Abstract
Herein an efficient and direct copper-catalyzed coupling of oxazoline-containing aryl bromides with electron-deficient secondary phosphine oxides is reported. The resulting tertiary phosphine oxides can be reduced to prepare a range of PHOX ligands. The presented strategy is a useful alternative to known methods for constructing PHOX derivatives.
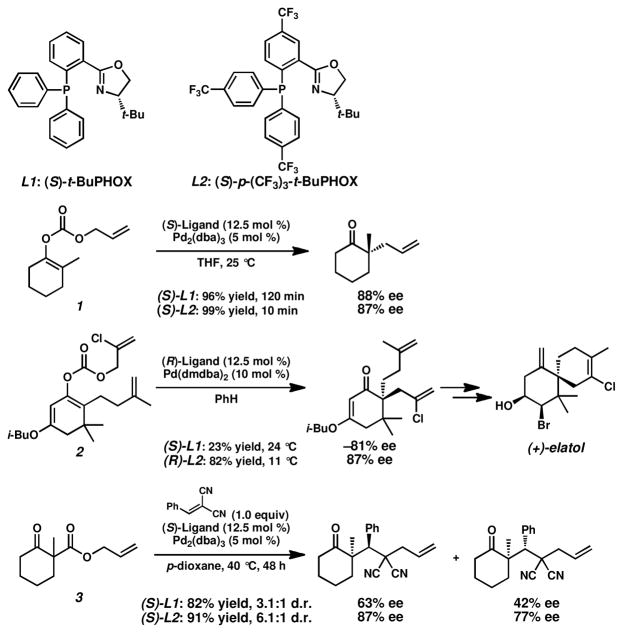
Phosphinooxazoline (PHOX) ligands have found broad applications in transition metal catalysis.1 Developed by Pfaltz,2 Helmchen,3 and Williams,4 PHOX ligands have become a preeminent class of P,N-ligands,5 with t-BuPHOX (L1, Scheme 1) representing a most prominent example.6 We have recently demonstrated the utility of t-BuPHOX in palladium-catalyzed enantioselective decarboxylative alkylation7 and protonation8 reactions. We, however, became aware of examples where t-BuPHOX provided only moderate results with respect to yields and enantioselectivities, and designed an electronically-modified version of this ligand, p-(CF3)3-t-BuPHOX (L2). In some cases, the electron-withdrawing trifluoromethyl groups affected the reactivity of the corresponding transition metal complex, leading to significantly shorter reaction times and enhanced selectivities. For example, we were able to achieve 99% yield and 87% ee in our palladium-catalyzed, enantioselective allylic alkylation reaction of allyl enol carbonate 1 within only 10 min at 25 °C with the use of (S)-L2, while the use of (S)-L1 required 120 min reaction time to give 96% yield and 88% ee.9 Further, this ligand was successfully applied in the catalytic asymmetric total synthesis of (+)-elatol where the key allylic alkylation of chloroallyl enol carbonate 2 was performed with (R)-L2, resulting in 82% yield of product in 87% ee, compared to only 81% ee and a poor 23% yield with the use of (S)-L1.10 Moreover, we recently published a palladium-catalyzed, enantioselective enolate alkylation cascade, which provides products with up to 99% enantiomeric excess,11 where (S)-p-(CF3)3-t-BuPHOX was far superior to (S)-t-BuPHOX for the alkylation of β-keto ester 3.
Scheme 1.
PHOX ligands and their use in synthesis and methodology development.
Previously, we published a convenient and scalable synthesis for t-BuPHOX,12 using an Ullmann-type coupling developed by Buchwald.13 While this approach proved useful for the coupling aryl halides and secondary phosphines, most substituted secondary phosphines are not commercially available. Similarly, substituted secondary phosphines (e.g., bis(4-(trifluoromethyl)phenyl)phosphine) are difficult to prepare in the required purity due to their propensity to oxidize upon exposure to air.14 Although the preparation of synthetically challenging PHOX variants was possible using our previously described conditions, a more efficient and higher yielding protocol was desired. Therefore a synthetic strategy for the synthesis of p-(CF3)3-t-BuPHOX (L2) that avoids phosphine intermediates was needed. We envisioned a preparative route toward this electron-deficient PHOX ligand in which an oxazoline-containing aryl bromide is joined directly with a secondary phosphine oxide.15,16 Herein, we demonstrate a copper-catalyzed coupling of aryl halides to secondary phosphine oxides for the synthesis of electron-deficient PHOX ligands.
