Abstract
HDL has many protective activities against atherosclerosis including its role in reverse cholesterol transport, and its antioxidant, anti-inflammatory, and endothelial cell maintenance functions. However, all HDL is not functionally equivalent. Recent studies have shown that infection, inflammation, diabetes, and coronary artery disease are associated with dysfunctional HDL. HDL can lose its protective activities through a variety of mechanisms including, but not limited to, altered protein composition, oxidative protein modification mediated by the enzyme myeloperoxidase, and lipid modification. Studies using bacterial endotoxin in humans and mice have directly demonstrated changes in HDL composition, loss of HDL’s cholesterol acceptor activity, and decreased hepatic processing and secretion of cholesterol. Although, a routine clinical assay for dysfunctional HDL is not currently available, the development of such an assay would be beneficial for a better understanding of the role that dysfunctional HDL plays as a risk factor for coronary artery disease, and for the determination of how various drug therapies effect HDL functionality.
Keywords: Reverse cholesterol transport, high density lipoprotein, apolipoprotein A-I, inflammation, lipopolysaccharide, cholesterol efflux
HDL-cholesterol is called the “good cholesterol” due to its inverse correlation with coronary artery disease (CAD) in epidemiological studies.1 HDL possesses several properties that protects against atherosclerosis, most prominently its role in mediating reverse cholesterol transport (RCT).2 In this pathway, cholesterol and phospholipids in arterial wall macrophage foam cells can be taken up by extracellular apolipoprotein A-I (apoAI), the major HDL protein, via the cell membrane protein ATP binding cassette protein A1 (ABCA1) to generate nascent HDL. HDL can accept more cholesterol from foam cells via the membrane protein ATP binding cassette protein G1 (ABCG1). Cholesterol in HDL can be converted into cholesterol esters by the plasma enzyme lecithin cholesterol acyl transferase (LCAT) to form mature spherical HDL. HDL can then deliver cholesterol ester directly to the liver through the receptor scavenger receptor BI (SR-BI), or indirectly through the action of cholesterol ester transfer protein (CETP) that transfers cholesterol esters to apoB containing lipoproteins, which can then be taken up by the hepatic LDL receptor. Once delivered to the liver, cholesterol can be excreted into the bile directly or first converted into bile acid for eventual excretion in feces. HDL also possesses antioxidant, anti-inflammatory, antithrombotic, and endothelial cell maintenance activities.
In Vitro Assays for HDL Function and the Emergence of the Concept of Dysfunctional HDL
HDL is heterogeneous in size and composition and one question that has arisen is whether all HDL is equally protective against atherosclerosis. Several in vitro assays can be used to assess HDL function. Alan Fogelman and colleagues developed both cell-based and cell-free assays to examine HDL’s anti-inflammatory and antioxidant activity.3,4 And through these studies, they’ve championed the concept that HDL can become dysfunctional. For example, LDL addition to endothelial cells co-cultured with smooth muscle cells induces cellular secretion of monocyte chemotactic factors leading to monocyte binding. While the addition of normal HDL is anti-inflammatory and can impede this monocyte chemotaxis, HDL from patients undergoing an acute phase reaction did not inhibit monocyte chemotaxis but actually increased it, demonstrating pro-inflammatory activity.5 Similarly, HDL from nondiabetic CAD subjects also has less anti-inflammatory activity compared to normal HDL.6
A simple assay related to plasma, HDL, or apoAI’s function in RCT is their ability to act as acceptors of cholesterol from cholesterol loaded macrophages. In this assay a subject’s plasma, or purified HDL or apoAI, is incubated with [3H]cholesterol loaded cultured macrophages, and cholesterol efflux is calculated as the % of the cellular [3H]cholesterol that appears in the media.7 For examples, plasma and HDL from sepsis patients have decreased cholesterol accepting activity, indicating that sepsis is associated with dysfunctional HDL.8
Assays related to HDLs endothelial maintenance and anti-inflammatory activities include the ability of HDL to promote NO production by cultured endothelial cells, the protection of cultured endothelial cells from apoptotic stimuli such as exposure to ultraviolet light, and the ability of HDL to reduce endothelial cell expression of the pro inflammatory adhesion protein VCAM-1 after treatment with an inflammatory cytokine.9,10 For example, HDL isolated from type 2 diabetic patients has reduced levels of these endothelial protective activities demonstrating HDL dysfunction.10
Mechanisms of apoAI and HDL dysfunction and the Role of Myeloperoxidase
