Abstract
Purpose
To determine whether lapatinib, a dual epidermal growth factor receptor (EGFR)/HER2 kinase inhibitor, can radiosensitize EGFR+ or HER2+ breast cancer xenografts.
Methods and Materials
Mice bearing xenografts of basal-like/EGFR+ SUM149 and HER2+ SUM225 breast cancer cells were treated with lapatinib and fractionated radiotherapy and tumor growth inhibition correlated with alterations in ERK1 and AKT activation by immunohistochemistry.
Results
Basal-like/EGFR+ SUM149 breast cancer tumors were completely resistant to treatment with lapatinib alone but highly growth impaired with lapatinib plus radiotherapy, exhibiting an enhancement ratio average of 2.75 and a fractional tumor product ratio average of 2.20 during the study period. In contrast, HER2+ SUM225 breast cancer tumors were highly responsive to treatment with lapatinib alone and yielded a relatively lower enhancement ratio average of 1.25 during the study period with lapatinib plus radiotherapy. Durable tumor control in the HER2+ SUM225 model was more effective with the combination treatment than either lapatinib or radiotherapy alone. Immunohistochemical analyses demonstrated that radiosensitization by lapatinib correlated with ERK1/2 inhibition in the EGFR+ SUM149 model and with AKT inhibition in the HER2+ SUM225 model.
Conclusion
Our data suggest that lapatinib combined with fractionated radiotherapy may be useful against EGFR+ and HER2+ breast cancers and that inhibition of downstream signaling to ERK1/2 and AKT correlates with sensitization in EGFR+ and HER2+ cells, respectively.
Keywords: Breast cancer, epidermal growth factor receptor, HER2, radiosensitization, lapatinib
Introduction
Breast cancer is the most commonly diagnosed cancer among women in the United States. It arises from different epithelial cell populations, resulting in tumors of basal or luminal origin, with the basal subtype conferring a worse prognosis (1, 2). Several groups have reported the use of traditional chemotherapy combined with radiotherapy (RT) in attempts to improve local control (3, 4), an approach that has proved to be beneficial in other cancers. However, because of the significant toxicities seen in these trials, a great deal of interest exists in using targeted biologic therapies combined with RT to improve local control rates while maintaining acceptable toxicity (5).
Currently, several targeted biologic therapies are of promising clinical interest against members of the epidermal growth factor receptor (EGFR/ErbB) family of proteins, including ErbB1/EGFR/HER1, ErbB2/HER2/neu, ErbB3/HER3, and ErbB4/HER4. All four receptors have been reported to play a role in tumorigenesis and are known to regulate epithelial cell proliferation, survival, and differentiation (6). The best studied among these family members remains EGFR and HER2, which are aberrantly expressed in a variety of human malignancies, including breast cancer. EGFR and HER2 play an important role in radioresistance, with EGFR positivity associated with a poor prognosis and an unfavorable response to therapy (7, 8).
Although it is well established that EGFR and HER2 signal to several downstream effector pathways, including MEK>ERK and PI3K>AKT, the key pathways that confer EGFR/HER2-mediated radioresistance are poorly understood (9, 10). Clarification of the pathways that mediate radiosensitization with inhibitors of EGFR/HER2 could potentially advance treatment options. For instance, with the development of EGFR inhibitor resistance, including the uncoupling of EGFR to downstream signaling pathways (18), direct inhibition of these pathways could provide alternative therapeutic targets. Additionally, identifying the relevant downstream effectors of EGFR/HER2 therapies as radiosensitizers could provide biomarkers to better predict the tumor response and improve patient selection.
Preclinical studies using a variety of EGFR inhibitors in different model systems have demonstrated their substantial promise as radiosensitizers (11). Cetuximab (Erbitux, Im-Clone Systems, New York, NY), a monoclonal antibody targeting EGFR, was the first biologic agent to show local control and survival advantage when used in combination with RT for patients with head-and-neck cancer (12). CI-1033 is a small molecule inhibitor that blocks all four EGFR family members that recently failed in a Phase II trial of metastatic breast cancer patients because of unacceptable toxicities (13). However, in preclinical studies with CI-1033, breast cancer cells were effectively radiosensitized with combination therapy (14), providing support for continued efforts to identify clinically efficacious EGFR inhibitors to radiosensitize breast cancer tumors. Lapatinib (Tykerb, GlaxoSmithKline, Middlesex, United Kingdom), a small molecule tyrosine kinase inhibitor with near-equipotent inhibition of both EGFR and HER2, has been approved for use by the Food and Drug Administration in patients with advanced or metastatic HER2-overexpressing breast cancer (15). Moreover, a subset of breast cancer patients exhibiting progression after previous therapy with trastuzumab (Herceptin, Genentech, Hoboken, NJ), a HER2 monoclonal antibody, have shown benefit with subsequent treatment with lapatinib (16, 17). Whether lapatinib can radiosensitize breast cancers that overexpress EGFR or HER2 is currently unknown. The purpose of the present study was to determine whether lapatinib could radiosensitize EGFR- or HER2-overexpressing breast cancer cells in mouse xenograft models and whether radiosensitization correlates with inhibition of downstream signaling to MEK>ERK and/or PI3K>AKT.