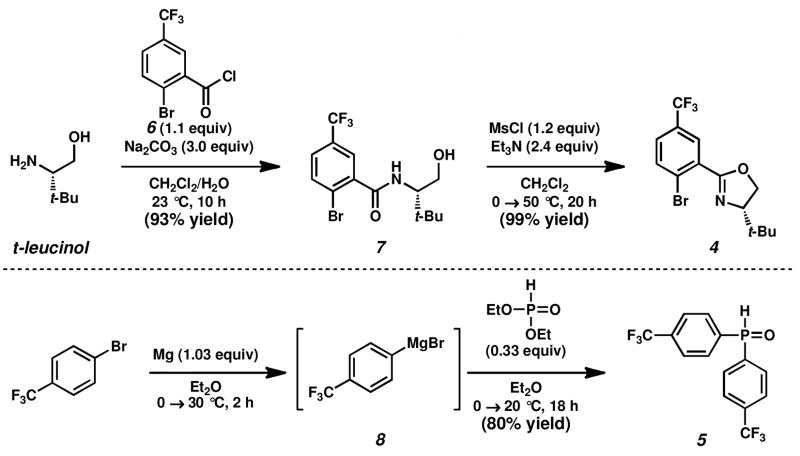
Oxazoline-containing aryl bromide 4 and secondary phosphine oxide 5 can be readily synthesized on multi-gram scale (Scheme 2). Aryl bromide 4 was prepared using modified conditions from a published route,10 requiring only one purification by flash chromatography. The treatment of (S)-t-leucinol with acid chloride 67 in the presence of sodium carbonate provided amide 7 in 93% yield.17 Subsequent mesylation of the free hydroxyl of 7, followed by in situ mesylate displacement results in formation of oxazoline 4 in 99% yield.18 Bis(4-(trifluoromethyl)phenyl)phosphine oxide 519 is produced in 80% yield by careful exposure of 4-(trifluoromethyl)phenylmagnesium bromide 8, synthesized via the Leazer method,20 to diethyl phosphite.21 Unlike the related secondary phosphine, phosphine oxide 5 can be purified by column chromatography and is stable to air at room temperature for several months.
Scheme 2.
Synthesis of oxazoline-containing aryl bromide 4 and secondary phosphine oxide 5.
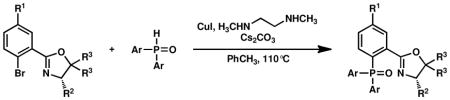
Our modification to Buchwald’s copper iodide-catalyzed conditions for the coupling of secondary phosphines with aryl bromides9,12 was tested for the coupling of secondary phosphine oxide 5 with oxazolinyl aryl bromide 4 (Table 1, entries 1 and 2). Gratifyingly, when a catalytic amount of CuI (12.5 mol %) was used in combination with N,N′-dimethylethylenediamine as ligand and Cs2CO3 as base, secondary phosphine oxide 5 could be successfully coupled with aryl bromide 4 to produce 9 in 57 % yield (entry 1). Reaction times could be reduced, and product yields improved to 65% yield with the use of a stoichiometric amount of CuI at a higher reaction concentration (entry 2).22 To our satisfaction, secondary phosphine oxide 5 could be coupled with other aryl bromides 10–12 in moderate to good yields using catalytic amounts of CuI (entries 3–5). Similarly, bis(3,5-bis(trifluoromethyl)phenyl)phosphine oxide (13) could be coupled with oxazoline-containing aryl bromides using catalytic loadings of CuI (entries 6 and 7).
Table 1.
Copper-catalyzed coupling of aryl bromides with secondary phosphine oxides.a
 | ||||
|---|---|---|---|---|
| Entry | Aryl bromide | Secondary phosphine oxide | Product | Yield (%)b |
| 1 2c |
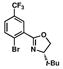 4 |
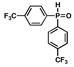 5 |
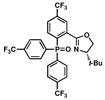 9 |
57 65 |
| 3 |
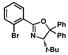 10 |
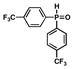 5 |
 14 |
85 |
| 4 |
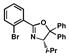 11 |
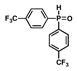 5 |
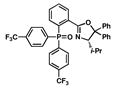 15 |
62 |
| 5 |
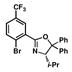 12 |
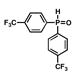 5 |
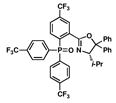 16 |
55 |
| 6 |
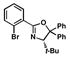 10 |
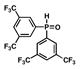 13 |
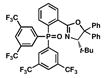 17 |
46 |
| 7 |
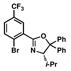 12 |
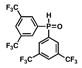 13 |
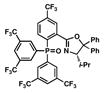 18 |
36 |
Reactions were performed with aryl bromide (1.0 equiv), secondary phosphine oxide (1.3 equiv), CuI (12.5 mol %), N,N′-dimethylethylenediamine (87.5% mol %), and Cs2CO3 (3.7 equiv) in PhCH3 (0.1 M) at 110 °C for 38–42 h.
