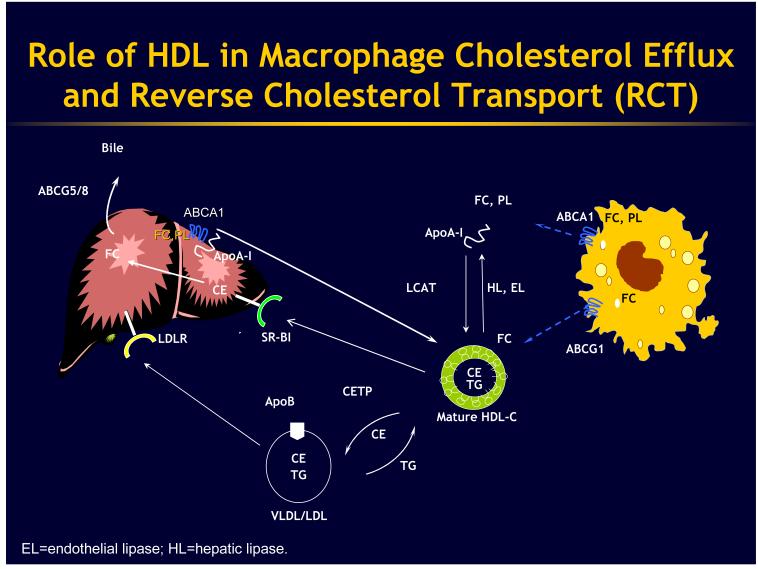
The story of HDL and its role in athero-protection begins in the artery wall where cholesterol-loaded macrophages have developed defense mechanisms to help them unload excess cholesterol. These defense mechanisms involve macrophage membrane proteins that utilize ATP (ATP-binding cassette transporters or ABCs) to promote cholesterol efflux onto HDL and its apolipoproteins 1. It is now clear that there are at least two major players in this regard. One is ABCA1, the molecule that is mutated in Tangier disease, and the other one is ABCG1. ABCA1 promotes efflux of cholesterol and phospholipids onto lipid-poor Apo-A1, the major protein constituent of HDL (Fig 1), while ABCG1 promotes cholesterol efflux onto HDL particles that have already been formed. ABCA1 and ABCG1 can act sequentially to promote cholesterol efflux, with ABCA1 serving to make HDL (this occurs mainly in the liver where ABCA1 adds free cholesterol and phospholipids to apoA-1 synthesized in the liver), and then ABCG1 acting to promote cholesterol efflux onto HDL particles. Interestingly, both ABCA1 and G1 are controlled transcriptionally, by a specialized transcription factor called LXR (liver-X-receptor) that acts in a complex with RXR (retinoid-X-receptor) so that when cells accumulate cholesterol some of that cholesterol gets turned into specific oxidized forms of cholesterol (oxysterols) that activate LXR and then up regulate ABCA1 and ABCG1. Very recently it has been discovered by Kathryn Moore and Carlos Fernandez-Hernando and their colleagues that there is another level of control of ABCA1 and ABCG1 involving a suppressor microRNA (a small kind of RNA that targets mRNAs for degradation) called miR-33 that acts to cause degradation of ABCA1 or ABCG1 protein and mRNA 2. miR-33 is embedded in the sterol regulatory element binding protein 2 (SREBP2) gene, the master transcriptional regulator of cholesterol biosynthetic genes, so when cells are deprived of cholesterol they up regulate their SREBP2 and in so doing they activate this micro RNA, which turns off ABCA1 and ABCG1, limiting cholesterol efflux and helping to conserve cellular cholesterol.
