Abstract
Evidence from well-defined cohort studies has shown that short sleep, through sleep fragmentation caused by obstructive sleep apnea (OSA) or behavioral sleep curtailment because of lifestyle choices, is associated with increased incidence of diabetes. In this report, we review epidemiologic and clinical data suggesting that OSA is involved in the pathogenesis of altered glucose metabolism. Evidence suggesting increased risk of developing diabetes resulting from curtailed sleep duration is also considered. Proposed mechanisms explaining associations between short sleep and diabetes are examined and clinical management of OSA among patients with diabetes is discussed.
Introduction
Findings from well-defined cohort studies (eg, the American Cancer Prevention Survey, the Nurses’ Health Study, the Sleep Heart Health Study, and the National Health and Nutrition Examination Survey [NHANES]) have shown that short or long sleep is associated with negative health outcomes [1–3]. Since the initial observation from the American Cancer Prevalence Survey that Americans were sleeping habitually 8 h to 9 h in 1979, self-reported sleep duration has declined gradually, with the current population modal sleep duration approximating 7 h [4, 5]. Although the population as a whole has been sleeping less [5], the number of Americans receiving a diagnosis of diabetes has dramatically increased in the same time span [6]. The concomitant decrease in sleep duration and the increased incidence of diabetes has led to the belief that short sleep (<5 h to 6 h) might be a risk factor for diabetes [7, 8]. This article examines evidence supporting associations of short sleep with diabetes and explores current approaches for managing patients with diabetes that target impaired sleep.
Prevalence of Diabetes
One of the characteristic features of diabetes mellitus is the inability to regulate serum glucose levels, resulting in impaired glucose tolerance, affecting 11% to 15.6% of the US population [6]. Research conducted to define the biochemistry of the insulin-response pathway has shown that type 1 and type 2 diabetes are associated with insulin deficiency, but type 2 diabetes is further complicated by cellular resistance to insulin action. According to data from the third NHANES, 5.1% of US adults have an existing diagnosis for diabetes; an additional 2.7% met criteria for the diagnosis, but have yet to receive one [6]. Analysis from the same research group indicated that 15.6% of American adults exhibit glucose intolerance (140 mg/dL) and 6.9% showed impaired fasting glucose levels (≥110 mg/dL) [6]. According to the National Institutes of Health, diabetes mellitus is the sixth leading cause of disease-related deaths in the United States [9].
Sleep Loss and Diabetes
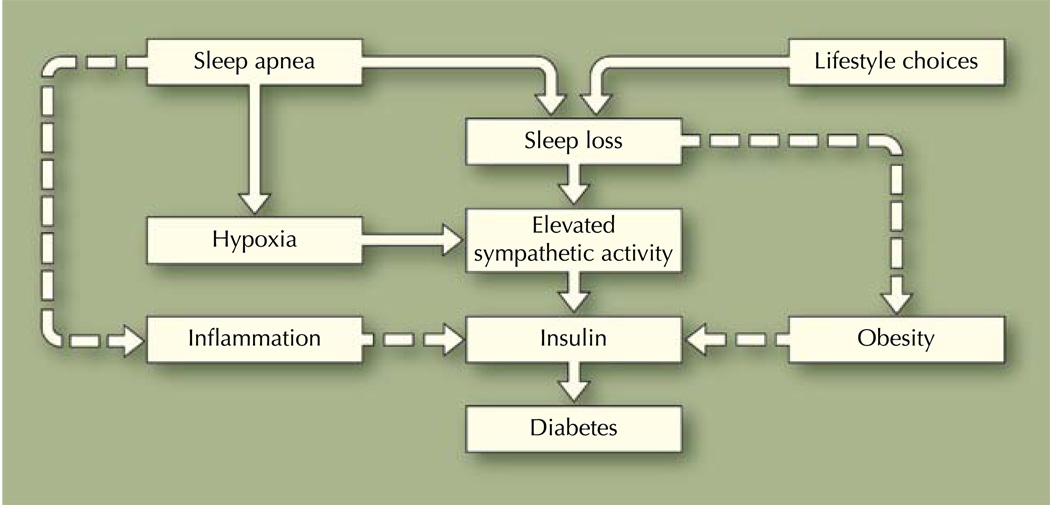
There is a growing body of evidence indicating that sleep loss, through sleep fragmentation caused by obstructive sleep apnea (OSA) or behavioral sleep curtailment because of lifestyle choices, is associated with diabetes (Fig. 1). OSA is a prevalent sleep-related breathing disorder, with estimates suggesting that 2% of middle-aged women and 4% of middle-aged men meet the criteria for a diagnosis [10, 11]. Data from the Wisconsin Sleep Cohort Study, an epidemiologic study surveying the US adult population, showed that sleep apnea affects as much as 15% of men and 5% of women between the ages of 30 years and 60 years [12]. More recent studies suggest that the number of adults at risk for OSA is increasing. According to the 2005 National Sleep Foundation survey, 26% of US adults (men, 31%; women, 21%) are at high risk for OSA [13].