Yield of isolated product.
Reaction was performed with 1.0 equiv of CuI and 3.0 equiv of N,N′-dimethylethylenediamine in PhCH3 (0.25 M) for 15 h.
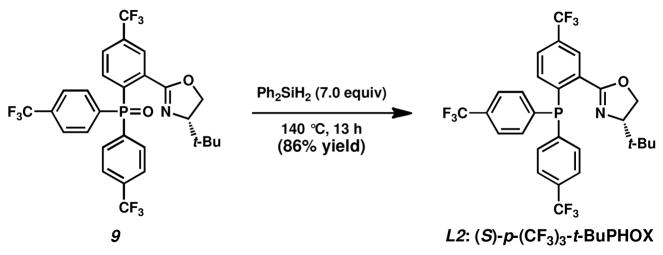
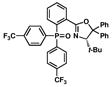
The resulting triarylphosphine oxides 9 and 14–18 can be smoothly purified by column chromatography and reduced to the corresponding PHOX ligands via silane reduction.23 For example, reduction of 9 to the desired p-(CF3)3-t-BuPHOX ligand was accomplished with neat diphenylsilane, yielding L2 in 86% isolated yield (Scheme 3).24
Scheme 3.
Reduction of 9 to afford (S)-p-(CF3)3-t-BuPHOX.
In conclusion, we have developed a rapid synthesis of p-(CF3)3-t-BuPHOX (L2) that results in an overall 51% yield starting from (S)-t-leucine under relatively mild conditions in four linear steps. The route utilizes a copper iodide-catalyzed coupling of oxazoline-containing aryl bromide 4 to secondary phosphine oxide 5, followed by a silane-mediated reduction of the resulting triarylphosphine oxide, to prepare the phosphinooxazoline core structure. We believe that the copper-catalyzed coupling of aryl bromides to secondary phosphine oxides provides a convenient alternative to the coupling of air-sensitive secondary phosphines for the preparation of tertiary phosphines.
Acknowledgments
This publication is based on work supported by Award No. KUS-11-006-02, made by King Abdullah University of Science and Technology (KAUST). Additionally, the authors wish to thank NIH-NIGMS (R01 GM 080269-01), the German Academic Exchange Service (DAAD, postdoctoral fellowship to J. S.), Abbott Laboratories, Amgen, the Gordon and Betty Moore Foundation, and Caltech for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.For reviews, see: McManus HA, Guiry PJ. Chem Rev. 2004;104:4151–4202. doi: 10.1021/cr040642v.Hargaden GC, Guiry PJ. Chem Rev. 2009;109:2505–2550. doi: 10.1021/cr800400z.
- 2.von Matt P, Pfaltz A. Angew Chem, Int Ed Engl. 1993;32:566–568. [Google Scholar]
- 3.Sprinz J, Helmchen G. Tetrahedron Lett. 1993;34:1769–1772. [Google Scholar]
- 4.Dawson GJ, Frost CG, Williams JMJ, Coote SJ. Tetrahedron Lett. 1993;34:3149–3150. [Google Scholar]
- 5.For a review, see: Helmchen G, Pfaltz A. Acc Chem Res. 2000;33:336–345. doi: 10.1021/ar9900865.See also: Pfaltz A. Acta Chem Scand B. 1996;50:189–194.Williams JMJ. Synlett. 1996:705–710.Helmchen G, Kudis S, Sennhenn P, Steinhagen H. Pure Appl Chem. 1997;69:513–518.
- 6.For selected examples of the use of t-BuPHOX in asymmetric reactions, see: Loiseleur O, Meier P, Pfaltz A. Angew Chem Int Ed Engl. 1996;35:200–202.Ripa L, Hallberg A. J Org Chem. 1997;62:595–602. doi: 10.1021/jo961832b.Sagasser I, Helmchen G. Tetrahedron Lett. 1998;39:261–264.Loiseleur O, Hayashi M, Keenan M, Schmees N, Pfaltz A. J Organomet Chem. 1999;576:16–22.Nilsson P, Gold H, Larhed M, Hallberg A. Synthesis. 2002:1611–1614.Hiroi K, Kazuhiro W. Tetrahedron: Asymmetry. 2002;13:1841–1843.Schulz SR, Blechert S. Angew Chem Int Ed. 2007;46:3966–3970. doi: 10.1002/anie.200604553.Cook MJ, Rovis T. J Am Chem Soc. 2007;129:9302–9303. doi: 10.1021/ja073269s.Linton E, Kozlowski MC. J Am Chem Soc. 2008;130:16162–16163. doi: 10.1021/ja807026z.