Then, in the whole organism this cholesterol efflux pathway becomes integrated into a larger pathway mediating reverse cholesterol transport (RCT). The concept derives from original work by John Glomset and the term “reverse cholesterol transport” was originally coined by Dan Steinberg. In the schematic of RCT the idea is that macrophage cholesterol efflux is followed by esterification of cholesterol-to-cholesterol esters (CEs) by the lecithin:cholesterol acyltransferase (LCAT) enzyme in HDL. CEs are transferred in plasma by cholesterol ester transfer protein (CETP) from HDL to triglyceride rich particles. The CETP pathway is especially important in humans. In rodents, in particular, HDL components may be directly removed from the plasma by the scavenger receptor B1 (SR-BI) pathway, which mediates a process of selective uptake of HDL CE in the liver, which may be followed by excretion of cholesterol into bile. One of the debates that is ongoing is whether the anti-atherogenic activity of HDL requires up regulation of all of the steps involved in reverse cholesterol transport (i.e. cholesterol efflux from arterial wall foam cells, transport in the plasma, uptake in the liver and excretion into bile) or whether the anti-atherogenic effect only requires increased cholesterol efflux from macrophage foam cells or other direct cellular activities of HDL such as anti-inflammatory effects.
Let me now discuss cholesterol ester transfer protein (CETP) and its inhibition in the overall context of RCT. When CETP is inhibited the overall rate of RCT is not changed. However, the route of RCT has changed. It is occurring through HDL instead of through VLDL and LDL. When CETP is inhibited, cholesterol efflux may be increased from macrophages but RCT overall is not increased. For that matter, niacin also increases HDL but probably does not increase fecal sterol output so it also does not increase overall reverse cholesterol transport. My personal view is that only the step at the interface between the artery wall and HDL needs to be up regulated to derive benefit.
Inhibiting CETP became a therapeutic strategy in 1989-90 when we and our colleagues in Japan characterized a genetic deficiency state of CETP and individuals who had homozygous deficiency were found to have remarkably elevated HDL levels of about 100-160 milligrams per deciliter3. Heterozygotes had an approximately 30 percent increase in HDL levels compared to unaffected family members. Thus, only 50 percent inhibition of CETP, equivalent to levels in heterozygotes would be required to raise HDL by as much as niacin, so this attracted a lot of interest. In addition, in that initial description we noted that homozygotes also had a 40 percent lowering of LDL cholesterol and ApoB, whereas heterozygotes showed a non-significant decrease in their LDL. This high HDL, low LDL phenotype is also seen after treatment of humans with potent CETP inhibitors.4 Such agents lower LDL appreciably, as well as raising HDL, whereas less potent CETP inhibitors raise HDL without much effect on LDL.
It is important to recognize that different therapeutic approaches to raising HDL have really different effects on the kind of HDL that accumulates5.The most desirable agent for HDL raising would be one that increased the synthesis of Apo-A1 in the liver but so far this has proved tricky. Fibrate drugs do increase Apo-A1 and even more so Apo-A2 synthesis in the liver and this is probably part of their HDL raising mechanism. However, as found in studies like ACCORD and FIELD the degree of HDL elevation with agents like fenofibrate is quite modest. Infusing Apo-A1 appears to be beneficial in animal models and in humans. Recently, small molecules have been described that increase ApoA-1 mRNA in the liver, and modestly increase apoA-1 and HDL levels in plasma.6 The next part of the HDL cycle would be to target the macrophage with LXR activators and this has been, in recent years, a Holy Grail because LXR activation up regulates cholesterol efflux and every step in reverse cholesterol transport, including up regulating CETP. The roadblock in this approach has been that LXR also up regulates SREBP1c and so very reproducibly causes fatty liver. Thus the task has become to make an LXR activator that is specific to the LXR-beta isoform which is expressed in the periphery more than the liver.7 Other agents, like PPAR gamma activators such as pioglitazone also have a modest effect to up regulate ABC transporters and this could be involved in their HDL elevating activity. Finally, some agent decrease catabolism of HDL and this includes CETP inhibitors and niacin, leading to accumulation of large HDL-2 type particles including those enriched in Apo-E. Ernie Schaefer has shown recently that niacin also increases the synthesis of Apo-A1 in hyperlipidemic subjects8. Earlier studies with niacin that were done in healthy, normolipidemic subjects found that there was a decrease in the turnover or catabolism of HDL, explaining the increase in HDL levels. Continuing on the CETP theme after we discovered the genetic deficiency with this dramatic phenotype of high HDL and low LDL, it became very important to ask, “What are the effects of CETP inhibition on atherosclerosis?” We and others tried very hard in animal models to get to the bottom of this and in mouse models the results have been conflicting and confusing. What appeared to be a beautiful experiment was to make a transgenic mouse that expresses CETP. This reproducibly reduced HDL levels but effects on atherosclerosis were variable in different studies, depending on what other genes were present in the model. In Apo-E knockout mice, CETP expression did increase atherosclerosis but in some other models with high triglycerides CETP expression either had no effect or even reduced atherosclerosis9.
