Abstract
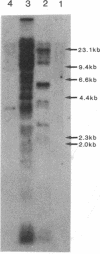
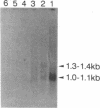
For isolation of the gene responsible for xeroderma pigmentosum (XP) complementation group A, plasmid pSV2gpt and genomic DNA from a mouse embryo were cotransfected into XP2OSSV cells, a group-A XP cell line. Two primary UV-resistant XP transfectants were isolated from about 1.6 X 10(5) pSV2gpt-transformed XP colonies. pSV2gpt and genomic DNA from the primary transfectants were again cotransfected into XP2OSSV cells and a secondary UV-resistant XP transfectant was obtained by screening about 4.8 X 10(5) pSV2gpt-transformed XP colonies. The secondary transfectant retained fewer mouse repetitive sequences. A mouse gene that complements the defect of XP2OSSV cells was cloned into an EMBL3 vector from the genome of a secondary transfectant. Transfections of the cloned DNA also conferred UV resistance on another group-A XP cell line but not on XP cell lines of group C, D, F, or G. Northern blot analysis of poly(A)+ RNA with a subfragment of cloned mouse DNA repair gene as the probe revealed that an approximately 1.0 kilobase mRNA was transcribed in the donor mouse embryo and secondary transfectant, and approximately 1.0- and approximately 1.3-kilobase mRNAs were transcribed in normal human cells, but none of these mRNAs was detected in three strains of group-A XP cells. These results suggest that the cloned DNA repair gene is specific for group-A XP and may be the mouse homologue of the group-A XP human gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clarkson J. M., Mitchell D. L., Adair G. M. The use of an immunological probe to measure the kinetics of DNA repair in normal and UV-sensitive mammalian cell lines. Mutat Res. 1983 Oct;112(5):287–299. doi: 10.1016/0167-8817(83)90004-4. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Defective repair replication of DNA in xeroderma pigmentosum. Nature. 1968 May 18;218(5142):652–656. doi: 10.1038/218652a0. [DOI] [PubMed] [Google Scholar]
- Corsaro C. M., Pearson M. L. Enhancing the efficiency of DNA-mediated gene transfer in mammalian cells. Somatic Cell Genet. 1981 Sep;7(5):603–616. doi: 10.1007/BF01549662. [DOI] [PubMed] [Google Scholar]
- De Weerd-Kastelein E. A., Keijzer W., Bootsma D. Genetic heterogeneity of xeroderma pigmentosum demonstrated by somatic cell hybridization. Nat New Biol. 1972 Jul 19;238(81):80–83. doi: 10.1038/newbio238080a0. [DOI] [PubMed] [Google Scholar]
- Fischer E., Keijzer W., Thielmann H. W., Popanda O., Bohnert E., Edler L., Jung E. G., Bootsma D. A ninth complementation group in xeroderma pigmentosum, XP I. Mutat Res. 1985 May;145(3):217–225. doi: 10.1016/0167-8817(85)90030-6. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Kohn K. W., Kann H. E., Jr DNA single-strand breaks during repair of UV damage in human fibroblasts and abnormalities of repair in xeroderma pigmentosum. Proc Natl Acad Sci U S A. 1976 Jan;73(1):39–43. doi: 10.1073/pnas.73.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto S., Tsuda T., Toda M., Yamagishi H. Transposon-like sequences in extrachromosomal circular DNA from mouse thymocytes. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2072–2076. doi: 10.1073/pnas.82.7.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Odijk H., Westerveld A. Differences between rodent and human cell lines in the amount of integrated DNA after transfection. Exp Cell Res. 1987 Mar;169(1):111–119. doi: 10.1016/0014-4827(87)90230-8. [DOI] [PubMed] [Google Scholar]
- Kondo S., Satoh Y., Kuroki T. Defect in UV-induced unscheduled DNA synthesis in cultured epidermal keratinocytes from xeroderma pigmentosum. Mutat Res. 1987 Jan;183(1):95–101. doi: 10.1016/0167-8817(87)90050-2. [DOI] [PubMed] [Google Scholar]
- Kraemer K. H., Lee M. M., Scotto J. Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch Dermatol. 1987 Feb;123(2):241–250. doi: 10.1001/archderm.123.2.241. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R., Kirk-Bell S., Arlett C. F., Paterson M. C., Lohman P. H., de Weerd-Kastelein E. A., Bootsma D. Xeroderma pigmentosum cells with normal levels of excision repair have a defect in DNA synthesis after UV-irradiation. Proc Natl Acad Sci U S A. 1975 Jan;72(1):219–223. doi: 10.1073/pnas.72.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayne L. V., Jones T., Dean S. W., Harcourt S. A., Lowe J. E., Priestley A., Steingrimsdottir H., Sykes H., Green M. H., Lehmann A. R. SV 40-transformed normal and DNA-repair-deficient human fibroblasts can be transfected with high frequency but retain only limited amounts of integrated DNA. Gene. 1988 Jun 15;66(1):65–76. doi: 10.1016/0378-1119(88)90225-9. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer-Pokora B., Haseltine W. A. Isolation of UV-resistant revertants from a xeroderma pigmentosum complementation group A cell line. 1984 Sep 27-Oct 3Nature. 311(5984):390–392. doi: 10.1038/311390a0. [DOI] [PubMed] [Google Scholar]
- Schultz R. A., Barbis D. P., Friedberg E. C. Studies on gene transfer and reversion to UV resistance in xeroderma pigmentosum cells. Somat Cell Mol Genet. 1985 Nov;11(6):617–624. doi: 10.1007/BF01534726. [DOI] [PubMed] [Google Scholar]
- Shows T. B., McAlpine P. J., Miller R. L. The 1983 catalogue of mapped human genetic markers and report of the Nomenclature Committee. Cytogenet Cell Genet. 1984;37(1-4):340–393. doi: 10.1159/000132014. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Sekiguchi M., Okada Y. Restoration of ultraviolet-induced unscheduled DNA synthesis of xeroderma pigmentosum cells by the concomitant treatment with bacteriophage T4 endonuclease V and HVJ (Sendai virus). Proc Natl Acad Sci U S A. 1975 Oct;72(10):4071–4075. doi: 10.1073/pnas.72.10.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielmann H. W., Edler L., Popanda O., Friemel S. Xeroderma pigmentosum patients from the Federal Republic of Germany: decrease in post-UV colony-forming ability in 30 xeroderma pigmentosum fibroblast strains is quantitatively correlated with a decrease in DNA-incising capacity. J Cancer Res Clin Oncol. 1985;109(3):227–240. doi: 10.1007/BF00390362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R. D., Robins P., Lindahl T. Complementation of the xeroderma pigmentosum DNA repair defect in cell-free extracts. Cell. 1988 Apr 8;53(1):97–106. doi: 10.1016/0092-8674(88)90491-6. [DOI] [PubMed] [Google Scholar]
- Zelle B., Lohman P. H. Repair of UV-endonuclease-susceptible sites in the 7 complementation groups of xeroderma pigmentosum A through G. Mutat Res. 1979 Sep;62(2):363–368. doi: 10.1016/0027-5107(79)90091-5. [DOI] [PubMed] [Google Scholar]
- de Jonge A. J., Vermeulen W., Keijzer W., Hoeijmakers J. H., Bootsma D. Microinjection of Micrococcus luteus UV-endonuclease restores UV-induced unscheduled DNA synthesis in cells of 9 xeroderma pigmentosum complementation groups. Mutat Res. 1985 Jun-Jul;150(1-2):99–105. doi: 10.1016/0027-5107(85)90106-x. [DOI] [PubMed] [Google Scholar]