- 7.(a) Behenna DC, Stoltz BM. J Am Chem Soc. 2004;126:15044–15045. doi: 10.1021/ja044812x. [DOI] [PubMed] [Google Scholar]; (b) Mohr JT, Behenna DC, Harned AM, Stoltz BM. Angew Chem, Int Ed. 2005;44:6924–6927. doi: 10.1002/anie.200502018. [DOI] [PubMed] [Google Scholar]; (c) Seto M, Roizen JL, Stoltz BM. Angew Chem, Int Ed. 2008;47:6873–6876. doi: 10.1002/anie.200801424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Mohr JT, Nishimata T, Behenna DC, Stoltz BM. J Am Chem Soc. 2006;128:11348–11349. doi: 10.1021/ja063335a. [DOI] [PubMed] [Google Scholar]; (b) Marinescu SC, Nishimata T, Mohr JT, Stoltz BM. Org Lett. 2008;10:1039–1042. doi: 10.1021/ol702821j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tani K, Behenna DC, McFadden RM, Stoltz BM. Org Lett. 2007;9:2529–2531. doi: 10.1021/ol070884s. [DOI] [PubMed] [Google Scholar]
- 10.White DE, Stewart IC, Grubbs RH, Stoltz BM. J Am Chem Soc. 2008;130:810–811. doi: 10.1021/ja710294k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Streuff J, White DE, Virgil SC, Stoltz BM. Nature Chem. 2010;2:192–196. doi: 10.1038/nchem.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krout MR, Mohr JT, Stoltz BM. Org Synth. 2009;86:181–193. doi: 10.15227/orgsyn.086.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelman D, Jiang L, Buchwald SL. Org Lett. 2003;5:2315–2318. doi: 10.1021/ol0346640. [DOI] [PubMed] [Google Scholar]
- 14.(a) Busacca CA, Lorenz JC, Grinberg N, Haddad N, Hrapchak M, Latli B, Lee H, Sabila P, Saha A, Sarvestani M, Shen S, Varsolona R, Wei X, Senanayake CH. Org Lett. 2005;7:4277–4280. doi: 10.1021/ol0517832. [DOI] [PubMed] [Google Scholar]; (b) Busacca CA, Lorenz JC, Sabila P, Haddad N, Senanyake CH. Org Synth. 2007;84:242–261. [Google Scholar]
- 15.For Pd-catalyzed couplings of secondary phosphine oxides with aryl halides and aryl triflates, see: Xu Y, Li Z, Xia J, Guo H, Huang Y. Synthesis. 1984:781–782.Uozumi Y, Tanahashi A, Lee SY, Hayashi T. J Org Chem. 1993;58:1945–1948.Kabachnik MM, Solntseva MD, Beletskaya IP. Russ Chem Bull. 1997;46:1491.Toffano M, Dobrota C, Fiaud JC. Eur J Org Chem. 2006:650–656.
- 16.For Cu-catalyzed couplings of secondary phoshine oxides with aryl halides, see: Rao H, Jin Y, Fu H, Jiang Y, Zhao Y. Chem Eur J. 2006;12:3636–3646. doi: 10.1002/chem.200501473.Huang C, Tang X, Fu H, Jiang Y, Zhao Y. J Org Chem. 2006;71:5020–5022. doi: 10.1021/jo060492j.Jiang D, Jiang Q, Fu H, Jiang Y, Zhao Y. Synthesis. 2008:3473–3477.
