Abstract
Oxidation of various carbohydrates to ammonium formate was investigated in the presence of hydrogen peroxide and ammonium hydroxide. Most of the examined carbohydrates except nonreducing sugars were efficiently converted into ammonium formate under environment friendly and mild conditions in aqueous media.
The conversion processes of biomass waste to value-added products are receiving great attention, especially those using environmentally benign methods to avoid environmental pollution and its consequences.1 For instance, carbohydrate biomass can be converted into formic acid, which is an important source of hydrogen used in fuel cells. In fact, direct formic acid fuel cells (DFAFC) can become an alternative to methanol based fuel cells because of their easier transportation and increased safety.2 Major producers of portable electronics in phones and computers are currently testing the efficiency of DFAFC in their devices.2d–f,3 With continuing development, there is potential for DFAFC to challenge traditional batteries as power sources for mobile electronic devices.2e Ammonium formate is also used in the Leuckart reaction, the reductive amination of aldehydes and ketones to amines,4 and the industrial preparation of formamide.5 As a result, there may be continuing demand for the large-scale preparation of formic acid from inexpensive and abundant materials such as biomass waste. The present work facilitates the efficient generation of formic acid from carbohydrate biomass under mild conditions.
Isbell et. al., published a pioneering series of reports on oxidative degradations of aldoses, ketoses and keto acids in the presence of alkaline hydrogen peroxide. The formic acid yields depended on the ratios of alkali bases and hydrogen peroxide. The reaction rate was low in the presence of bases with the gradual addition of peroxide, and the conversion was accelerated to some degree in the presence of hydrogen peroxide with the gradual addition of bases.6–8 Most reducing sugars were converted to formic acid chemoselectively and efficiently, however there were a few practical shortcomings.
In order to keep the chemoselectivity high, the reactions were run over a long period of time at lower temperatures such as 0 °C. For example, glucose and galactose were converted to formic acid chemoselectively, but 4 weeks of reaction time were necessary to achieve an 84% yield from glucose, and similarly 5 days were required for a 94% yield from galactose. Shorter reaction times of two days or less resulted in yields of approximately 20 – 40%.7c–d,9 Later studies using Fe(II) salts10 showed that these reactions could be accelerated somewhat although many carbohydrates including glucose still required long reaction times at low temperatures. Therefore, these methods proved to be far from practical.
Another recent report showed significant improvements in hydrothermal oxidation of carbohydrates in the presence of alkali, however chemoselectivity was not as good as the previous methods. Besides, this hydrothermal protocol required harsh conditions including high temperatures (i.e., 250 °C), and the maximum reported yield was 75%.11
Though the aforementioned studies are remarkable initiatives in the conversion of carbohydrates into formic acid, further research is required to mitigate the common concerns such as high or low temperatures, long reaction hours, and the use of strong bases. Thus, we embarked on the study to explore potential solutions by varying reagents and conditions, and ultimately learned that the use of ammonium hydroxide instead of alkali metal bases provided significantly improved methods with pragmatic conditions such as room temperature, short reaction time (i.e., 1 h), and the use of water as the solvent of choice.
As shown in the Table 1, we sought optimal ratios of hydrogen peroxide and ammonium hydroxide, and learned that the increase in ammonium hydroxide enhanced the formation of ammonium formate (entries 1–2). While this trend was reiterated in entries 3–5, no conversion was observed without ammonium hydroxide (entry 6), underscoring the important role of ammonium hydroxide in comparison with alkali metal bases used in the previously known methods. The amounts of hydrogen peroxide also affected the results significantly (entries 1 vs. 4, and entries 2 vs. 5), with the optimal conditions as shown in entry 5. Thus we successfully converted glucose to formate under mild conditions in a chemoselective manner with an excellent conversion (99%, entry 5).
Table 1.
Optimization of oxidative degradation of glucose
| Entry | 30% H2O2(μL) | 25% NH4OH (μL) | Conversion toa Formate (%)b |
|---|---|---|---|
| 1 | 130 | 10 | 17 |
| 2 | 130 | 30 | 41 |
| 3 | 150 | 05 | 23 |
| 4 | 150 | 10 | 44 |
| 5 | 150 | 30 | 99 |
| 6 | 300 | 00 | 00c |
| 7 | 300 | 30 | 99d |
Reaction conditions: galucose (0.028 mmol), 30% H2O2 (150 μL), 25% NH4OH (30 μL), RT for 1 h.