Fig. 1.
Pathway linking sleep loss to insulin resistance and diabetes
OSA is characterized by recurrent apnea and hypopnea episodes resulting from partial or complete closure of the upper airway during sleep [14]. Resulting breathing pauses lead to intermittent nighttime hypoxemia, which in turn causes recurrent cortical arousals associated with sleep fragmentation, loud snoring [15], and hemodynamic changes [16, 17]. Consequences of untreated OSA include altered sleep architecture [18], cognitive deficits and poor performance [19], excessive daytime sleepiness and fatigue [20], increased cardiovascular morbidity [21–24], automobile accidents [23, 25], and excess mortality [22, 26].
OSA and Diabetes
Evidence suggests that OSA is involved in the pathogenesis of altered glucose metabolism [27, 28]. Several epidemiologic and experimental studies have shown that patients with OSA have increased glucose levels and increased insulin resistance that predispose genetically predetermined individuals to developing type 2 diabetes [8]. Cross-sectional data suggest that associations of OSA with glucose intolerance and increased insulin resistance might be independent of the presence of obesity [29], an important risk factor for OSA and diabetes [30, 31]. Obese and nonobese patients with sleep apnea are insulin resistant, whereas not all apnea patients are obese [29–31].
Investigators have proposed the following scenario to explain the underlying pathway linking OSA to insulin resistance and type 2 diabetes. Intermittent hypoxemia and sleep fragmentation caused by OSA engender a cascade of pathophysiologic events, which include autonomic activation, alterations in neuroendocrine function, and release of proinflammatory mediators (eg, tumor necrosis factor-α and interleukin-6) [8, 29]. Investigators have also shown elevated circulating levels of C-reactive protein, reactive oxygen species, and advanced glycation end products among patients with OSA [32]. In summary, OSA produces an increase in sympathetic activity [17], and increased sympathetic activity impairs glucose homeostasis by enhancing glycogen breakdown and gluconeogenesis [33]. Thus, recurrent hypoxemia along with abnormal sympathetic activity and the proinflammatory state, commonly observed among patients with OSA, might mediate the relationships between insulin resistance and OSA (Fig. 1).
OSA’s severity is determined by the apnea-hypopnea index (AHI). AHI refers to the total number of apneas (complete cessation of breathing lasting ≥10 s) and hypopneas (50% reduction in airflow lasting ≥10 s, followed by Sa02 desaturations) divided by the patient’s total sleep time; an AHI less than 5 is considered normal. Data from the Sleep Heart Health Study indicated that the prevalence of 2-h glucose tolerance increased from 9.3% among patients with an AHI less than 5–15% among those with an AHI greater than 15 [34].
