Abstract
We report that N-4-nosyl-3-phenyloxaziridine is an effective terminal oxidant for copper(II)-catalyzed oxyamination recently developed in our labs. This oxaziridine can be prepared on multi-gram scale and is easily purified by recrystallization. The products of oxyamination using this oxaziridine bear protecting groups that can be readily removed in high yields under mild conditions.
Keywords: Oxaziridines, Oxyamination, Copper, Amino alcohols
A great variety of natural products, bioactive small molecules, and chiral reagents for synthesis feature vicinal aminoalcohols as an important structural element.i Among the most straightforward methods for the construction of this substitution pattern is the Sharpless aminohydroxylation, which utilizes an osmium catalyst to effect the vicinal difunctionalization of simple alkenes.ii Over the past several years, there has been increasing interest in the development of complementary, osmium-free methods to effect the same transformation.iii
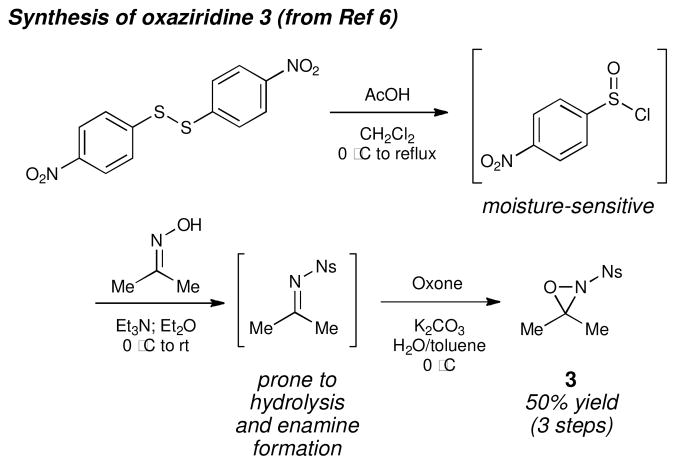
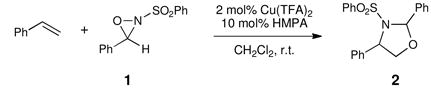
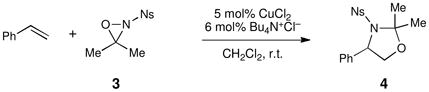
Our group has been developing a novel copper(II)-catalyzed olefin oxyamination reaction that uses N-sulfonyl oxaziridines as the terminal oxidant.iv We originally reported oxyaminations utilizing N-benzenesulfonyl 3-phenyloxaziridine 1v (i.e., Davis’ oxaziridine). The oxyaminated products resulting from this transformation (2, eq 1) bear N-benzenesulfonyl groups that require relatively harsh reducing conditions for their removal. More recently, we reported conditions that would enable N-4-nosyl-3,3-dimethyloxaziridine 3 to be utilized as the terminal oxidant.vi This oxaziridine produces oxyamination products bearing a more easily cleaved protecting group on nitrogen; however, given the sensitivity of the intermediate imine, the synthesis of 3 is relatively technically demanding and gives somewhat moderate yields (Figure 1).vii
Figure 1.
Synthesis of oxaziridine 3.
Ref 4:
 |
(1) |
Ref 6:
 |
(2) |
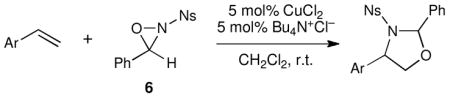
Thus, we wondered whether we could perform copper(II)-catalyzed oxyamination reactions using oxaziridines that (1) bear easily removable N-sulfonyl protecting groups than the benzenesulfonyl group of oxaziridine 1 and (2) are synthesized using simpler and higher-yielding protocols than oxaziridine 3. We thus became interested in exploring the reactivity of N-tertbutylsulfonyl 3-phenyloxaziridine 5 and N-p-nosyl 3-phenyloxaziridine 6 (Figure 2). We expected that the oxyamination products arising from reactions using these oxaziridines would bear N-protecting groups that have been reported to be removable under acid-catalyzedviii and nucleophilicix conditions, respectively.
Figure 2.

3-Phenyloxaziridines with varied N-sulfonyl groups. Bus = t-BuSO2; Ns = 4-NO2PhSO2.
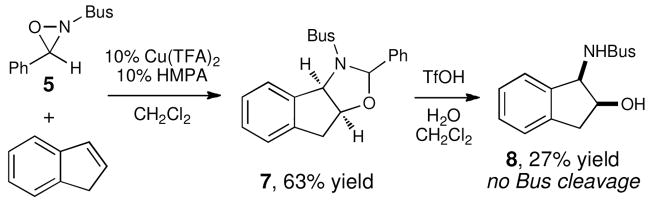
At the outset of this study, the syntheses of 5 and 6 had previously been reported by García Ruano and coworkers.x First, we used this method to prepare 5 and then assessed its utility in the oxyamination. Reactions using 5 proved to be considerably slower than the corresponding reactions using the less sterically demanding oxaziridine 1. Using 10 mol% Cu(TFA)2, we were able to react indene with 5 and isolate 78% yield of the desired oxyamination product 7 after 6 h (Scheme 1). Unfortunately, deprotection of the product to afford the free amino alcohol proved to be problematic. When 7 was subjected to a variety of acidic conditions, the benzylidene aminal could be easily removed, but the N-t-butylsulfonyl moiety remained intact. Attempts using forcing conditions led only to unproductive decomposition.