The remaining % was accounted for by unreacted starting material.
No reaction.
Glucose (0.028 mmol), 30% H2O2 (300 μL), 25% NH4OH (30μL), RT for 24 h.
The conversion yields were calculated based on the assumption that the theoretical yield would be six equivalents of formic acid from each glucose. In all the cases, formic acid was an exclusive product, and the remaining mass balance was starting material or the lower aldoses6–9. We could find little or no over oxidation or decomposition of ammonium formate, even under extreme conditions such as higher concentration of hydrogen peroxide and longer hours (entry 7). The product was compared with commercially available ammonium formate and formic acid. Previous reports showed that the reactions run in the presence of bases formed the corresponding formate salts.6–9 Under the present reaction conditions, ammonium hydroxide also converted formic acid to ammonium formate. Oxidative degradation of glucose was done on a one gram scale, and the yields of the product were reproducible. The reaction was smooth and efficient at room temperature in an aqueous medium, therefore these conditions were practical and environment friendly. 1H NMR of the reaction mixture showed that the percentage conversion to formic acid increased with time, and usually the reaction went to completion or stopped in 1 h. Having determined the optimal conditions, various aldoses, ketoses, disaccharides, and trisaccharides were examined by our oxidative degradation methods in the presence of ammonium hydroxide as a base. The percentage yield calculations were done using a known volume of methanol in D2O.
Under the developed conditions, monomeric aldoses including D-erythrose, D-xylose, D-ribose, D-glucose, and D-galactose were smoothly converted into formic acid in 32, 92, 96, 99, and 93% yields, respectively (Table 2, entries 1–5). However, oxidative degradation of ketoses such as 1,3-dihydroxyacetone dimer, D-tagatose, and D-fructose were degraded into glycolic acid and formic acid.3c The conversion yields were 99, 83, and 46%, respectively (entries 6–8). When the reaction was performed with glycolic acid under similar conditions, no degradation or oxidation products were observed. Hence, it would be natural to assume that two carbons in the ketose were converted into one equivalent of glycolic acid and the remaining carbons were transformed into formic acid. In fact, the ratios of these two degradation products were well aligned with the expectations, showing a 1:1 ratio from dihydroxyacetone and 4:1 ratios from hexoses as illustrated in Table 3.
Table 2.
Oxidative degradation of carbohydrates to ammonium formate
| Entry | Carbohydrate | Conversion to Formate (%)a,d |
|---|---|---|
| 1 | D-erythrose | 32 |
| 2 | D-xylose | 92 |
| 3 | D-(−)-ribose | 96 |
| 4 | D-glucose | 99 |
| 5 | D-galactose | 93 |
| 6 | Dihydroxyacetonee | 99 |
| 7 | D-tagatosee | 83 |
| 8 | D-fructosee | 46 |
| 9 | Sucrose | 00bc |
| 10 | α-D-lactose | 99b |
| 11 | Maltose | 99b |
| 12 | D-(+)-cellobiose | 70b |
| 13 | Raffinose | 00 |
| 14 | Melizitose | 26 |
Reaction conditions: Aldose/Ketose/Disacchride/Trisaccharide(5mg), 30% H2O2 (150 μL 25% NH4OH (30 μl RT for 1 h;
Time taken 24 h at RT;
Time taken 48 h at 60 °C.
The remaining percentage was unreacted starting material except in D-erythrose.
Yields calculated based on the lower aldose formed during oxidative degradation.
Table 3.
Oxidative degradation of ketoses to ammonium formate and ammonium glycolate.
| Entry | Reactant | Ratio of formate/Glycolatea |
|---|---|---|
| 1 | Dihydroxyacetone | 1/1 |
| 2 | D-Tagatose | 4/1 |
| 3 | D-Fructose | 4/1 |
Reaction conditions: Ketose (5mg), 30% H2O2 (150 ul), 25% NH4OH (30 ul, RT for l h.