In contrast to the mouse models, in the rabbit CETP expression is pro-atherogenic9. CETP inhibition in approximately 20 different studies using various approaches in rabbits, with one exception in a Watanabe rabbit study, has been associated with reduced atherosclerosis. This also included a large study that was done in cholesterol-fed rabbits with Pfizer’s CETP inhibitor torcetrapib, which reduced aortic atherosclerosis10. Despite this torcetrapib failed in human clinical trials. It is not known whether torcetrapib in rabbits caused hypertension and hyperaldosteronism as it did in humans.
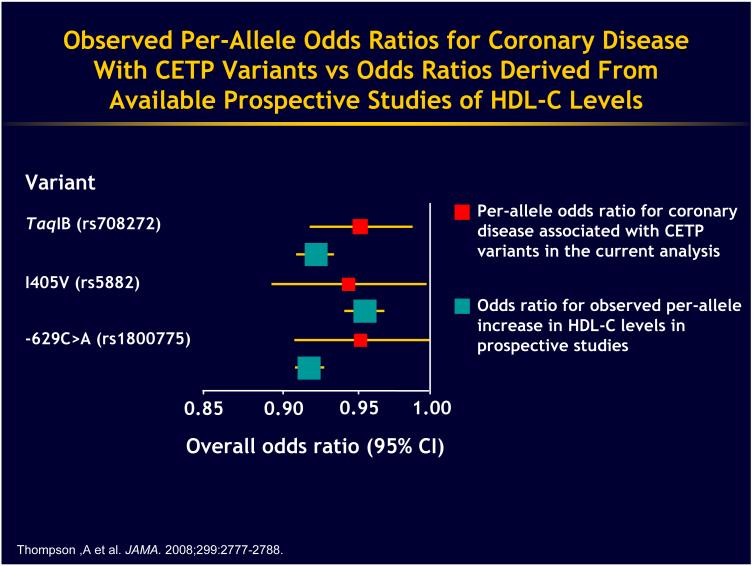
The ultimate experiment to assess the effects of CETP inhibition must be done in humans and I wish I could stand up here and say there was a clear message. We studied the genetic deficiency in Japan, and basically the results of those studies are inconclusive. There was a well performed epidemiological meta-analysis carried out by John Danesh and his colleagues11. They did a meta-analysis of the Taq1B variant, which is a single nucleotide polymorphism (snp i.e. a change in a single nucleotide of a gene that is commonly observed in the general popualtion) in the first intron of the CETP gene. They also looked at two other snps in the CETP gene, one that changes an amino acid in a conservative fashion and then a third one. The overall pattern is that reduction in CETP levels and the modest increase in HDL associated with these snps was also associated in this meta-analysis with a reduction in coronary heart disease risk (Fig 2). These polymorphisms are associated with small changes in HDL in the general population, about a 5-10 percent elevation of HDL. These effects were not different to the protective effect of HDL in the general population. You will note, of course, that the confidence intervals for these relationships almost touch the non-significant 1.0 risk ratio so these are modest effects but they are in the right direction. The Taq1B protective effect is statistically significant and certainly there is no indication here or in any other large study of CETP polymorphisms that there is an excessive risk so the studies overall indicate either a neutral effect or reduction in coronary heart disease risk.