There are many ways in which HDL and apoAI can become dysfunctional. For example inflammation changes hepatic gene expression and leads to changes in the protein composition of HDL (also called the HDL proteome). During inflammation or in experimental models where lipopolysaccharide (LPS), a bacterial endotoxin, has been utilized, the levels of apoAI and CETP on HDL are decreased, while the levels of the acute phase reactant proteins serum amyloid A (SAA) and secretory phospholipase A2 (sPLA2) on HDL are markedly increased; and, these changes are associated with decreased cholesterol acceptor activity of HDL11. Proteomic HDL studies have shown that the antioxidant activity of HDL is associated with the levels of paraoxonase proteins.12 After apoAI, the second most abundant protein in HDL is apolipoprotein A-II (apoAII), and, Lusis has described that HDL from transgenic mice that over express apoAII has diminished anti-inflammatory activity.13 Similarly, HDL derived from influenza virus infected mice also has diminished anti-inflammatory activity.14
In addition to altering the protein content of HDL, enzymatic alteration of HDL lipids can also alter its function. For example, treatment of HDL with 15-lipoxygenase reduces HDL lipid acceptor activity and its anti inflammatory activity.15
Enzymes can also modify HDL proteins. Myeloperoxidase (MPO) is found in neutrophils and monocytes and it plays an important role in killing microorganisms. It performs this activity by using chloride ions and cell generated hydrogen peroxide to create hypochlorous acid, the same chemical that is in household bleach. Bleach is an efficient antibacterial agent, but it can also perform many damaging reactions against host proteins by covalently modifying the amino acid residues cysteine, methionine, tyrosine, tryptophan, and lysine. In collaboration with Hazen, we discovered that plasma apoAI is a selective target for MPO mediated protein modification yielding high levels of covalent modifications compared to other plasma proteins.16 This selectivity can be accounted for by the direct binding of MPO to apoAI, with the highly reactive MPO derived oxidants formed locally and reacting with the adjacent apoAI. An important observation was made by Alan Daugherty and colleagues, who showed that MPO is present at high levels in human atheroma, the site where HDL needs to retain full functionality in order to remove cholesterol from the lesion in the first step of the RCT pathway.17
Hazen and colleagues have shown that the MPO levels in blood are higher in patients with heart disease than controls.18 And furthermore, stratification of blood MPO levels in consecutive patients presenting with chest pain was a dramatic predictor of who was going to have a subsequent major adverse coronary event within the next 6 months. Those in the top quartile of MPO levels were at 4.7-fold risk compared to those in the lowest MPO quartile.18 Among these subjects, MPO levels were more predictive than C-reactive protein (CRP) levels, with those in the highest CRP quartile having 1.8-fold increased risk compared to those in the lowest CRP quartile.18
Thus, MPO may be a risk factor for CAD, and it can bind to and modify apoAI. The MPO mediated modifications of apoAI include apoAI cross linking that can be observed on SDS denaturing gel electrophoresis, with MPO altering apoAI from a small monomer form to dimer and multimer cross linked forms19 Other MPO products of apoAI modification are the formation of chlorotyrosine and nitrotyrosine from tyrosine residues, methionine sulfide from methionine residues, mono- and di-hydroxytryptophan from tryptophan residues, and amino adipic acid and chlorolysine from lysine residues.16,19–22 The level of apoAI chlorotyrosine levels can be used as a fingerprint of MPO reactivity, because chlorotyrosine is only formed by the activity of MPO and not by any other enzymatic or nonenzymatic activities.23 Thus, one can use the chlorotyrosine content in apoAI to assay its extent of MPO modification and to determine whether the extent of modification is correlated to apoAI function. We identified chlorotyrosine modified apoAI in human plasma, and even higher levels of this modification in apoAI isolated from atheroma.16,20
Work by the Hazen and Smith groups in Cleveland, and independently by Heinecke’s group in Seattle, have determined that apoAI chlorotyrosine levels are higher in subjects with CAD than in control subjects.16,24 Furthermore, both teams have shown that MPO modification of apoAI in vitro leads to loss of apoAI cholesterol acceptor activity.16,25 And, for apoAI isolated from human plasma, there is an inverse correlation between its chlorotyrosine content and cholesterol acceptor activity, such that the apoAI preparations with higher chlorotyrosine contents have less ability to act as cholesterol acceptors from cholesterol-loaded macrophages.16 Thus, apoAI becomes dysfunctional in its ability to mediate RCT by either in vitro or in vivo modification by MPO.