Methods And Materials
Cell lines and reagents
Lapatinib was synthesized and generously provided by GlaxoSmithKline and formulated in sulfo-butyl-ether-β-cyclodextrin as a 10% aqueous solution. The human breast cancer cell lines SUM149 and SUM225 were cultured as previously described (18).
Xenograft treatment and tumor harvesting
Animal experiments were performed in accordance with the University of North Carolina Institutional Animal Care and Use Committee guidelines. Cells (5–10 × 106) were suspended in 200 μL of a 1:1 ratio of phosphate-buffered saline/Matrigel (BD Biosciences, Franklin Lakes, NJ) before injection into the flanks of 4–5-week-old female C.B-17 Fox Chase severe combined immunodeficient mice (Charles River Laboratories, Wilmington, MA). For optimization of dosing studies, tumors were grown to a diameter of 10 mm and then treated with lapatinib (0, 30, or 100 mg/kg twice daily at 6-h intervals) for a total of five treatments within 2.5 days, as previously described (19). The mice were sacrificed by carbon dioxide inhalation, and the tumors harvested 4 h after the last dose of lapatinib and flash-frozen until processing for immunoprecipitation.
For tumor radiosensitization and immunohistochemical studies, tumors were grown to a volume of 100 mm3, randomized (n = 8 mice/group), and treated with vehicle, lapatinib, RT, or both lapatinib and RT. Lapatinib (100 mg/kg twice daily at 6-h intervals) or vehicle (10% sulfo-butyl-ether-β-cyclodextrin) was administered by oral gavage for 10 days starting at Day –10. RT was administered at 2 Gy/fraction to anesthetized mice for 3 consecutive days starting at Day –4 and delivered by a linear accelerator (Primus, Siemens, New York, NY) using 6-MeV electrons and a custom lead cutout.
Tumors were measured at regular 3-day intervals, and tumor volumes calculated by width × length/2. The fold-change in tumor volume was normalized to baseline (Day –10) size and plotted over the indicated points to generate tumor growth graphs using GraphPad Prism, version 5.0. Statistical significance was determined using two-way analysis of variance. Enhancement ratios were determined by dividing the average tumor volumes of tumors receiving RT alone by those receiving RT plus lapatinib. Tumor growth rates were derived by determining the slopes of the growth curves for each treatment group.
To assess the antagonistic, additive, and synergistic effects, we used the fractional tumor product method using values averaged for the study duration starting at Day 0 (20, 21), where a value >1 suggested that the combined treatments were effectively synergistic, <1 antagonistic, and equal to 1 additive.
Immunohistochemistry
For immunohistochemical analyses, tumors were harvested within 72 h of the last treatment at Day –1, fixed in 10% buffered formaldehyde for 24 h, embedded in paraffin, and processed using antigen retrieval buffer (BioGenex, San Ramon, CA). The antibodies used included anti-phospho-ERK1/2, anti-total ERK1/2, anti-phospho AKT, and anti-total AKT (all from Cell Signaling Technologies, Beverely, MA), which were incubated overnight at 4°C, along with appropriate secondary antibodies, and visualized by VECTASTAIN Elite biotin-avidin complex reaction (Vector Laboratories, Burlingame, CA) with nickel-enhanced diaminobenzidine (Pierce Invitrogen, Carlsbad, CA.) used as the chromogen and hematoxylin used as counterstain. All samples were stained in triplicate, and the intensity and percentage of stained cells scored by a pathologist blinded to the treatment groups and multiplied together to derive a total immunohistochemical score for phosphorylated ERK1/2 and phosphorylated AKT. Statistical analyses were performed using one-way analysis of variance (GraphPad Prism).
Protein extraction, immunoprecipitation, and Western blotting
To determine EGFR phosphorylation levels in tumor tissue, flash-frozen tissue was pulverized with mortar and pestle, and lysates prepared and processed with anti-EGFR (polyclonal rabbit 22 antisera), as previously described (18). Western blotting was performed using anti-phospho-tyrosine antibody (Santa Cruz Biotechnology, Santa Cruz, CA) or anti-EGFR antisera (polyclonal rabbit 22).
Results
We have previously shown that EGFR and HER2 activation are blocked by lapatinib in EGFR-overexpressing (EGFR+) basal-like SUM149 and HER2-overexpressing (HER2+) SUM225 breast tumor cells in vitro (18). In the present study, we sought to determine whether lapatinib could radiosensitize these cells in vivo and whether the response to therapy would correlate with the inhibition of downstream signaling.