Another approach involving CETP snps has come out of the genome-wide association studies. Perhaps amongst many different fields the most successful application of genome-wide association studies has been in the elucidation of the genetic architecture of plasma lipoprotein levels in humans. Quite a few of these studies have been carried out by Sek Kathiresan at the Massachusetts General Hospital12. Something like 20-30 percent of the genetic variance in plasma lipoprotein levels has been explained by snps that have been identified in about 100 different genes, some of them known many of them unknown or previously unsuspected.13 One such study looked at snps involving known genes regulating LDL and HDL metabolism and related them to plasma lipoprotein levels and also, to risk of coronary heart disease12. The snp that has the biggest effect on the variance of HDL levels in the general European population is in the CETP gene. In terms of LDL the Apo-E gene had the largest effect on variance in LDL. They made a genotype score where they took the the risk allele for LDL that would cause higher LDL or for HDL that would cause lower HDL and made a composite score for the LDL and HDL snps. The higher your score, the worse you did in this nested case control CHD study. The supplementary information for this paper showed that both the HDL snps and the LDL snps independently predicted risk. The story that has come out of genome-wide association studies looking at relationships of snps in single genes associated with LDL or HDL levels to CHD is somewhat muddy. However, more recent analyses indicate that LDL, triglyceride and HDL genes all have relationships in some cases to coronary artery disease risk.13 However, the LDL genes have the most consistent and strongest relationship.
Is the HDL that is associated with CETP inhibition or deficiency dysfunctional? We tried to look at this in ex vivo studies using HDL from subjects treated with CETP inhibitors or with genetic deficiency of CETP and we found that that this HDL showed increased cholesterol efflux14. This goes along with the finding that in a post hoc analysis carried out by Steve Nicholls of the ILLUSTRATE study, the imaging study done with torcetrapib, subjects in the top quartile of HDL on treatment actually, had regression of their atherosclerosis15. Finally, in recent studies we have examined HDL from subjects treated with the CETP inhibitor anacetrapib in an in vitro macrophage inflammation study16. In this experiment we pre-treated the macrophages with the HDL and then we add an inflammatory stimulus i.e. lipopolysaccharide. The HDL isolated from subjects following anacetrapib treatment was somewhat better than the pretreatment HDL in suppressing the inflammatory response. In ABCA1/ G1 knockout macrophages that extra beneficial effect was. Thus, the HDL is less effective as an anti-inflammatory agent in cells lacking the transporters but it still works.