Although the Cleveland and Seattle groups agree on the finding that the MPO interaction with apoAI is selective and leads to apoAI loss of function, there are differing opinions in the precise apoAI modification that leads to apoAI dysfunction.26 Heinecke’s findings imply that tyrosine-192 chlorination and methionine modification combine to make it dysfunctional. They reached this conclusion through the use of a recombinant apoAI isoform in which tyrosine-192 was converted to a phenylalanine. Although this variant was susceptible to loss of function by MPO treatment, if the modified apoAI was then treated with a bacterial enzyme, methionine sulfide reductase, the native methionine was regenerated and the cholesterol acceptor activity of apoAI was restored.26
On the other hand, the Cleveland group came to a different conclusion based on the study of a series of site directed recombinant apoAI isoforms. First, we created an apoAI variant in which all seven tyrosine residues were replaced by phenylalanine, a hydrophobic residue that is relatively resistant to MPO oxidation. This variant has full cholesterol acceptor activity, but when treated in vitro with MPO, we observed loss of function at the same dose response as the wild type isoform, accompanied by protein cross linking, implying that apoAI tyrosine modification cannot be responsible for its loss of function by MPO treatment.19 Next we made an apoAI variant in which the three methionine residues were replaced by valine residues. Again, this variant had full cholesterol acceptor activity. When it was treated with increasing strengths of MPO modification in vitro, this variant was more sensitive than the wild type isoform in its loss of cholesterol acceptor activity, implying that the methionine residues are protective against apoAI’s loss of function.21 This data agreed with an earlier study which reported that the formation of methionine sulfoxide residues on apoAI does not impair its function.27 We then chemically modified apoAI lysine residues by reductive methylation, turning lysine’s primary amines into secondary and tertiary amines, which retain their positive charge but are less reactive to MPO oxidation than unmodified lysine.28 This methylated apoAI retained its cholesterol acceptor activity; and, it was equally susceptible as wild type apoAI to the MPO mediated loss of function, implying that lysine modification is not responsible for the MPO mediated apoAI dysfunction.21 Then we created apoAI variants in which the four tryptophan residues were replaced by either leucine or phenylalanine residues. We tested these variants for their ability to act as cholesterol acceptors and found that the leucine variant lost its activity while the phenylalanine variant retained its activity; thus, only another bulky aromatic residue could be substituted for apoAI tryptophan residues.21 When the variant with four tryptophan residues replaced with phenylalanines (called the 4WF isoform) was treated with increasing strengths of MPO modification, it was apparent that the 4WF isoform was highly resistant to losing its cholesterol acceptor activity.21 Thus, we demonstrated that MPO mediated tryptophan modification to its hydroxylated products is primarily responsible for apoAI’s loss of function. We speculated that the 4WF isoform of apoAI might be useful as a therapeutic to promote atherosclerosis regression, as it would be resistant to the abundant MPO in the atheroma, and thus be better able to mediate RCT in this environment.21