Multivariate-adjusted analysis of data obtained from participants in the Wisconsin Sleep Cohort showed that the odds ratio (OR) for having a physician-diagnosed type 2 diabetes with an AHI ≥ 15 referenced to an AHI less than 5 was 2.30 [35]. The OR for developing type 2 diabetes within 4 years with an AHI ≥ 15 compared with an AHI less than 5 was 1.62 [35]. This is consistent with analysis of the Sleep Heart Health Study data showing that the OR for having an abnormal glucose tolerance was 1.44 among patients with an AHI ≥ 15 [34]. Analysis of those data also indicated that insulin resistance was also greatest among the latter group. That study provided evidence that apnea-induced hypoxemia is associated with glucose intolerance and insulin resistance [34]. Moreover, data from the Nurses’ Health Study suggest that short sleep resulting from sleep fragmentation induced by OSA may also lead to the development or exacerbation of type 2 diabetes [36].
Short Sleep Duration and Diabetes
According to data from the Sleep Heart Health Study, short sleep duration induced by behavioral restriction of bedtime may also place individuals at risk for diabetes [7]. Several large-scale studies have investigated the link of sleep duration to insulin resistance and diabetes [7, 37•, 38–40, 41••]. Analysis of data from the NHANES I indicated that individuals reporting sleep durations ≤5 h had significantly greater odds (OR, 1.47) of having incident diabetes over the 10-year follow-up period than those sleeping 7 h habitually [41••]. This is consistent with results of a 12-year follow-up investigation, which found that the relative risk of developing diabetes was higher among individuals with short sleep duration (<5 h; OR, 2.8) [39]. Data from the Sleep Heart Health Study suggest that compared with individuals sleeping 7 h to 8 h habitually, individuals sleeping ≤ 5 h or less than 6 h had adjusted ORs for diabetes of 2.51 and 1.66, respectively [42].
Although the preponderance of studies demonstrated associations of short sleep duration with diabetes, a few reports have indicated that individuals sleeping ≥ 9 h might also be at risk for diabetes (OR, 1.52) [37•, 39]. Our own analysis of data from the 2005 National Health Interview Survey, sampling over 30,000 adults of representative gender and race/ethnicity, has shown the adjusted risk of long sleep associated with diabetes was 1.48 [43]. Thus, more definitive explanation of associations of habitual sleep with insulin resistance and diabetes awaits further empirical studies.
Whether short sleep is caused by OSA or behaviorally determined, short sleepers appear to be at greater risk for insulin resistance and diabetes. Although several possible mechanisms have been advanced to explain the short-sleep diabetes link—increased sympathetic activity, increased cortisol levels resulting from elevated hypothalamic-pituitary-adrenal axis activity, and dysregulated leptin and ghrelin, which may lead to obesity and hypertension (two important markers for diabetes [37•])—an explanation for the long-sleep diabetes link is much less convincing. Results from two independent studies have led investigators to posit that long sleep observed among patients with diabetes might result from the sleep-inducing effects of diabetes itself and/or proinflammatory cytokines [37•, 41••].
Other investigators explaining the long-sleep diabetes association point to the high prevalence of depression among patients with diabetes [44, 45]. On balance, we should note that evidence also indicates that adults with depression have a 37% increased risk of developing type 2 diabetes [46]. It may be that individuals with diabetes and who are also depressed are more prone to experiencing long sleep. Whereas short sleep is believed a risk factor for diabetes, long sleep may be one of the many symptoms experienced by individuals with diabetes.
Treating OSA Among Individuals with Diabetes
Continuous positive airway pressure (CPAP) is a therapeutic method that requires the patient to wear a sealed mask over the nose, or in certain cases both the nose and mouth while sleeping. The patient receives forced room air via the mask (that has been fitted by a technician), titrating the pressure in the oropharyngeal airway, which helps to maintain airway patency; the patient is able to breathe easier, thus diminishing sleep fragmentation. Use of CPAP as a therapeutic modality for sleep apnea is often coupled with some form of behavior modification, targeting individual’s weight [47, 48]. Thus, treatment objectives include eradicating not only physiologic abnormalities including sleep fragmentation, apneic episodes, and oxygen desaturations, but also symptoms such as snoring and daytime sleepiness and reducing risk for comorbid conditions. This constitutes the standard of care according to the American Academy of Sleep Medicine [49]. CPAP is used routinely to treat OSA, leading to improvement in daytime sleepiness, improved quality of life, and improved daytime performance [49].