RT plus lapatinib synergistically inhibited tumor growth in basal-like/EGFR+ SUM149 xenografts in vivo
To determine the dose of lapatinib needed to inhibit EGFR in vivo, we evaluated the levels of phosphorylated EGFR in SUM149 xenografts treated with lapatinib using a dosing scheme commonly used for HER2+ breast cancer xenograft mouse models (19, 22). Partial inhibition of EGFR phosphorylation was evident after treatment with 30 mg/kg of lapatinib, and full inhibition occurred with 100 mg/kg (Fig. 1A). This showed that the dosing regimen needed to inhibit activation of EGFR is similar to that needed to inhibit HER2 in vivo. Thus, we chose to use the 100 mg/kg dosing regimen for all subsequent breast cancer xenograft radiosensitization studies.
Fig. 1.
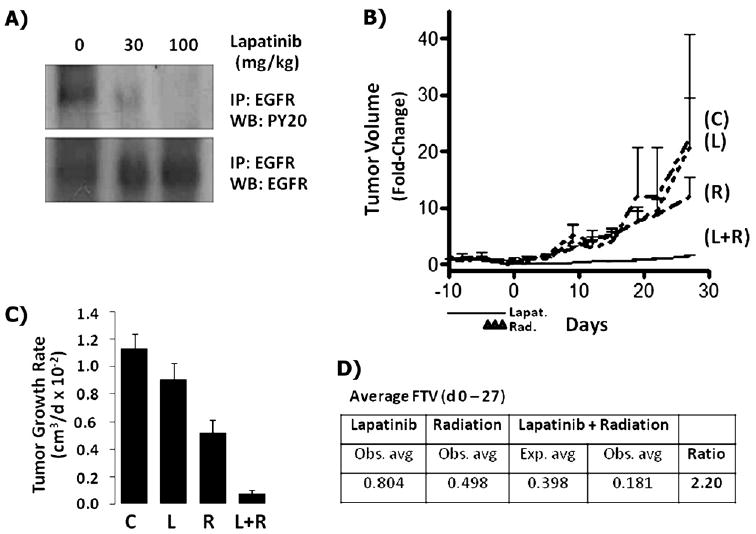
Lapatinib-mediated radiosensitization of SUM149 basal-like/epidermal growth factor receptor-positive (EGFR+) breast cancer xenografts. (A) SUM149 tumors were grown to a tumor diameter of 10 mm, treated with lapatinib (30 or 100 mg/kg twice daily at 6-h intervals) or vehicle for total of five treatments within 2.5 days. EGFR was immunoprecipitated and analyzed by Western blotting with anti-phosphotyrosine antiserum. (B) Tumors grown to 100 mm3 and treated with either lapatinib (100 mg/kg twice daily) or vehicle for 10 days starting at Day –10 and radiotherapy at 2 Gy/fraction administered for 3 consecutive days starting at Day –4. Tumor volume changes were normalized to baseline (Day –10) and plotted over time for each treatment group. C = vehicle control; L = lapatinib; R = radiotherapy; L+R = lapatinib plus radiotherapy. (C) Tumor growth rates = the slopes of growth curves during study duration for each treatment group. (D) Synergy assessment = the ratio of expected/observed average fractional tumor volumes (FTV) during study duration for lapatinib plus radiotherapy.
Next, to investigate whether lapatinib could radiosensitize basal-like EGFR+ SUM149 cells, the xenografts were allowed to develop palpable tumors and then treated with vehicle, lapatinib, radiotherapy, or lapatinib plus radiotherapy. No significant difference in tumor growth was seen between the vehicle and lapatinib-treated xenografts during the study duration (27 days). In contrast, treatment with RT alone or lapatinib plus RT resulted in tumor growth delay (Fig. 1B). The average fold- increase in tumor volume at study termination (Day 27) was significantly reduced in the mice treated with lapatinib plus RT (1.53; p <.001) compared with that in the control mice (20.8) or those treated with lapatinib (22.5) or RT (12.1) alone. Comparing the average rate of tumor growth per day (Fig. 1C) also showed a significant reduction with lapatinib plus RT vs. RT alone. The enhancement ratio of the tumors treated with lapatinib plus RT averaged 2.75 during the study duration (Supplementary Fig. e1) and was greatest immediately after completion of the study treatments at Day 0 (3.24) and Day 19 (3.20), demonstrating immediate and durable tumor control. To determine whether the enhanced interaction with lapatinib plus RT was additive or synergistic, the fractional product method was used and gave an expected/observed fractional tumor volume ratio average of 2.20 during the study duration (Fig. 1D), consistent with a synergistic interaction.