Scheme 1.
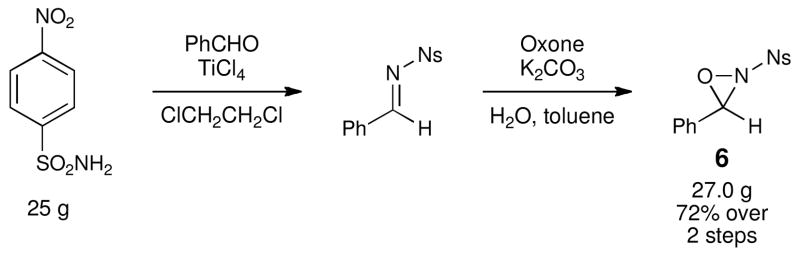
Thus, we next turned our attention to oxyaminations using N-nosyl oxaziridine 6. However, García Ruano had reported that the yield of 6 using his protocol was rather modest (57%), and we were unable to improve the yield further upon extensive optimization. Thus, we performed a straightforward modification of the procedure reported by Davisv for preparation of 1 (Scheme 2). Lewis acid catalyzed condensation of 4-nitrobenzenesulfonamide with benzaldehyde affords a crystalline imine intermediate, which can be oxidized by treatment with aqueous Oxone.xi This two-step procedure is high-yielding, reliable, operationally facile, and easily performed on multigram scale without purification of the intermediate. The oxaziridine is a crystalline air- and moisture-stable solid, samples of which we have been able to store in a refrigerator for months without noticeable decomposition.
Scheme 2.
We probed the copper(II)-catalyzed oxyaminations of a variety of styrenes using oxaziridine 6 as the terminal oxidant; the results of these experiments are summarized in Table 1. In general, we found that the reactions using 6 provided slightly lower yields than the corresponding oxyamination reactions using 1 or 3 under identical conditions. Nevertheless, we found that the tolerance of substitution on the styrene substrate to be essentially the same.
Table 1.
Oxyaminations of styrenes using 6.a
 | ||||
|---|---|---|---|---|
| entry | styrene | product | time | yield |
| 1 | 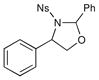 |
3 h | 75% | |
| 2 | 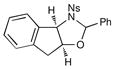 |
45 min | 52% | |
| 3 | 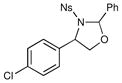 |
3 h | 54% | |
| 4 | 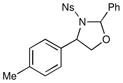 |
1 h | 70% | |
| 5 | 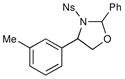 |
3 h | 75% | |
| 6 | 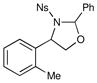 |
2.5 h | 65% | |
| 7 | 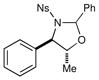 |
1 h | 84% | |
| 8 | 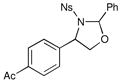 |
1.5 h | 59% | |
Conditions: 1 equiv of styrene, 1.5 equiv of 6, 5 mol% CuCl2, 5 mol% Bu4N+Cl−, CH2Cl2 (0.2 M in styrene), ambient temperature.
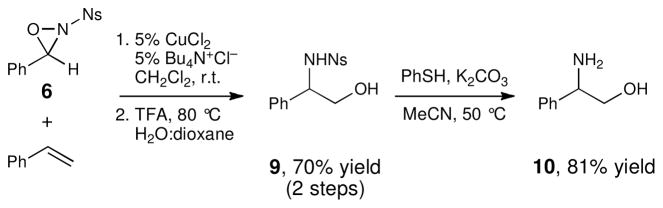
In contrast to the N-t-butylsulfonyl group, we found that removal of the 4-nosyl group proceeded easily under the nucleophilic conditions reported by Fukuyama and coworkers. Our optimized process for the oxyamination–deprotection sequence is outlined in Scheme 3. First, styrene was subjected to oxyamination in the presence of 6, 5 mol% CuCl2, and 5 mol% Bu4N+Cl−, and the benzylidene aminal of the crude product was cleaved in good yield upon acid hydrolysis. Next, the sulfonyl group was removed using PhSH and K2CO3. This deprotection procedure is high yielding and is milder and operationally more convenient than the strongly reducing conditions required for the removal of the N-benzenesulfonyl group. The amino alcohol product could be easily isolated in analytically pure form by standard chromatography techniques.
Scheme 3.
Thus, we have discovered that oxaziridine 5 is a useful alternate terminal oxidant for copper(II)-catalyzed oxyamination of olefins. It is easily synthesized on multi-gram scale in high yield, purified by recrystallization, and stored for long periods at low temperature. Oxyamination reactions using 5 produce aminal products that can be easily deprotected under mild conditions to reveal the free amino alcohol. This protocol should therefore serve as a useful alternative to the methods we have previously reported.