Intervention studies aiming to treat OSA with nasal CPAP have further demonstrated the link of short sleep (caused by OSA) to diabetes. New clinical evidence suggests that diabetes control can be achieved through treatment of OSA. In one clinical study investigating the effects of an 8-week CPAP trial, it was found that patients adhering to the prescribed regimen exhibited improved insulin sensitivity, in addition to improved blood pressure, total cholesterol, and tumor necrosis factor-α [50•]. CPAP therapy also has been shown to eliminate apneic episodes and associated hemodynamic changes occurring during sleep that might increase risk for insulin resistance and diabetes [17]. Using a 72-h continuous glucose monitoring system, another CPAP study showed a significant reduction in hemoglobin A1c level (9.2%±2.0% to 8.6%±1.8%) [51].
Conclusions
Review of the extant literature does not yield any evidence supporting a cause-and-effect relationship between habitual sleep and diabetes. However, data suggest that OSA is independently associated with altered glucose metabolism and may predispose genetically predisposed individuals to developing type 2 diabetes. If causal associations between OSA and impaired glucose metabolism can be shown, this might offer another mechanism to explain increased cardiovascular morbidity. It seems clear at this point that CPAP therapy holds great promise in enhancing diabetes control. CPAP studies have shown significant improvement in insulin sensitivity, blood pressure, lipid profile, and left ventricular function [8, 17, 51]. CPAP therapy can also normalize leptin and ghrelin levels [52], thereby reducing central obesity, an important risk factor of type 2 diabetes.
Some studies have not shown significant improvement in metabolic disorders subsequent to CPAP treatment, but those clinical studies had several limitations [53]. These include limited treatment compliance, inadequate duration of treatment, and some may have been underpowered to detect statistical effects. In light of such evidence, therapeutic approaches might integrate methods to increase sleep time via reduction of OSA severity and through lifestyle modifications. Synergistically, these therapeutic strategies may confer significant benefits in managing cardiovascular complications, resulting from uncontrolled diabetes and/or untreated sleep apnea.
Acknowledgments
This research was supported by funding from the National Institutes of Health/National Center on Minority Health and Health Disparities (R01MD004113, P20MD005092).
Footnotes
Disclosure No other potential conflicts of interest relevant to this article were reported.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Kripke DF, Garfinkel L, Wingard DL, et al. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 2.Patel SR, Ayas NT, Malhotra MR, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–444. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 3.Tamakoshi A, Ohno Y. Self-reported sleep duration as a predictor of all-cause mortality: results from the JACC study, Japan. Sleep. 2004;27:51–54. [PubMed] [Google Scholar]
- 4.National Sleep Foundation. Sleep in American Poll. Washington, DC: National Sleep Foundation; 2008. [Google Scholar]
- 5.Jean-Louis G, Kripke DF, Ancoli-Israel S, et al. Sleep duration, illumination, and activity patterns in a population sample: effects of gender and ethnicity. Biol Psychiatry. 2000;47:921–927. doi: 10.1016/s0006-3223(99)00169-9. [DOI] [PubMed] [Google Scholar]
- 6.Harris MI. Diabetes in America: epidemiology and scope of the problem. Diabetes Care. 1998;21 Suppl 3:C11–C14. doi: 10.2337/diacare.21.3.c11. [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165:863–867. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 8.Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. Association of sleep time with diabetes mellitus and impaired glucose tolerance. J Appl Physiol. 2005;99:1998–2007. doi: 10.1152/japplphysiol.00695.2005. [DOI] [PubMed] [Google Scholar]
- 9.Jemal A, Ward E, Hao Y, Thun M. Trends in the leading causes of death in the United States, 1970–2002. JAMA. 2005;294:1255–1259. doi: 10.1001/jama.294.10.1255. [DOI] [PubMed] [Google Scholar]
- 10.Young T, Finn L. Epidemiological insights into the public health burden of sleep disordered breathing: sex differences in survival among sleep clinic patients. Thorax. 1998;53 Suppl 3:S16–S19. doi: 10.1136/thx.53.2008.s16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young T, Peppard P. Sleep-disordered breathing and cardiovascular disease: epidemiologic evidence for a relationship. Sleep. 2000;23 Suppl 4:S122–S126. [PubMed] [Google Scholar]