HER2+ SUM225 xenografts are lapatinib sensitive and exhibited enhanced growth delay when combined with RT
In the HER2+ SUM225 xenografts, the average fold- increase in tumor volume early in the study at Day 21 was significantly reduced in the mice treated with lapatinib alone (4.44; p <.01) compared with that in the control mice (12.68). At Day 21, the combination of lapatinib plus RT did not provide a statistically significant difference in the fold- increase in tumor volume compared with RT alone (3.19 vs. 4.89, p = NS), indicating that lapatinib did not provide radiosensitization at early points in the SUM225 xenografts (Fig. 2A). This was supported by analyses during the initial 21-day growth period in which the interaction of lapatinib plus RT was less than additive using the fractional tumor product method (data not shown). However, although tumors from the control mice and lapatinib-only treatment arms could not be assessed beyond Days 45 and 81, respectively, tumor regrowth in the RT only and lapatinib plus RT groups increasingly diverged during the remaining study duration (138 days), with statistically significant differences in the fold- increase in tumor volume (13.99 vs. 3.66, p <.01) starting at Day 97. Comparisons of the average rate of tumor growth daily (Fig. 2B) was also significantly reduced with lapatinib plus RT vs. RT alone. The enhancement ratios in the mice treated with lapatinib plus RT averaged 1.25 during the study duration (Days 0–138; Supplementary Fig. e1) and was greatest immediately after completion of the study treatments (Days 0–10; enhancement ratio, 2.3) and toward study termination at 3 months (Days 97–138; enhancement ratio, 1.43).
Fig. 2.
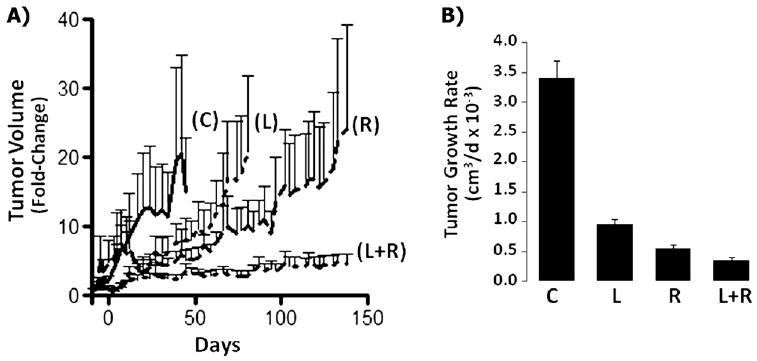
Lapatinib-mediated radiosensitization of SUM225 HER2+ breast cancer xenografts. (A) Tumors were treated as described in Fig. 1, and tumor volume changes normalized to baseline (Day –10) and plotted over time for each treatment group. C = vehicle control; L = lapatinib; R = radiotherapy; L+R = lapatinib plus radiotherapy. (B) Tumor growth rates = the slopes of growth curves for study duration for each treatment group.
Lapatinib-mediated radiosensitization correlates with inhibition of ERK1/2 in basal-like/EGFR+ SUM149 and AKT in HER2+ SUM225 xenograft models
We next sought to determine whether lapatinib-mediated radiosensitization correlated with inhibition of downstream signaling through the MEK>ERK and PI3K>AKT pathways. For these analyses, tumors were obtained on completion of the study treatments from the companion mice in each treatment group and analyzed using immunohistochemistry. Treatment with lapatinib plus RT in the basal-like/EGFR+ SUM149 xenograft tumors showed a reproducible and significant decrease in cells staining positive for phosphorylated ERK1/2 with a reduced phosphorylated ERK1/2 score (3.7 ± 0.6) compared with RT alone (9.3 ± 1.0; Fig. 3). No statistically significant difference was found in phosphorylated ERK1/2 levels between RT alone (9.3 ± 1.0) or lapatinib alone (8.7 ± 0.8) and the control (8.8 ± 0.8). In addition, no change in phosphorylated AKT levels was seen in any treatment group (data not shown).
Fig. 3.
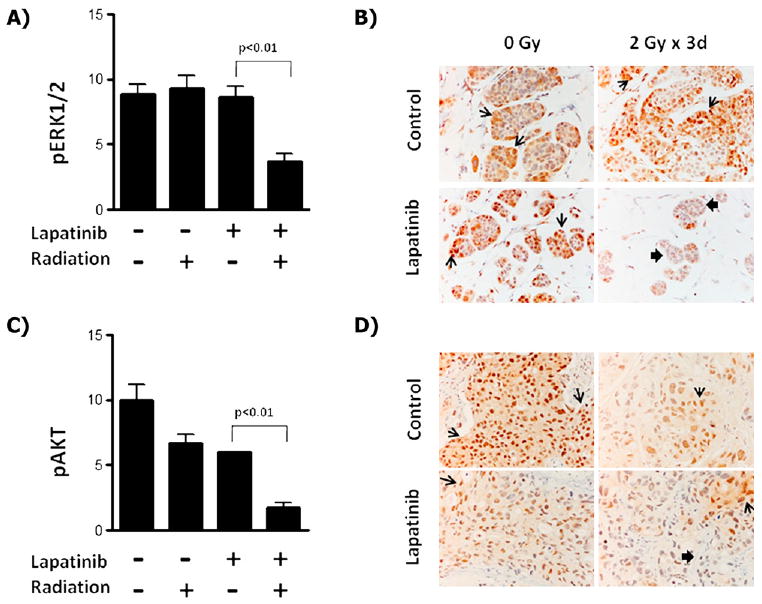
Radiosensitization by lapatinib correlates with inhibition of ERK1/2 in EGFR+/basal-like cells and with AKT in HER2+ breast cancer cells. (A) Tumors from basal-like/EGFR+ SUM149 xenografts were processed for immunohistochemistry with phosphorylated ERK1/2 antiserum and quantified from mice treated with lapatinib, radiotherapy, lapatinib plus radiotherapy, or vehicle control. (B) Sample immunohistochemistry staining of SUM149 tumors with phosphorylated ERK1/2 serum at 400×. Similarly, tumors from HER2+ SUM225 xenografts were processed for immunohistochemistry with phosphorylated AKT antiserum and (C) quantified. (D) Sample immunohistochemistry of SUM225 tumors with phosphorylated AKT serum at 400×. Open arrows indicate areas of increased staining; solid arrows, areas of reduced staining.
