at the end of preservation, 1 hour after graft reflow, and at autopsy, then fixed with 10% formalin and stained with hematoxylin-eosin.
Statistics
Student’s t test was used for the comparison of group means, in which a P value less than .05 was considered to be significant.
RESULTS
As shown in Table 1, 100% survival rate could be obtained in 72-hour UW preserved kidneys. In contrast, only 17% of animals could survive when preserved with EC solution. Serum creatinine levels in long survivors started to decrease within 1 week after surgery. The renal blood flow after revascularization was significantly higher in UW kidneys when compared to that of EC. As shown in Fig 1 and Table 2, UW solution could completely protect both cortical and medullary microcirculation from 72-hour cold ischemic insult. When the ischemic time was prolonged up to 120 hours, however, the medullary circulation became poor in spite of showing well-maintained renal blood flow and fairly intact cortical perfusion. Consequently, 5 out of 6 kidneys in this group failed within 2 weeks. Histology revealed the well-preserved microvascular structures, in particular the muscular layer of the arteries, even in 120-hour UW preserved kidney, suggesting the remarkably protective effects of UW solution on renal vascular components.
Table 1.
Survival Time and Blood Flow of Canine Renal Autografts After Transplantation
| Renal blood flow after reperfusion (mL/min/g kid wt) | |||||
|---|---|---|---|---|---|
| Mean survival | |||||
| Group | n | Preservation | (d) | 0.5 h | 3.0 h |
| 2 | 6 | EC 72-h | 7.50 ± 4.9 | 1.46 ± 1.06 | 1.27 ± 0.60 |
| 3 | 6 | UW 72-h | 15.00 ± 0.0* | 2.55 ± 0.98†† | 2.00 ± 0.28† |
| 5 | 6 | UW 120-h | 6.00 ± 4.7 | 1.90 ± 0.76 | 1.82 ± 0.44 |
All values are presented as Mean ± SD.
P < .001.
P < .02.
P < .05; as compared with group 2.
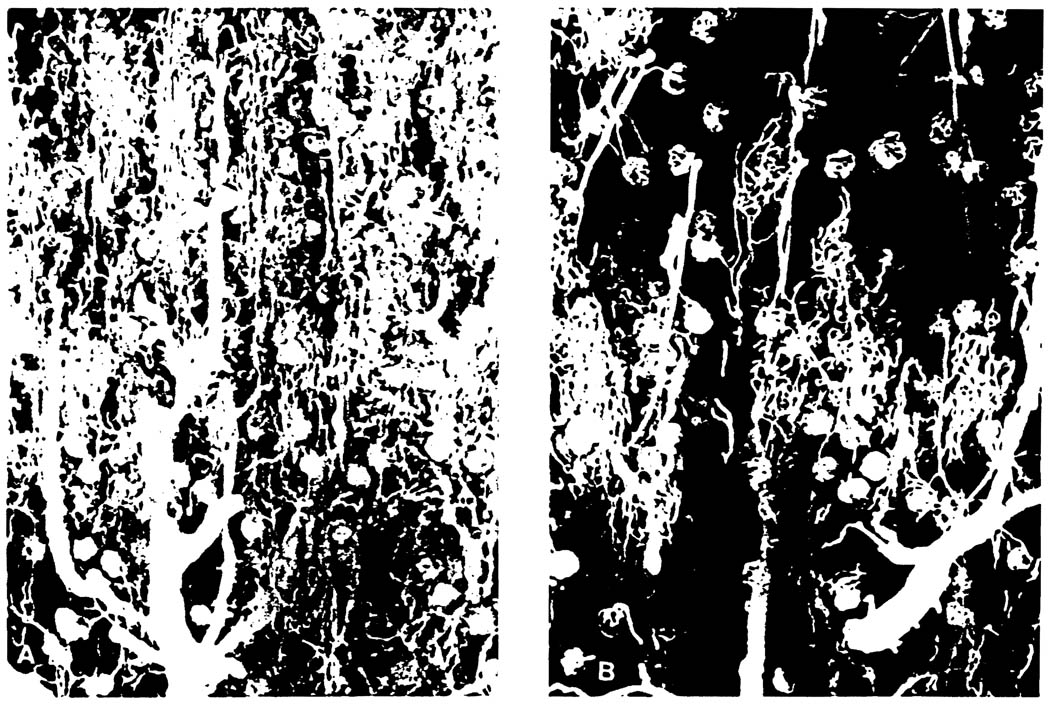
Fig 1.