- 12.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746–1752. [PubMed] [Google Scholar]
- 13.Hiestand DM, Britz P, Goldman M, et al. Prevalence of symptoms and risk of sleep apnea in the US population: results from the national sleep foundation sleep in America 2005 poll. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Chest. 2006;130:780–786. doi: 10.1378/chest.130.3.780. [DOI] [PubMed] [Google Scholar]
- 14.Hudgel DW. Mechanisms of obstructive sleep apnea. Chest. 1992;101:541–549. doi: 10.1378/chest.101.2.541. [DOI] [PubMed] [Google Scholar]
- 15.Patil SP, Schneider H, Schwartz AR, Smith PL. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest. 2007;132:325–337. doi: 10.1378/chest.07-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinto JM, Garpestad E, Weiss JW, et al. Hemodynamic changes associated with obstructive sleep apnea followed by arousal in a porcine model. J Appl Physiol. 1993;75:1439–1443. doi: 10.1152/jappl.1993.75.4.1439. [DOI] [PubMed] [Google Scholar]
- 17.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 19.El Ad B, Lavie P. Effect of sleep apnea on cognition and mood. Int Rev Psychiatry. 2005;17:277–282. doi: 10.1080/09540260500104508. [DOI] [PubMed] [Google Scholar]
- 20.Guilleminault C, Stoohs R, Clerk A, et al. A cause of excessive daytime sleepiness. The upper airway resistance syndrome. Chest. 1993;104:781–787. doi: 10.1378/chest.104.3.781. [DOI] [PubMed] [Google Scholar]
- 21.Kiely JL, McNicholas WT, Zgierska A, et al. Cardiovascular risk factors in patients with obstructive sleep apnoea syndrome. Eur Respir J. 2000;16:128–133. doi: 10.1034/j.1399-3003.2000.16a23.x. [DOI] [PubMed] [Google Scholar]
- 22.Wright J, Johns R, Watt I, et al. Health effects of obstructive sleep apnoea and the effectiveness of continuous positive airways pressure: a systematic review of the research evidence. BMJ. 1997;314:851–860. doi: 10.1136/bmj.314.7084.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Findley LJ, Weiss JW, Jabour ER. Drivers with untreated sleep apnea. A cause of death and serious injury. Arch Intern Med. 1991;151:1451–1452. [PubMed] [Google Scholar]
- 24.Newman AB, Nieto FJ, Guidry U, et al. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am J Epidemiol. 2001;154:50–59. doi: 10.1093/aje/154.1.50. [DOI] [PubMed] [Google Scholar]
- 25.Stoohs RA, Guilleminault C, Itoi A, Dement WC. Traffic accidents in commercial long-haul truck drivers: the influence of sleep-disordered breathing and obesity. Sleep. 1994;17:619–623. [PubMed] [Google Scholar]
- 26.Lavie P, Herer P, Peled R, et al. Mortality in sleep apnea patients: a multivariate analysis of risk factors. Sleep. 1995;18:149–157. doi: 10.1093/sleep/18.3.149. [DOI] [PubMed] [Google Scholar]
- 27.Spiegel R, Knudtson K, Leproult R, et al. Sleep loss: a novel risk factor for insulin resistance and type II diabetes. J Appl Physiol. 2005;99:2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 28.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 29.Punjabi NM, Sorkin JD, Katzel LI, et al. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165:677–682. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 30.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–1158. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 31.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–1599. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 32.Shamsuzzaman AS, Winnicki M, Lanfranchi P, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–2464. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 33.Punjabi NM, Ahmed MM, Polotsky VY, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance. Respir Physiol Neurobiol. 2003;136:167–178. doi: 10.1016/s1569-9048(03)00079-x. [DOI] [PubMed] [Google Scholar]
- 34.Punjabi NM, Shahar E, Redline S, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 35.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172:1590–1595. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ayas NT, White DP, Al Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–384. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 37. Beihl DA, Liese AD, Haffner SM. Sleep duration as a risk factor for incident type 2 diabetes in a multiethnic cohort. Ann Epidemiol. 2009;19:351–357. doi: 10.1016/j.annepidem.2008.12.001. This is a study that assessed associations between sleep duration and type 2 diabetes, considering insulin sensitivity and secretion, two important risk factors for diabetes.