In contrast, the HER2+ SUM225 xenografts showed no change in phosphorylated ERK1/2 levels in any of the treatment arms (data not shown). However, a statistically significant decrease in phosphorylated AKT was observed in the lapatinib-alone and RT-alone treatment groups (6.0 ± 0.0 and 6.7 ± 0.7, respectively) compared with the control group (10.0 ± 1.15), with the largest decrease in phosphorylated AKT observed in the combined lapatinib plus RT group (1.8 ± 0.4). These data suggest that the mechanism of lapatinib-mediated radiosensitization differs between breast cancer subtypes, such that basal-like/EGFR+ cells are sensitized through inhibition of ERK1/2, while in HER2+ cells, sensitization occurs through inhibition of AKT.
Discussion
We have demonstrated that in mouse xenograft models, the dual EGFR/HER2 inhibitor, lapatinib, can radiosensitize both HER2+ and basal-like/EGFR+ breast cancer cell lines. Specifically, we have shown that tumors from SUM149 basal-like/EGFR+ cells were insensitive to lapatinib monotherapy treatment but were radiosensitized when lapatinib was combined with RT. In contrast, tumors from SUM225 HER2+ cells were highly sensitive to lapatinib monotherapy alone and, like the basal-like/EGFR+ cells, showed an enhanced therapeutic response when lapatinib was combined with RT. In addition, we found that inhibition of phosphorylated ERK1/2 correlated with radiosensitization in the basal-like/EGFR+ tumors and inhibition of phosphorylated AKT correlated with the response in HER2+ tumors. We hypothesized that a subset of breast cancer patients could benefit from a therapeutic regimen in which RT is combined with lapatinib for both basal-like/EGFR+ and HER2+ breast cancers and that the downstream biomarkers of response will differ by subtype.
Inhibitors against the EGFR family of proteins have shown promise as radiosensitizers in preclinical studies for a variety of cancer types and in clinical studies of head-and-neck cancer (11, 12). Although lapatinib has been approved by the Food and Drug Administration for the treatment of HER2+ metastatic breast cancer, the EGFR pathway has recently become a potential target for the basal-like subtype, in which ≥50% of basal-like tumors have been shown to overexpress EGFR as assessed by immunohistochemistry (23, 24). Basal-like breast cancers represent 16% of all breast cancers, and patients with this subtype of breast cancer face a worse prognosis and are not candidates for therapies commonly used for other breast cancer subtypes, such as anti-estrogen receptor or anti-HER2 therapies, because they are typically estrogen receptor, HER2, and progesterone receptor negative (25). Instead, these patients are treated with conventional chemotherapy regimens, which have shown limited efficacy. Consistent with our previous in vitro studies, which showed lapatinib-mediated inhibition of proliferation in SUM149 cells (18), other groups have also shown that other EGFR inhibitors, including Iressa (an EGFR-specific inhibitor) and CI-1033 (an inhibitor that blocks all four ErbB family members), inhibit proliferation and anchorage-independent growth, as well as radiosensitize SUM149 cells in vitro (14, 24). Basal-like breast cancers are found in approximately 40% of patients with inflammatory breast cancer, representing one of the most aggressive forms of breast cancer (26). In a recent Phase II clinical trial of patients with EGFR+ inflammatory breast cancer, only 1 of 15 patients responded to lapatinib monotherapy (27). Consistent with this finding, our analysis of the basal-like/EGFR+ SUM149 cells showed that lapatinib monotherapy was ineffective at inhibiting tumor growth. In contrast, when lapatinib was combined with RT, a synergistic interaction was seen, causing a significant reduction in tumor growth, with an average enhancement ratio of 2.75 for the study duration.