- 17.(S)-2-Bromo-N-(1-hydroxy-3,3-dimethylbutan-2-yl)-5-(trifluoromethyl)benzamide (7). To a 250 mL, round-bottom flask charged with a magnetic stirring bar and (S)-t-leucinol (2.10 g, 17.9 mmol, 1.0 equiv) was added methylene chloride (60 mL). To the mixture was added a solution of sodium carbonate (5.70 g, 53.8 mmol, 3.0 equiv) in water (45 mL). The biphasic mixture is vigorously stirred at 23 °C. To the mixture was dropwisely added 2-bromo-5-(trifluoromethyl)benzoyl chloride 69(5.66 g, 19.7 mmol, 1.1 equiv) over 15 min. The reaction mixture was vigorously stirred at 23 °C for 10 h. The layers were separated, and the aqueous layer was extracted with methylene chloride (4 × 50 mL). The combined organic layers were stirred with a 1 N potassium hydroxide solution in methanol (10 mL) for 30 min, then acidified to neutral pH with 1 N hydrochloric acid (~8 mL). To the mixture was added water (15 mL), and the layers were separated. The aqueous layer was extracted with methylene chloride (4 × 20 mL). The combined organic layers were washed with brine, dried over sodium sulfate and concentrated. The resulting residue 7 (6.10 g, 16.6 mmol, 93% yield) was found to be pure by 1H-NMR, and used without further purification. Analytical data matched literature values for the corresponding (R)-enantiomer.10
- 18.(S)-2-(2-Bromo-5-(trifluoromethyl)phenyl)-4-tert-butyl-4,5-dihydrooxazole (4). To a flame-dried, 250 mL three-necked flask charged with a magnetic stirring bar and (S)-2-bromo-N-(1-hydroxy-3,3-dimethylbutan-2-yl)-5-(trifluoromethyl)benzamide 7 (6.10 g, 16.6 mmol, 1.0 equiv) was added methylene chloride (86 mL) and freshly distilled triethylamine (5.60 mL, 40.0 mmol, 2.4 equiv). The solution was cooled to 0 °C in an ice bath, and methanesulfonyl chloride (1.50 mL, 19.4 mmol, 1.2 equiv) was dropwisely added over 3 min. A reflux condenser was attached to the flask, and the reaction was heated to 50 °C with stirring for 20 h. The crude reaction mixture was allowed to cool to ambient temperature, and an aqueous saturated sodium bicarbonate solution (30 mL) was added with vigorous stirring for 5 min. The layers were separated, and the aqueous phase was extracted with methylene chloride (3 × 20 mL). The combined organic layers were washed with brine, dried over magnesium sulfate, and concentrated. The resulting residue was subjected to silica gel chromatography, eluted with hexanes/ethyl acetate (9:1 → 6:1) to afford 4 (5.76 g, 16.4 mmol, 99% yield) as a colorless oil. Analytical data matched literature values.9
- 19.Grayson M, Farley CE, Streuli CA. Tetrahedron. 1967;23:1065–1078. [Google Scholar]
- 20.Leazer JL, Jr, Cvetovich R, Tsay FR, Dolling U, Vickery T, Bachert D. J Org Chem. 2003;68:3695–3698. doi: 10.1021/jo026903n. [DOI] [PubMed] [Google Scholar]
- 21.Bis(4-(trifluoromethyl)phenyl)phosphine oxide (5). To a flame-dried, 50 mL flask charged with magnesium metal (607 mg, 25.0 mmol, 3.1 equiv) was added diethyl ether (12 mL). The mixture was cooled to 0 °C and 4-bromobenzotrifluoride (3.38 mL, 24.2 mmol, 3.0 equiv) was dropwisely added over 25 min, during which the mixture changed from colorless to yellow to black. A reflux condenser was attached to the flask, and the mixture was allowed to warm to 23 °C over 20 min, then heated to 30 °C for 90 min. The Grignard solution was transferred via cannula to a flame-dried, 25 mL flask to remove excess magnesium metal. The resulting Grignard solution was cooled to 0 °C, and diethyl phosphite (1.04 mL, 8.05 mmol, 1.0 equiv) was dropwisely added over 5 min. The reaction mixture was allowed to slowly warm to 23 °C with stirring over 18 h. The reaction mixture was cooled to 0 °C and treated with 2 M aqueous hydrochloric acid (10 mL). The mixture was allowed to warm to ambient temperature and extracted with ethyl acetate (3 × 25 mL). The combined organic layers were washed with brine, dried over sodium sulfate and concentrated. The resulting residue was subjected to silica gel chromatography, eluted with hexane/ethyl acetate (1:1 → 1:9), to give 5 (2.18 g, 6.46 mmol, 80% yield) as a pale yellow solid. Mp: 67–69 °C (lit. 65–67 °C); Rf = 0.29 (hexane/ethyl acetate, 1/1); 1H-NMR (300 MHz, CDCl3) δ 8.19 (d, JHP = 492 Hz, 1H), 7.83–7.90 (m, 4H), 7.78–7.82 (m, 4H); 31P-NMR (121 MHz, CDCl3) δ 17.80 (JPH = 490 Hz); 19F-NMR (282 MHz, CDCl3) δ −63.35; FTIR (neat film, NaCl) 3482, 3042, 2341, 1401, 1325, 1171, 1128, 1103, 1062, 1018, 942, 832, 711 cm−1; HRMS (FAB, Pos) m/z calc’d for C14H10OPF6 [M+H]+: 339.0373, found 339.0387.