As shown in the Table 2, several disaccharides were examined under similar conditions. Reducing disaccharides such as α-D-lactose monohydrate, maltose, and D-(+)-cellobiose underwent oxidative degradation completely in 24 h to generate formate in 99, 99, and 70% yields, respectively, while non-reducing sucrose remained intact (entries 9–12). The reaction did not proceed with sucrose even at 60 °C, implying that the conversion of non-reducing carbohydrates would not be feasible due to mechanistic mismatch. Nonetheless, these conditions furnished an improved method to oxidatively degrade reducing carbohydrates rapidly at ambient temperatures with reliable chemoselectivities.
Earlier reports show that the degradation of carbohydrates follow five different paths such as α-hydroxy hydroperoxide-cleavage mechanism, the Baeyer Villiger mechanism, the ester mechanism, the dihydroxy-epoxide mechanism, and a radical mechanism.8 Based on these reported mechanisms,8 the alkaline hydrogen peroxide degrades reducing sugars by nucleophilic addition of hydrogen peroxide to the carbonyl group. Likewise under our conditions, the adduct, α-hydroxy hydroperoxide would decompose rapidly into formic acid and the next lower aldoses by α-hydroxy hydroperoxide cleavage (Scheme 1). These lower aldoses would further follow the similar degradation steps until completely oxidized to formic acid.6–12 In a separate experiment where glucose was treated with triethylamine N-oxide with or without ammonium hydroxide or hydrogen peroxide, we observed no oxidation or degradaton products. This may imply that the N-oxide is not involved in the oxidative degradation, further supporting the nucleophilic addition of hydroperoxide.
Scheme 1.
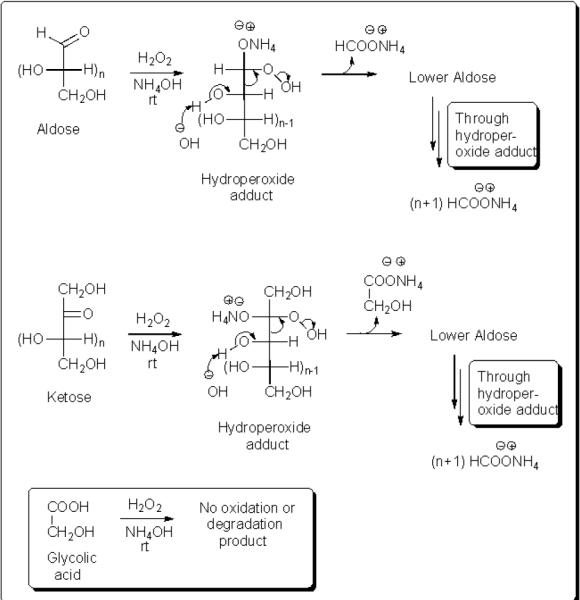
Possible mechanism for the oxidative degradation of aldose and ketose to ammonium formate
On the other hand, ketoses would give rise to the formation of glycolic acid and lower aldoses, which further react to give one equivalent of glycolic acid and the remaining carbon number of formic acid. Our results corroborate this potential pathway (Table 3). In addition, the oxidation results of disaccharides (Table 2, entries10–12) can also be explained by the proposed mechanism, which allow only the hemiacetal part of the disaccharide to afford the acyclic reducing form, ultimately generating formic acid.9a Sucrose being a non-reducing sugar with an acetal linkage between glucose and fructose does not undergo ring opening to give an acyclic aldose. Therefore, sucrose does not undergo oxidation under the given conditions to give ammonium formate.
Based on these concepts, two different trisaccharides were investigated (Table 2, entries 13 – 14). Non-reducing trisaccharide, raffinose was not hydrolysed under the present conditions to the corresponding acyclic form, therefore showing no reactivity towards oxidative degradation. In contrast, reducing trisaccharide melizitose was partially hydrolysed to undergo oxidative degradation, which accounted for the marginal yield of ammonium formate.