In the SUM225 HER2+ xenografts, tumor growth was strongly inhibited by lapatinib alone, with durable growth inhibition evident long after drug withdrawal. This result is consistent with in vitro data showing that lapatinib greatly inhibits the growth of HER2+ breast cancer cells (28) and with recent clinical trials in which HER2+ breast cancer patients responded to lapatinib monotherapy (29, 30). Although we did not observe radiosensitization with lapatinib during early points in the SUM225 xenografts, statistically significant tumor growth inhibition was observed starting at Day 97 in the lapatinib plus RT arm and persisted through study termination (Day 138), producing an enhancement ratio of 1.43 from Days 97 to 138 and an overall average enhancement ratio of 1.25 for the entire study duration (Days 0–138). The degree of radiosensitization by lapatinib for both the EGFR+ SUM149 and HER2+ SUM225 xenographs was similar to what has been reported in preclinical radiosensitization studies of head-and-neck cancer cell lines with cetuximab (31, 32), an EGFR inhibitor clinically shown to radiosensitize and improve survival (12).
Thus, our study results support the feasibility of combining RT with pharmacologic inhibitors that target EGFR or HER2 in breast cancer patients. We recently reported the results from a small Phase II study that evaluated trastuzumab (Herceptin), an anti-HER2 antibody, plus RT followed by surgery in heavily pretreated, chemotherapy-refractory, HER2+ breast cancer patients. Of the 7 patients who underwent combined trastuzumab plus RT followed by surgery, 3 (43%) showed a pathologic response (complete response or microscopic residual disease) compared with a response of 5% in a comparison cohort (2 of 38 patients) (33). It remains important to assess whether lapatinib can also radiosensitize breast cancer and whether lapatinib can radiosensitize trastuzumab-resistant breast cancer.
As a drug class, EGFR inhibitors have shown clinical efficacy against various cancer types; however, their use has been limited by a lack of biomarkers to predict and better select those patients most likely to respond to therapy (10, 34, 35). In the basal-like/EGFR+ SUM149 xenograft model, lapatinib-mediated radiosensitization correlated with inhibition of downstream signaling to ERK1/2, which was not observed with either lapatinib or RT alone. Recent studies from our laboratory (36) have demonstrated that radioresistance in basal-like/EGFR+ breast cancer cells results from activation of the Raf>MEK>ERK pathway and that both lapatinib and the MEK-inhibitor CI-1040 can radiosensitize these cell lines in vitro. Consistent with our data, an analysis of 46 breast tumor cell lines by Mirzoeva et al. (37) showed that cell lines of the basal-like subtype were more sensitive to inhibitors of MEK than those of luminal origin.
Early studies in xenografts using HER2+ BT474 breast tumor cells showed lapatinib response correlated with partial inhibition of ERK1/2 and complete inhibition of AKT (28, 38). In our HER2+ SUM225 xenografts, radiosensitization by lapatinib correlated with inhibition of downstream signaling of AKT, but not ERK1/2, suggesting that AKT is a better marker of lapatinib response in HER2+ breast cancers. In addition, the high degree of lapatinib sensitivity in the SUM225 cells could be imparted through the combined inhibition of HER2 and EGFR, because these cells are known to express extremely high levels of HER2 with some concurrent, but much lower, levels of EGFR. Given that approximately 35% of HER2+ breast cancers are also EGFR+ (39), profiling breast cancer patients to include EGFR status, along with estrogen receptor, progesterone receptor, and HER2 status, would allow for better selection among the various therapeutic agents that target the EGFR family of receptors.
Conclusion
Although EGFR and HER2 activate common downstream signaling pathways, our studies have shown that fundamental differences exist between EGFR and HER2 response to RT, providing insight into the divergent consequences of EGFR and HER2 signaling and inhibition. A model based on the present study correlates lapatinib-mediated radiosensitization of EGFR+ cells with ERK1/2 inhibition in basal-like/EGFR+ cells and with AKT inhibition in HER2+ cells. Importantly, our results suggest that although EGFR+ breast cancers appear unresponsive to lapatinib monotherapy, the combination of lapatinib plus RT might provide a therapeutic option for patients with basal-like/EGFR+ breast cancers, who currently have few therapeutic options (40). In addition, HER2+ breast cancer patients who are candidates for adjuvant RT could experience better outcomes with longer response durations with combined RT and lapatinib.
Supplementary Material
Acknowledgments
Supported by Grant CA83753 (to C. I. Sartor), Grant CA115888 (to C. I. Sartor and J. M. Shields), National Institutes of Health, and GlaxoSmithKline.
Footnotes
Conflict of interest: none.