To summarize, we may again ask the question: was the failure of torcetrapib due to the molecule or the mechanism? (Fig 3) Since that failure in 2006 it has been shown that there were significant off-target effects causing increased blood pressure, aldosterone, and potassium levels17. There was an excess of death from sepsis that could be mechanism related, perhaps related to immunosuppressive effects of HDL, or might be just a statistical aberration. It is also clear that in vitro, at least, HDL is not dysfunctional and with high HDL reduction of coronary atherosclerosis would suggest benefit. Of course, this can only be settled with further clinical trials and these are ongoing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tall AR. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J Intern Med. 2008;263:256–73. doi: 10.1111/j.1365-2796.2007.01898.x. [DOI] [PubMed] [Google Scholar]
- 2.Rayner K, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro M, Fisher EA, Moore KJ, Fernandez-Hernando C. miR-33 coordinates genes regulating cholesterol homeostasis. Science. 2010 doi: 10.1126/science.1189862. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inazu A, Brown ML, Hesler CB, et al. Increased high-density lipoprotein levels caused by a common cholesteryl-ester transfer protein gene mutation. N Engl J Med. 1990;323:1234–8. doi: 10.1056/NEJM199011013231803. [DOI] [PubMed] [Google Scholar]
- 4.Krishna R, Anderson MS, Bergman AJ, et al. Effect of the cholesteryl ester transfer protein inhibitor, anacetrapib, on lipoproteins in patients with dyslipidaemia and on 24-h ambulatory blood pressure in healthy individuals: two double-blind, randomised placebo-controlled phase I studies. Lancet. 2007;370:1907–14. doi: 10.1016/S0140-6736(07)61813-3. [DOI] [PubMed] [Google Scholar]
- 5.Linsel-Nitschke P, Tall AR. HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat Rev Drug Discov. 2005;4:193–205. doi: 10.1038/nrd1658. [DOI] [PubMed] [Google Scholar]
- 6.Bailey D, Jahagirdar R, Gordon A, et al. RVX-208: a small molecule that increases apolipoprotein A-I and high-density lipoprotein cholesterol in vitro and in vivo. J Am Coll Cardiol. 2010;55:2580–9. doi: 10.1016/j.jacc.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 7.Bradley MN, Hong C, Chen M, et al. Ligand activation of LXR beta reverses atherosclerosis and cellular cholesterol overload in mice lacking LXR alpha and apoE. J Clin Invest. 2007;117:2337–46. doi: 10.1172/JCI31909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamon-Fava S, Diffenderfer MR, Barrett PH, et al. Extended-release niacin alters the metabolism of plasma apolipoprotein (Apo) A-I and ApoB-containing lipoproteins. Arterioscler Thromb Vasc Biol. 2008;28:1672–8. doi: 10.1161/ATVBAHA.108.164541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barter PJ, Brewer HB, Jr., Chapman MJ, Hennekens CH, Rader DJ, Tall AR. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:160–7. doi: 10.1161/01.atv.0000054658.91146.64. [DOI] [PubMed] [Google Scholar]
- 10.Morehouse LA, Sugarman ED, Bourassa PA, et al. Inhibition of CETP activity by torcetrapib reduces susceptibility to diet-induced atherosclerosis in New Zealand White rabbits. J Lipid Res. 2007;48:1263–72. doi: 10.1194/jlr.M600332-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Thompson A, Di Angelantonio E, Sarwar N, et al. Association of cholesteryl ester transfer protein genotypes with CETP mass and activity, lipid levels, and coronary risk. Jama. 2008;299:2777–88. doi: 10.1001/jama.299.23.2777. [DOI] [PubMed] [Google Scholar]
- 12.Kathiresan S, Melander O, Anevski D, et al. Polymorphisms associated with cholesterol and risk of cardiovascular events. N Engl J Med. 2008;358:1240–9. doi: 10.1056/NEJMoa0706728. [DOI] [PubMed] [Google Scholar]
- 13.Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–13. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuura F, Wang N, Chen W, Jiang XC, Tall AR. HDL from CETP-deficient subjects shows enhanced ability to promote cholesterol efflux from macrophages in an apoE- and ABCG1-dependent pathway. J Clin Invest. 2006;116:1435–42. doi: 10.1172/JCI27602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicholls SJ, Tuzcu EM, Brennan DM, Tardif JC, Nissen SE. Cholesteryl ester transfer protein inhibition, high-density lipoprotein raising, and progression of coronary atherosclerosis: insights from ILLUSTRATE (Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation) Circulation. 2008;118:2506–14. doi: 10.1161/CIRCULATIONAHA.108.790733. [DOI] [PubMed] [Google Scholar]
- 16.Yvan-Charvet L, Kling J, Pagler T, et al. Cholesterol Efflux Potential and Anti-Inflammatory Properties of High-Density Lipoprotein After Treatment With Niacin or Anacetrapib. Arterioscler Thromb Vasc Biol. 2010 doi: 10.1161/ATVBAHA.110.207142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–22. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]