In conclusion, oxidative degradation of reducing sugars to ammonium formate was established under mild conditions in the presence of 30% aqueous hydrogen peroxide and 25% ammonium hydroxide at room temperature. The number of equivalents of produced ammonium formate was equivalent to the number of carbons present in the corresponding aldoses. For instance, glucose and ribose upon oxidative degradation gave six and five equivalents of ammonium formate, respectively. In the case of ketoses, oxidative degradation led to ammonium formate and glycolic acid in predictable ratios. However, the oxidative degradation of disaccharides and trisaccharides to ammonium formate depended on the presence of acetal or hemiacetal linkages between the monosaccharide units. The presence of a hemiacetal facilitated hydrolysis of reducing sugars to acyclic forms, which underwent oxidation to give ammonium formate whereas non-reducing sugars were resistant to oxidative degradation. We believe that the conditions developed herein are more practical and useful compared to the previously known methods due to the efficiency at ambient temperature, short reaction time, environment friendly solvent, and high chemoselectivities.
Supplementary Material
Scheme 2.
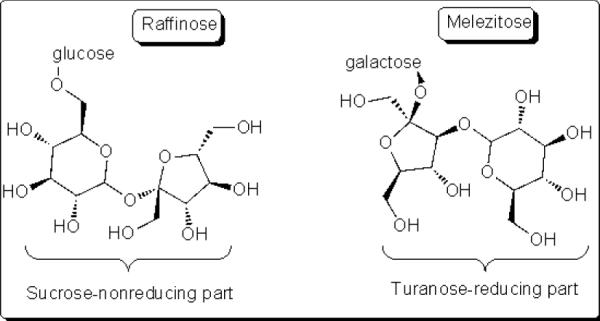
Nonreducing trisaccharides.
Acknowledgments
We would like to acknowledge Joo Ho Lee, Thomas Mathew, Richard Giles, and Victor Hadi for editing the manuscript and for their valuable suggestions. We would like to thank the Hydrocarbon Research Foundation and the National Institute of Health (S10 RR025432) for generous financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material Supplementary Material includes the 1H wet 1D NMR's of all entries in Tables 1, 2, and 3, reaction mixtures of the compounds in scheme 2, ammonium formate, and a mixture of ammonium formate and formic acid.
References
- 1.(a) Soetaert W, Vandamme E. Biofuels. Wiley; Chichester, U.K: 2009. [Google Scholar]; (b) Kozlowski R, Zailkov G, Pudel F. “Renewable resources: obtaining, processing, and applying” Hauppauge. Nova Science Publisher; New York: 2009. [Google Scholar]; (c) Jim F, Enamoto H. BioResources. 2009;4:704–713. [Google Scholar]
- 2.(a) Uhm S, Chung ST, Lee J. J. Power Sources. 2008;178:34–43. [Google Scholar]; (b) Jung WS, Han J, Nam SW, Lim TH, Oh IH, Hong SA. Hwahak Konghak. 2009;47:362–367. [Google Scholar]; (c) Ma DN, Yuan QY, Tang YW, Gao Y, Chu YY, Lu TH. Yingyong Huaxue. 2008;25:1125–1129. [Google Scholar]; (d) Lu TH, Zhang LL, Tang LL, Gao Ya-wen, Gao Y. Dianchi Gongye. 2007;12:119–121. [Google Scholar]; (e) Ha S, Larsen R, Zhu Y, Masel RI. Fuel Cells. 2004;4:337–343. [Google Scholar]; (f) Shu Y, Ha SY, Masel RI. J. of Power Sources. 2004;130:8–14. [Google Scholar]; (g) Rice C, Ha S, Masel RI, Waszczuk P, Wieckowski A, Barnard T. J. Power Sources. 2003;115:229–235. [Google Scholar]; (h) Rhee Y, Ha S, Masel RI. J. Power Sources. 2003;117:35–38. [Google Scholar]