Acknowledgments
Financial support for this research was provided by the NIH (R01-GM084022), the NSF (CHE-0645447), and the University of Wisconsin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- i.Bergmeier SC. Tetrahedron. 2000;56:2561–2571. [Google Scholar]
- ii.For reviews of the scope of the Sharpless asymmetric aminohydroxylation and its use in synthesis, see: O’Brien P. Angew Chem Int Ed. 1999;38:326–329. doi: 10.1002/(SICI)1521-3773(19990201)38:3<326::AID-ANIE326>3.0.CO;2-T.Nilov D, Reiser O. Adv Synth Catal. 2002;344:1169–1173.Bodkin JA, McLeod MD. J Chem Soc, Perkin Trans 1. 2002:2733–2746.
- iii.(a) Noack M, Göttlich R. Chem Commun. 2002:536–537. doi: 10.1039/b111656h. [DOI] [PubMed] [Google Scholar]; (b) Chikkanna D, Han H. Synlett. 2004:2311–2314. [Google Scholar]; (c) Mahoney JM, Smith CR, Johnston JN. J Am Chem Soc. 2005;127:1354–1355. doi: 10.1021/ja045608c. [DOI] [PubMed] [Google Scholar]; (d) Alexanian EJ, Lee C, Sorensen EJ. J Am Chem Soc. 2005;127:7690–7691. doi: 10.1021/ja051406k. [DOI] [PubMed] [Google Scholar]; (e) Szolcsányi P, Gracza T. Chem Commun. 2005:3948–3950. doi: 10.1039/b506731f. [DOI] [PubMed] [Google Scholar]; (f) Correa A, Tellitu I, Domínguez E, SanMartin R. J Org Chem. 2006;71:8316–8319. doi: 10.1021/jo061486q. [DOI] [PubMed] [Google Scholar]; (g) Liu G, Stahl SS. J Am Chem Soc. 2006;128:7179–7181. doi: 10.1021/ja061706h. [DOI] [PubMed] [Google Scholar]; (h) Schultz MJ, Sigman MS. J Am Chem Soc. 2006;128:1460–1461. doi: 10.1021/ja0579053. [DOI] [PubMed] [Google Scholar]; (i) Desai LV, Sanford MS. Angew Chem Int Ed. 2007;46:5737–5740. doi: 10.1002/anie.200701454. [DOI] [PubMed] [Google Scholar]; (j) Cochran BM, Michael FE. Org Lett. 2008;10:5039–5042. doi: 10.1021/ol8022165. [DOI] [PubMed] [Google Scholar]; (k) Beaumont S, Pons V, Retailleau P, Dodd RH, Dauban P. Angew Chem Int Ed. 2010;49:1634–1637. doi: 10.1002/anie.200906650. [DOI] [PubMed] [Google Scholar]; (l) Lovick HM, Michael FE. J Am Chem Soc. 2010;132:1249–1251. doi: 10.1021/ja906648w. [DOI] [PubMed] [Google Scholar]
- iv.(a) Michaelis DJ, Shaffer CJ, Yoon TP. J Am Chem Soc. 2007;129:1866–1867. doi: 10.1021/ja067894t. [DOI] [PubMed] [Google Scholar]; (b) Michaelis DJ, Ischay MA, Yoon TP. J Am Chem Soc. 2008;130:6610–6615. doi: 10.1021/ja800495r. [DOI] [PubMed] [Google Scholar]
- v.(a) Davis FA, Nadir U, Kluger EW. J Chem Soc Chem Comm. 1977:25–26. [Google Scholar]; (b) Davis FA, Lamendola J, Nadir U, Kluger EW, Sedergran TC, Panunto TW, Billmers R, Jenkins R, Turchi IJ, Watson WH, Chen JS, Kimura M. J Am Chem Soc. 1980;102:2000–2005. [Google Scholar]
- vi.Benkovics T, Du J, Guzei I, Yoon TP. J Org Chem. 2009;74:5545–5552. doi: 10.1021/jo900902k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vii.Our synthesis of 3 was adapted from: Jennings WB, Watson SP, Boyd DR. J Chem Soc, Chem Commun. 1988:931–932.
- viii.Sun P, Weinreb SM, Shang M. J Org Chem. 1997;62:8604–8608. doi: 10.1021/jo971455i. [DOI] [PubMed] [Google Scholar]
- ix.(a) Fukuyama T, Jow CK, Cheung M. Tetrahedron Lett. 1995;36:6373–6374. [Google Scholar]; (b) Maligres PE, See MM, Askin D, Reider PJ. Tetrahedron Lett. 1997;38:5253–5256. [Google Scholar]
- x.García Ruano JL, Alemán J, Fajardo C, Parra A. Org Lett. 2005;7:5493–5496. doi: 10.1021/ol052250w. [DOI] [PubMed] [Google Scholar]
- xi.We have now reported a detailed procedure for the gram-sacle synthesis of 6 in the context of a related study: Partridge KM, Anzovino ME, Yoon TP. J Am Chem Soc. 2008;130:2920–2921. doi: 10.1021/ja711335d.