Introduction
This protocol can be used for analysis of protein expression during Ae. aegypti development. Embryos, larvae, and pupae that have been prepared as described in the accompanying tissue preparation protocol can be stained using this technique. This methodology will aid in the analysis of Ae. aegypti developmental proteins of interest. It will also be critical for the development of markers for particular developing tissues.
Materials
Equipment
Dissecting microscope
Microfuge tubes
Micropipetter
Transfer pipets
Timer
Reagents
Antibodies (primary and secondary)
Blocking Solution (5% NGS in PT)
DAB (with or without Ni)
Glycerol (50% and 70%)
Hydrogen peroxide
Methanol
PBS <R>
Primary antibody
PT <R>
SDS
Tissues (dissected embryos, sonicated/dissected larvae or pupae)
Method
Rehydration
-
1.
Obtain embryos, larvae, or pupae that were previously fixed and prepared for immunohistochemical analysis. The tissue can remain in microfuge tubes for the duration of the experiment. Unless otherwise indicated, use 1 mL rinse/wash volumes throughout this procedure. If the tissue has been stored in methanol in the freezer, remove the methanol and rehydrate the tissue with 50% methanol / 50% PBS for 5 min. Proceed with a 5 min rinse in PBS and 2× 10 min rinses in PT.
Primary Antibody Reaction
-
2.
Wash the tissue 3X 10 min with PT. Specifically for pupae, first wash the tissue with PT for 5 min, followed by a 30 min incubation in PT + 0.3% SDS at room temperature.
-
3.
Incubate the animals in PT-NGS for 30 min at room temperature.
-
4.
Remove the PT-NGS, add 100 μL of primary antibody (diluted to the appropriate concentration in PT-NGS), and place at 4 °C overnight (no more than 16 hrs).
Secondary Antibody Reaction
-
5.
Rinse the tissue through a series of PT washes (3X 5 min, then 4X 30 min). Tubes should be rotated during the wash steps.
-
6.
Incubate the tissue in PT-NGS for 30 min at room temperature.
-
7.
Remove the PT-NGS and add 200 μL of the secondary antibody diluted to the appropriate concentration in PT-NGS. For robust antibodies, it may be possible to leave the secondary on for 2 hrs. at room temperature. However, we typically leave the secondary antibody on the tissue at 4 °C overnight (no more than 16 hrs).
Color reaction
-
8.
Rinse the tissue through a series of PT washes (3X 5 min, then 4X 30 min).
-
9.
If you are using fluorescent secondary antibodies, you can proceed to step 12. If you are using HRP-conjugated secondaries, proceed as follows: Add 300 μl of DAB (with Ni for a black reaction product, without Ni for brown). Mix gently and allow the tube to stand for 5 min.
-
10.
To begin the reaction, add 30 μL of 0.3% H2O2 to each tube. Gently mix the solution with the pipette tip. Observe the reaction under a dissecting microscope to monitor the color change.
-
11.
Stop the reaction by removing the DAB and continuing with a series of 3× 5 min washes with PT. NOTE: DAB waste must be disposed of properly.
-
12.
Wash 2 × 2 min with PBS.
-
13.
Replace the last PBS wash with 50% glycerol. Allow the tissue to clear in 50% glycerol (minimally) overnight. Replace the glycerol with 70% glycerol the following day. Tissues can be stored and mounted in 70% glycerol. Examples of embryos that have been processed as described are shown in Fig. 5.
Troubleshooting
Problem: poor staining of tissues (step 10); Solutions: If tissues are under- or over-stained, the concentration of the primary or secondary antibodies may need to be adjusted. Also, be certain that tissues have not become desiccated or stuck to the sides of the tube during the procedure, either of which can lead to poor staining. Please see Patel (1994) for an excellent comprehensive discussion of troubleshooting immunohistochemistry experiments in flies; many of his suggestions can be applied to staining mosquitoes.
Discussion
This protocol is an adaptation of Patel's (1994) Drosophila immunohistochemical methodology which has been tailored for staining Ae. aegypti. Modifications of the basic protocol are required for successful staining of a variety of developmental stages (embryos, larvae, or pupae), and these modifications are noted at appropriate steps in the procedure. For example, staining is enhanced by extended incubation with the secondary antibody (step 7), and in the case of pupae, pre-treatment with detergent (step 2). The approach described here is robust and has yielded excellent results in Ae. aegypti (Simanton et al., 2009).
Figure. Protein expression analysis in Ae. aegypti during embryonic development.