Dissecting photomicrographs of renal cortical vasculatures visualized with silicon rubber compound at 1 hour after reperfusion of the graft (×50). (A) 72-hour EC kidney. Notice patchy distribution of avascular area, irregular and deformed pattern of interlobular artery and glomerulus. (B) 72-hour UW kidney. The glomerulus and capillary networks are fully filled with silicon rubber.
Table 2.
Morphological Evaluation of Silicon Rubber Filling in Renal Microvasculatures 1 h After Graft Reperfusion
| Cortex |
Medulla |
|||||
|---|---|---|---|---|---|---|
| Group | n | Preservation | Outer | Inner | Outer | Inner |
| 1 | 8 | none | 3.2 ± 0.6 | 3.6 ± 0.5 | 3.6 ± 0.5 | 3.2 ± 0.8 |
| 2 | 8 | EC 72-h | 1.4 ± 0.7* | 1.6 ± 0.7* | 0.0 ± 0.0* | 0.0 ± 0.0* |
| 3 | 8 | UW 72-h | 3.1 ± 0.7* | 3.4 ± 0.7* | 2.9 ± 0.9*† | 2.1 ± 1.0*† |
| 5 | 8 | UW 120-h | 2.7 ± 0.5 | 2.8 ± 0.4 | 1.8 ± 0.8† | 0.8† ± 0.7† |
Overall filling grade was determined as follows: 4, excellent; 3, good; 2, fair; 1, poor; 0, filling defect. All values were presented as Mean ± SD.
P < .0001.
P < .005.
DISCUSSION
Postischemic cortical hypoperfusion as a result of a damaged vascular system can cause a decrease in glomerular filtration rate, tubular obstruction, and back leaking. Disturbances in oxygen and substrate delivery make these changes irreversible.3,4 The present study demonstrated that the beneficial effects of UW solution might be attributed in part to its protection of the microcirculation and the consequent prompt relief of ischemia after revascularization. However, in 120-hour UW preserved kidney, there was a major discrepancy between perfusion of the cortex and medulla. This might be caused by a swelling of medullary tubular cells, which ·are most susceptible to ischemia.5–7 Hence, it still remains to be investigated what is a key distinction between the tolerance of microvascular system and parenchymal tissue to ischemia. Correction of intrarenal blood maldistribution might be important in successfully preserving the kidney for 120 hours.
Acknowledgments
This work was supported by research grants from the Veterans Administration and project grant DK 29961 from the National Institutes of Health, Bethesda, MD.
REFERENCES
- 1.Barger AC, Herd JA. N Engl J Med. 1971;284:482. doi: 10.1056/NEJM197103042840907. [DOI] [PubMed] [Google Scholar]
- 2.Anaise D, Bachvaroff RJ, Sato K, Rapaport Ff. Transplantation. 1984;38:570. doi: 10.1097/00007890-198412000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Finn WF, Arendshorst WJ, Gottschalk CW. Cire Res. 1975;36:675. doi: 10.1161/01.res.36.6.675. [DOI] [PubMed] [Google Scholar]
- 4.Brezis M, Rosen S, Silva P, Epstein FH. Kidney Int. 1984;26:375. doi: 10.1038/ki.1984.185. [DOI] [PubMed] [Google Scholar]
- 5.Carriere S, Geoffrey D, Thorburn MB, Barger AC. Cire Res. 1966;19:167. [Google Scholar]
- 6.Vetterlein F, Petho A, Schmidt G. Am J Physiol. 1986;251:H510. doi: 10.1152/ajpheart.1986.251.3.H510. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto K, Wilson DR, Baumal R. Am J Physiol. 1984;116:253. [PMC free article] [PubMed] [Google Scholar]