- 3.(a) Wolfe MD, Lipscomb JD. J. Biolog. Chem. 2003;278:829–835. doi: 10.1074/jbc.M209604200. [DOI] [PubMed] [Google Scholar]; (b) Eugeneyan Y, Schwartz FW. Environ. Sci. Technol. 2000;34:2535–2541. [Google Scholar]; (c) Brooks CD, Huang LC, McCarron AW, Jhonstone Chem.Commun. 1999:37–38. [Google Scholar]; (d) Stefan MI, Bolton JR. Environ. Sci. Technol. 1998;32:1588. [Google Scholar]; (e) Sasaki K, Okamoto T, Oka S. Chem. Eng. Comm. 1989;83:111–116. [Google Scholar]; (f) Okamoto T, Sasaki K, Oka S. J. Am. Chem. Soc. 1988;110:1187–1196. [Google Scholar]; (g) Kumar A. J. Am. Chem. Soc. 1981;103:5179–5182. [Google Scholar]; (h) Venturello C, Ricci M. J. Org. Chem. 1986;51:1599–1602. [Google Scholar]; (i) Hockett RC, Fletcher HG., Jr. J. Am. Chem. Soc. 1944;66:469–472. [Google Scholar]
- 4.(a) Xia B, Tu M, Zheng M, Shen D. Jingxi Huagong Zhongjianti. 2008;38:30–31. 50. [Google Scholar]; (b) Yang L, Deng Y, Dai R, Liu W. Xiandai Huagong. 2008;28:44–46. 48. [Google Scholar]; (c) Wu H, Yin Q, Zhang L. Yingyong Huagong. 2006;35:357–358. [Google Scholar]
- 5.Freer PC, Sherman PL., Jr American Chemical Journal. 1898;20:223–8. [Google Scholar]
- 6.Moody GJ. Advan. Carbohydrate Chem. 1964;19:149–79. doi: 10.1016/s0096-5332(08)60281-7. [DOI] [PubMed] [Google Scholar]
- 7.(a) Isbell HS. “Enolization and Oxidation Reactions of Reducing Sugars”, in “Carbohydrates in Solution”. Advan. Chem. Ser. 1973;117:70–87. [Google Scholar]; (b) Isbell HS, Frush HL, Martin ET. Carbohyd. Res. 1973;26:287–295. [Google Scholar]; (c) Isbell HS, Frush HL. Carbohyd. Res. 1973;28:295–301. [Google Scholar]; (d) Isbell HS, Frush L. Carbohyd. Res. 1975;45:197–204. [Google Scholar]
- 8.(a) Isbell HS, Frush HL. Carbohyd. Res. 1975;45:197–204. See all references of this article. [Google Scholar]; (b) Arts SJHF, Mombarg EJM, Bekkum HV, Sheldon RA. Synthesis. 1996:597–613. [Google Scholar]
- 9.(a) Isbell HS, Harriet L, Naves R. Carbohyd. Res. 1974;36:C1–C4. [Google Scholar]; (b) Isbell HS, Frush HL. Carbohyd. Res. 1976;49:C1–C4. [Google Scholar]
- 10.Isbell HS, Frush L. Carbohyd. Res. 1976;51 [Google Scholar]
- 11.(a) Jun F, Zhou Z, Moriya T, Kishida H, Higashizhima H, Enomoto H. Environ. Sic. Technol. 2005;39:1893–1902. doi: 10.1021/es048867a. [DOI] [PubMed] [Google Scholar]; (b) Jin F, Yun J, Li G, Kishita A, Tohji K, Enomoto H. Water Dynamics. AIP Conference.2008. pp. 139–142. [Google Scholar]; (c) Gin F, Yun J, Li G, Kishita A, Tohji E, Enomoto H. Green Chem. 2008;10:612–615. [Google Scholar]
- 12.(a) Isbell HS, Frush HL. Carbohyd. Res. 1973;26:287–295. [Google Scholar]; (b) Isbell HS. Adv. Chem. Ser. 1973;117:70–87. [Google Scholar]; (c) Isbell HS, Frush HL, Matin ET. Carbohyd. Res. 1973;26:287–295. [Google Scholar]; (d) Isbell HS, Frush HL, Matin ET. Carbohyd. Res. 1973;26:287. [Google Scholar]; (e) Naves R, Soontracharoen P. Carbohyd. Res. 1981;90:111–122. [Google Scholar]; (f) Salam MA, Isbell HS. Carbohyd. Res. 1980;82:253–261. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