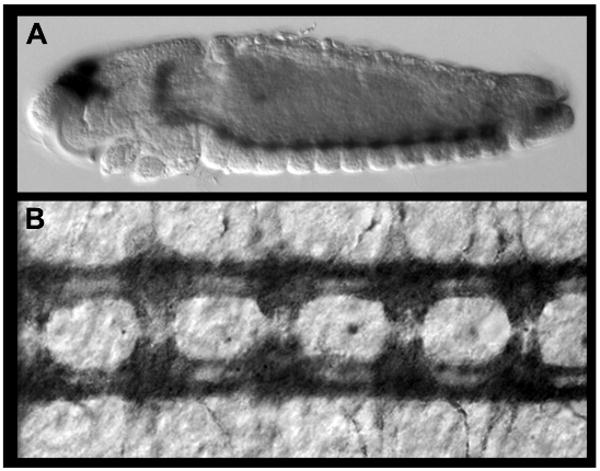
(A,B) Acetylated tubulin expression labels the axons of the developing nerve cord. A lateral view of a 55 hr whole-mount embryo stained with the accompanying protocol is shown in A (anterior is oriented left). A filleted nerve cord from a 55 hr embryo is shown in B (anterior is oriented up).
Acknowledgments
Development of the protocol described was funded by the following awards to MDS: NIH/NIAID Award R01 AI 081795-01 and NIH/NINDS Award R15 NS 048904-0.
Recipes
DAB and DAB-NI Solutions
| Reagent | Quantity (for 33 ml) | Final concentration |
|---|---|---|
| 1× PBS | 33 ml | 1× |
| Tween-20 | 16.5 μl | 0.05% |
| 3,3′-Diaminobenzidine (DAB) | 10 mg | 0.3 mg/ml |
Add one 10 mg DAB tablet (Sigma Cat. D-5905) to PBS and Tween-20 in a 50 ml tube. Rock in the dark for 30 min. Filter through a 0.22 μm filter to remove particulate matter and store in 1 ml aliquots at -20° C. Aliquots should be used immediately after thawing and should not be refrozen. Use of this DAB mixture will yield a brown color reaction product. For a black reaction product, thaw DAB and then add 8 μl of 8% nickel chloride per 1 ml DAB aliquot. Mix well and use immediately.
Glycerol Solution
50% and 70% glycerol solutions can be prepared by mixing the appropriate volumes of ultrapure glycerol with 1× PBS. Place the solution on a rocker at room temperature for 30 min to ensure thorough mixing of PBS and glycerol. Check the final pH to be sure that it is near 7.4. Store at room temperature.
PBS (10× stock)
| Reagent | Quantity (for 1 L) | Final concentration |
|---|---|---|
| Na2HPO4 | 11.9 g | 84.1 mM |
| NaH2PO4 (anhydrous) | 2.23 g | 18.6 mM |
| NaCl | 102.2 g | 1.75 M |
Bring the volume to 1 L with distilled water. Adjust the pH to 7.4 and autoclave before use. Prepare the working strength solution (1×, simply referred to as PBS in the protocol) by diluting 1:10 with sterile dH20. Both 1× and 10× PBS are stored at room temperature.
PT
| Reagent | Quantity (for 1 L) | Final concentration |
|---|---|---|
| 10× PBS | 100 ml | 1× |
| 100% Triton X-100 | 1 ml | 0.1% |
Bring final volume to 1 L with sterile dH20. Mix and store at room temperature.
PT-NGS
Heat inactivate NGS at 56°C for 30 min and sterilize by filtering through a 0.22 μm filter while the NGS is still warm. Store in 1 ml aliquots at -20° C. After thawing, the aliquots are stable for several months at 4° C. To make PT-NGS, mix 950 μl of PT (see above) with 50 μl of NGS. Store at 4° C for no more than a week. Discard if the solution is cloudy.
Footnotes
Conflicts of interest: none declared
References
- Patel NH. Imaging neuronal subsets and other cell types in whole-mount Drosophila embryos and larvae using antibody probes. Methods Cell Biol. 1994;44:445–487. doi: 10.1016/s0091-679x(08)60927-9. [DOI] [PubMed] [Google Scholar]
- Simanton W, Clark S, Clemons A, Jacowski C, Farrell-VanZomeren A, Beach P, Browne WE, Duman-Scheel M. Conservation of arthropod midline netrin accumulation revealed with a cross-reactive antibody provides evidence for midline cell homology. Evol Dev. 2009;11:260–268. doi: 10.1111/j.1525-142X.2009.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