- 38.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 39.Mallon L, Broman JE, Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care. 2005;28:2762–2767. doi: 10.2337/diacare.28.11.2762. [DOI] [PubMed] [Google Scholar]
- 40.Nilsson PM, Roost M, Engstrom G, et al. Incidence of diabetes in middle-aged men is related to sleep disturbances. Diabetes Care. 2004;27:2464–2469. doi: 10.2337/diacare.27.10.2464. [DOI] [PubMed] [Google Scholar]
- 41. Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30:1667–1673. doi: 10.1093/sleep/30.12.1667. This study explored relationships between sleep duration and diabetes incidence over an 8- to 10-year follow-up period in data from the First National Health and Nutrition Examination Survey.
- 42.Sanders MH, Givelber R. Sleep disordered breathing may not be an independent risk factor for diabetes, but diabetes may contribute to the occurrence of periodic breathing in sleep. Sleep Med. 2003;4:349–350. doi: 10.1016/s1389-9457(03)00118-7. [DOI] [PubMed] [Google Scholar]
- 43.Zizi F, Jean-Louis G, Fernandez S, et al. Sleep duration and risk of diabetes: analysis of the National Health Interview Survey. Sleep. 2009;32:152. [abstract] [Google Scholar]
- 44.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31:2383–2390. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 46.Knol MJ, Twisk JW, Beekman AT, et al. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49:837–845. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- 47.Edinger JD, Hoelscher TJ, Marsh GR, et al. A cognitive-behavioral therapy for sleep-maintenance insomnia in older adults. Psychol Aging. 1992;7:282–289. doi: 10.1037//0882-7974.7.2.282. [DOI] [PubMed] [Google Scholar]
- 48.Edinger JD, Carwile S, Miller P, et al. Psychological status, syndromatic measures, and compliance with nasal CPAP therapy for sleep apnea. Percept Mot Skills. 1994;78:1116–1118. doi: 10.2466/pms.1994.78.3c.1116. [DOI] [PubMed] [Google Scholar]
- 49. [Accessed August 24, 2002];The American Academy of Sleep Medicine: Practice Parameters. Available at http://www.aasmnet.org/PracticeParameters.aspx.
- 50. Dorkova Z, Petrasova D, Molcanyiova A, et al. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest. 2008;134:686–692. doi: 10.1378/chest.08-0556. This study examined the effects of 8 weeks of continuous positive airway pressure therapy on glucose and lipid profile, systemic inflammation, oxidative stress, and global cardiovascular disease risk among patients with OSA and metabolic syndrome.
- 51.Babu AR, Herdegen J, Fogelfeld L, et al. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005;165:447–452. doi: 10.1001/archinte.165.4.447. [DOI] [PubMed] [Google Scholar]
- 52.McNicholas WT, Javaheri S. Pathophysiologic mechanisms of cardiovascular disease in obstructive sleep apnea. Sleep Med Clin. 2007;2:539–547. [Google Scholar]
- 53.Jean-Louis G, Zizi F, Clark LT, Kossotis J. Associations of sleep apnea to cardiovascular diseases: role of diabetes and metabolic syndrome. In: Clark LT, editor. In Cardiovascular Disease and Diabetes: Contemporary Management. New York: McGraw-Hill; 2007. pp. 472–505. [Google Scholar]