MPO can not only make lipid free apoAI dysfunctional (as described above), it can also make reconstituted HDL (rHDL) dysfunctional and proinflammatory. Hazen has shown that rHDL’s ability to accept cellular cholesterol, which is mediated by ABCG1, SR-BI, and passive diffusion, is also impaired by MPO treatment.9 We demonstrated that MPO binds to apoAI in helix 8 (residues 190 to 203).16 Plasma LCAT binds to and is activated by apoAI. LCAT converts HDL cholesterol to cholesterol ester, in the process of forming spherical mature HDL, and allows HDL to absorb more cholesterol from peripheral cells. Hazen mapped the LCAT binding site on apoAI in rHDL to residues 159–180, just adjacent to the MPO binding site. rHDL treated with MPO loses its ability to activate LCAT, which is associated with apoAI tyrosine 166 modification.29 Heinecke has demonstrated that rHDL made from an apoAI variant in which methionine-148 was replaced with a leucine is partially protected from the MPO mediated loss of LCAT activation.30
HDL and rHDL possess anti apoptotic and anti inflammatory activities on endothelial cells that are mediated via the HDL receptor SR-BI. When rHDL was incubated with MPO, Hazen and colleagues demonstrated these activities were diminished accompanied by the loss of SR-BI binding.9 Even more striking, as rHDL is modified at increasing doses of MPO, it gains a proinflammatory activity, inducing the expression of the adhesion molecule VCAM1 in endothelial cells, which is mediated by activation of the transcription factor NFκB.9 Endothelial VCAM1 expression promotes atherosclerosis by participating in monocyte entry into the artery wall during atherogenesis.
Inflammation and Dysfunctional HDL: Human Studies
There have been several studies in humans that examine the effect of inflammation on RCT. First of all, one needs to establish which fractions of human plasma contain the major cholesterol acceptors that can extract lipids from cholesterol-loaded macrophages. Rothblat and colleagues determined that the small lipid-poor pre-beta HDL, which migrates on native gels the same as lipid-free apoAI, possesses the major cholesterol acceptor activity when incubated with macrophages; and, this process is mediated by the membrane protein ABCA1.7 These results imply that cholesterol efflux via ABCA1 to lipid-free apoAI may be a key pathway in RCT from macrophages. When either human or mouse macrophages are treated with the bacterial endotoxin LPS in vitro, their ability to efflux cholesterol is inhibited due to down regulation of the transporters ABCA1 and ABCG1.31,32 These studies lend support to the hypothesis that inflammation due to LPS or other mediators that use similar cellular signaling pathways would inhibit RCT. Reilly and colleagues tested this hypothesis directly by administering low doses (3ng/kg) of LPS to human volunteers.31 As expected, they observed increased plasma levels of the acute phase reactant proteins serum amyloid A and CRP, with HDL remodeling. The HDL recovered from these subjects had decreased cholesterol acceptor activity, thus demonstrating in humans that that inflammation impairs the ability of HDL to act as a cholesterol acceptor. Tietge and colleagues studied human sepsis patients, and found that this severe inflammation was associated with a 73% reduction in the plasma cholesterol acceptor activity, compared to normal control plasma, and that this reduced cholesterol acceptor activity of the patient plasma substantially improved 3 weeks after resolution of the sepsis.8 The reduction of plasma cholesterol acceptor activity during sepsis was accompanied by decreased HDL levels and HDL remodeling with increased plasma levels of SAA, sPLA2, and MPO. They isolated HDL from the patients with sepsis and found that it had decreased cholesterol acceptor activity compared to the HDL isolated after the patients recovered, again demonstrating dysfunctional HDL associated with inflammation. Landmesser and colleagues found that HDL isolated from type 2 diabetic patients, compared to normal subjects, has less activity to promote endothelial cell nitric oxide production, endothelium dependent relaxation, and endothelial progenitor cell dependent endothelial repair.10 These impairments were associated with increased HDL lipid peroxidation and MPO content. All these activities of HDL were improved upon treatment of the diabetic subjects with extended-release niacin. Thus, some treatments that raise HDL, such as niacin, may also improve HDL functionality.