- 22.(S)-2-(2-(Bis(4-(trifluoromethyl)phenyl)phosphoryl)-5-(trifluoromethyl)phenyl)-4-tert-butyl-4,5-dihydrooxazole (9). To a 50 mL Schlenk flask charged with a magnetic stirring bar, CuI (976 mg, 5.12 mmol, 1.0 equiv) and bis(4-(trifluoromethyl)phenyl)phosphine oxide 5 (2.25 g, 6.66 mmol, 1.3 equiv) was added toluene (15 mL) under an Ar atmosphere. To the mixture was added N,N′-dimethylethylenediamine (1.65 mL, 15.4 mmol, 3.0 equiv) and the resulting mixture was stirred for 20 min. To the mixture was then added (S)-2-(2-bromo-5-(trifluoromethyl)phenyl)-4-tert-butyl-4,5-dihydrooxazole 4 (1.79 g, 5.12 mmol, 1.0 equiv), Cs2CO3 (6.18 g, 19.0 mmol, 3.7 equiv) and 5 mL toluene. The Schlenk flask was sealed and the reaction was heated to 110 °C with stirring for 15 h. The crude reaction mixture was allowed to cool to ambient temperature and concentrated under reduced pressure. 1H-NMR of the mixture showed ~70% conversion to the desired product with formation of ~30% of debrominated 4. The concentrated reaction mixture was subjected to silica gel chromatography, eluted with hexanes/ethyl acetate (3:1 → 1:1) to provide 9 (1.97 g, 3.33 mmol, 65% yield) as white crystals. Mp: 159–161 °C; Rf = 0.60 (hexane/ethyl acetate, 1/1); 1H-NMR (300 MHz, CDCl3) δ 8.20 (br s, 1 H), 7.80–8.03 (m, 4 H), 7.72 (s, 1H), 7.70–7.78 (m, 5H), 3.89 (dd, J = 17.1, 8.7 Hz, 1H), 3.86 (dd, J = 18.9, 8.7 Hz, 1H), 3.38 (app t, J = 9.6 Hz, 1H), 0.71 (s, 9H); 31P-NMR (121 MHz, CDCl3) δ 27.96; 19F-NMR (282 MHz, CDCl3) δ −63.26, −63.26, −63.42; FTIR (neat film, NaCl) 3435, 2105, 1644, 1479, 1399, 1323, 1173, 1131, 1062 cm−1; HRMS: HRMS (FAB, Pos) m/z calc’d for C28H24O2PNF9 [M+H]+: 608.1401, found 608.1414; [a]25D = −56.3 ° (c 1.03, CH2Cl2).
- 23.Koch G, Lloyd-Jones GC, Loiseleur O, Pfaltz A, Pretot R, Schaffner S, Schnider P, von Matt P. Recl Trav Chim Pays-Bas. 1995;114:206–210. [Google Scholar]
- 24.(S)-2-(2-(bis(4-(trifluoromethyl)phenyl)phosphino)-5-(trifluoromethyl)phenyl)-4-tert-butyl-4,5-dihydrooxazole, (L2). To a flame-dried 50 mL Schlenk tube charged with a magnetic stirring bar and (S)-2-(2-(bis(4-(trifluoromethyl)phenyl)phosphoryl)-5-(trifluoromethyl)phenyl)-4-tert-butyl-4,5-dihydro-oxazole 9 (2.02 g, 3.33 mmol, 1.0 equiv) under an Ar atmosphere was added diphenylsilane (4.30 mL, 23.3 mmol, 7.0 equiv). The Schlenk tube was sealed and the clear solution was heated to 140 °C with stirring for 13 h. The crude reaction mixture was allowed to cool to ambient temperature, then subjected directly to silica gel chromatography, eluted with hexanes and methylene chloride (3:1), to provide ligand L2 as a colorless, semi-crystalline oil. Crystallization was induced with pentane (4 mL) at −20 °C to yield L2 (1.69 g, 2.86 mmol, 86% yield) as colorless crystals. Analytical data matched literature values.9,10