References
- 1.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 2.Fadare O, Tavassoli FA. Clinical and pathologic aspects of basal-like breast cancers. Nat Clin Pract Oncol. 2008;5:149–159. doi: 10.1038/ncponc1038. [DOI] [PubMed] [Google Scholar]
- 3.Karasawa K, Katsui K, Seki K, et al. Radiotherapy with concurrent docetaxel for advanced and recurrent breast cancer. Breast Cancer. 2003;10:268–274. doi: 10.1007/BF02966728. [DOI] [PubMed] [Google Scholar]
- 4.Suh WW, Schott AF, Hayman JA, et al. A phase I dose escalation trial of gemcitabine with radiotherapy for breast cancer in the treatment of unresectable chest wall recurrences. Breast J. 2004;10:204–210. doi: 10.1111/j.1075-122X.2004.21305.x. [DOI] [PubMed] [Google Scholar]
- 5.Tepper JE, Sartor CI. Radiation therapy and biologics: A ripe opportunity? Nat Clin Pract Oncol. 2006;3:463. doi: 10.1038/ncponc0565. [DOI] [PubMed] [Google Scholar]
- 6.Holbro T, Hynes NE. ErbB receptors: Directing key signaling networks throughout life. Annu Rev Pharmacol Toxicol. 2004;44:195–217. doi: 10.1146/annurev.pharmtox.44.101802.121440. [DOI] [PubMed] [Google Scholar]
- 7.Sartor CI. Epidermal growth factor family receptors and inhibitors: Radiation response modulators. Semin Radiat Oncol. 2003;13:22–30. doi: 10.1053/srao.2003.50003. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt-Ullrich RK, Contessa JN, Lammering G, et al. ERBB receptor tyrosine kinases and cellular radiation responses. Oncogene. 2003;22:5855–5865. doi: 10.1038/sj.onc.1206698. [DOI] [PubMed] [Google Scholar]
- 9.Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol. 2009;21:177–184. doi: 10.1016/j.ceb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Gusterson BA, Hunter KD. Should we be surprised at the paucity of response to EGFR inhibitors? Lancet Oncol. 2009;10:522–527. doi: 10.1016/S1470-2045(09)70034-8. [DOI] [PubMed] [Google Scholar]
- 11.Sartor CI. Mechanisms of disease: Radiosensitization by epidermal growth factor receptor inhibitors. Nat Clin Pract Oncol. 2004;1:80–87. doi: 10.1038/ncponc0048. [DOI] [PubMed] [Google Scholar]
- 12.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 13.Rixe O, Franco SX, Yardley DA, et al. A randomized, phase II, dose-finding study of the pan-ErbB receptor tyrosine-kinase inhibitor CI-1033 in patients with pretreated metastatic breast cancer. Cancer Chemother Pharmacol. 2009;64:1139–1148. doi: 10.1007/s00280-009-0975-z. [DOI] [PubMed] [Google Scholar]
- 14.Rao GS, Murray S, Ethier SP. Radiosensitization of human breast cancer cells by a novel ErbB family receptor tyrosine kinase inhibitor. Int J Radiat Oncol Biol Phys. 2000;48:1519–1528. doi: 10.1016/s0360-3016(00)01358-4. [DOI] [PubMed] [Google Scholar]
- 15.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 16.Blackwell KL, Pegram MD, Tan-Chiu E, et al. Single-agent lapatinib for HER2-overexpressing advanced or metastatic breast cancer that progressed on first- or second-line trastuzumab-containing regimens. Ann Oncol. 2009;20:1026–1031. doi: 10.1093/annonc/mdn759. [DOI] [PubMed] [Google Scholar]
- 17.Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: Updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112:533–543. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- 18.Zhou H, Kim YS, Peletier A, et al. Effects of the EGFR/HER2 kinase inhibitor GW572016 on EGFR- and HER2-overexpressing breast cancer cell line proliferation, radiosensitization, and resistance. Int J Radiat Oncol Biol Phys. 2004;58:344–352. doi: 10.1016/j.ijrobp.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 19.Molina JR, Kaufmann SH, Reid JM, et al. Evaluation of lapatinib and topotecan combination therapy: Tissue culture, murine xenograft, and phase I clinical trial data. Clin Cancer Res. 2008;14:7900–7908. doi: 10.1158/1078-0432.CCR-08-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matar P, Rojo F, Cassia R, et al. Combined epidermal growth factor receptor targeting with the tyrosine kinase inhibitor gefitinib (ZD1839) and the monoclonal antibody cetuximab (IMC-C225): Superiority over single-agent receptor targeting. Clin Cancer Res. 2004;10:6487–6501. doi: 10.1158/1078-0432.CCR-04-0870. [DOI] [PubMed] [Google Scholar]
- 21.Yokoyama Y, Dhanabal M, Griffioen AW, et al. Synergy between angiostatin and endostatin: Inhibition of ovarian cancer growth. Cancer Res. 2000;60:2190–2196. [PubMed] [Google Scholar]
- 22.Gril B, Palmieri D, Bronder JL, et al. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J Natl Cancer Inst. 2008;100:1092–1103. doi: 10.1093/jnci/djn216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoadley KA, Weigman VJ, Fan C, et al. EGFR associated expression profiles vary with breast tumor subtype. BMC Genomics. 2007;8:258. doi: 10.1186/1471-2164-8-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stratford AL, Habibi G, Astanehe A, et al. Epidermal growth factor receptor (EGFR) is transcriptionally induced by the Y-box binding protein-1 (YB-1) and can be inhibited with Iressa in basal-like breast cancer, providing a potential target for therapy. Breast Cancer Res. 2007;9:R61. doi: 10.1186/bcr1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rakha EA, Reis-Filho JS, Ellis IO. Basal-like breast cancer: A critical review. J Clin Oncol. 2008;26:2568–2581. doi: 10.1200/JCO.2007.13.1748. [DOI] [PubMed] [Google Scholar]
- 26.Van Laere SJ, Van den Eynden GG, Van der Auwera I, et al. Identification of cell-of-origin breast tumor subtypes in inflammatory breast cancer by gene expression profiling. Breast Cancer Res Treat. 2006;95:243–255. doi: 10.1007/s10549-005-9015-9. [DOI] [PubMed] [Google Scholar]
- 27.Johnston S, Trudeau M, Kaufman B, et al. Phase II study of predictive biomarker profiles for response targeting human epidermal growth factor receptor 2 (HER-2) in advanced inflammatory breast cancer with lapatinib monotherapy. J Clin Oncol. 2008;26:1066–1072. doi: 10.1200/JCO.2007.13.9949. [DOI] [PubMed] [Google Scholar]
- 28.Xia W, Mullin RJ, Keith BR, et al. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21:6255–6263. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 29.Kaufman B, Trudeau M, Awada A, et al. Lapatinib monotherapy in patients with HER2-overexpressing relapsed or refractory inflammatory breast cancer: Final results and survival of the expanded HER2+ cohort in EGF103009, a phase II study. Lancet Oncol. 2009;10:581–588. doi: 10.1016/S1470-2045(09)70087-7. [DOI] [PubMed] [Google Scholar]
- 30.Spector NL, Xia W, Burris H, III, et al. Study of the biologic effects of lapatinib, a reversible inhibitor of ErbB1 and ErbB2 tyrosine kinases, on tumor growth and survival pathways in patients with advanced malignancies. J Clin Oncol. 2005;23:2502–2512. doi: 10.1200/JCO.2005.12.157. [DOI] [PubMed] [Google Scholar]
- 31.Krause M, Schutze C, Petersen C, et al. Different classes of EGFR inhibitors may have different potential to improve local tumour control after fractionated irradiation: A study on C225 in FaDu hSCC. Radiother Oncol. 2005;74:109–115. doi: 10.1016/j.radonc.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Nakata E, Hunter N, Mason K, et al. C225 antiepidermal growth factor receptor antibody enhances the efficacy of docetaxel chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:1163–1173. doi: 10.1016/j.ijrobp.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 33.Horton JK, Halle J, Ferraro M, et al. Radiosensitization of chemotherapy-refractory, locally advanced or locally recurrent breast cancer with trastuzumab: A phase II trial. Int J Radiat Oncol Biol Phys. 2009 Jun 26; doi: 10.1016/j.ijrobp.2009.03.027. In press. [DOI] [PubMed] [Google Scholar]
- 34.Krause M, Baumann M. Clinical biomarkers of kinase activity: Examples from EGFR inhibition trials. Cancer Metastasis Rev. 2008;27:387–402. doi: 10.1007/s10555-008-9141-z. [DOI] [PubMed] [Google Scholar]
- 35.Loong HH, Ma BB, Chan AT. Update in antiepidermal growth factor receptor therapy in the management of metastatic colorectal cancer. J Oncol. 2009 doi: 10.1155/2009/967920. 967920. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambade MJ, Camp JT, Kimple RJ, et al. Mechanism of lapatinib-mediated radiosensitization of breast cancer cells is primarily by inhibition of the Raf>MEK>ERK mitogen-activated protein kinase cascade and radiosensitization of lapatinib-resistant cells restored by direct inhibition of MEK. Radiother Oncol. 2009;93:639–644. doi: 10.1016/j.radonc.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mirzoeva OK, Das D, Heiser LM, et al. Basal subtype and MAPK/ERK kinase (MEK)-phosphoinositide 3-kinase feedback signaling determine susceptibility of breast cancer cells to MEK inhibition. Cancer Res. 2009;69:565–572. doi: 10.1158/0008-5472.CAN-08-3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rusnak DW, Lackey K, Affleck K, et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther. 2001;1:85–94. [PubMed] [Google Scholar]
- 39.DiGiovanna MP, Stern DF, Edgerton SM, et al. Relationship of epidermal growth factor receptor expression to ErbB-2 signaling activity and prognosis in breast cancer patients. J Clin Oncol. 2005;23:1152–1160. doi: 10.1200/JCO.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 40.Viale G, Rotmensz N, Maisonneuve P, et al. Invasive ductal carcinoma of the breast with the “triple-negative” phenotype: prognostic implications of EGFR immunoreactivity. Breast Cancer Res Treat. 2009;116:317–328. doi: 10.1007/s10549-008-0206-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