Inflammation and Dysfunctional HDL: Animal Models
Mouse models are increasingly useful to study disease pathogenesis and mechanisms. Dan Rader and colleagues first described a simple but elegant way to assay RCT in vivo in mice.33 Cultured macrophages are cholesterol loaded with [3H]cholesterol, which are then injected i.p. into recipient mice. The [3H]cholesterol radioactivity is followed over time as it enters the plasma, liver, and bile compartments, and then enters the feces. In the initial description of this protocol, Rader demonstrated that over expression of apoAI led to higher levels of HDL and increased [3H]cholesterol flowing from the macrophages to the plasma, liver, bile and feces.33 Both Reilly’s and Tietge’s groups injected mice with LPS in order to induce inflammation, and then studied the effects on RCT.8,31 Four and 24-hours after LPS injection, they found decreased [3H]cholesterol mobilization from the labeled macrophages to the plasma compartment. This effect on RCT to the plasma compartment resolved by the 48 hour time point. The reduction in RCT to the plasma was associated with reduced cholesterol acceptor activity of the HDL from those early time points. However, the major block in RCT that they observed at later time points was not at the level of the plasma. Instead, there was a dramatic decrease in the ability of the liver to excrete cholesterol, synthesize bile acids, and excrete the bile acids. These hepatic changes are associated with decreased levels of the mRNAs encoding ABCG5 and G8 (sterol excreting transporters), ABCB11 (bile acid excreting transporter), and CYP7A1 (bile acid synthesis enzyme).8,31 However, a major drawback of these studies is that the LPS injections make the mice quite sick, with reduced feeding and a 34 percent decrease in fecal output.8 Thus, any conclusion about the effects of LPS in RCT to the feces must be considered in the light of the significant inhibition of feces production. Tietge’s group performed an experiment in which MPO was directly injected i.v. into mice.8 This led to a small reduction in plasma HDL levels and the appearance of cross linked apoAI. The MPO injections also led to significant reductions in RCT to the plasma and fecal compartments, thus demonstrating a direct effect of MPO on RCT in vivo.8
We wondered if other mediators of inflammation would also impair RCT. We used zymosan, a polysaccharide derived from yeast cell walls, which induces inflammation and also activates neutrophils to undergo a respiratory burst and release reactive oxygen species. We were able to titrate the zymosan dose such that the mice had reduced food intake and fecal output on the first day, but completely recovered total fecal output by the second day; and, RCT could be assessed up to five days later. Zymosan treatment led to a 25 percent decrease in RCT from the macrophages to the liver, plasma and feces.34 But unlike the effects of LPS where the major block was in the liver, the major block with the zymosan model of inflammation occurred in the first step, getting the cholesterol from the macrophage to the plasma. Zymosan treatment was associated with HDL remodeling. We observed an increase in SAA, a small decrease in apoAI levels, and a significant 35 percent decrease in the cholesterol acceptor activity of the plasma. Thus, it appears that different models in inflammation can create dysfunctional HDL and block RCT by partially overlapping mechanisms.
The in vivo assay for RCT in mice, developed by Rader, is an excellent assay and has been adapted by many groups.33 However, it does have a limitation in that it can only study the unidirectional trafficking of the labeled cholesterol from the macrophage foam cells to the plasma, liver, and feces. This unidirectional view of RCT may not be reflective of what’s occurring in the foam cell, as cholesterol trafficking is bidirectional, going both into and out from the cells. Thus, we developed a novel foam cell regression/progression assay that combines the [3H]cholesterol RCT assay with an assay in which we can directly measure foam cell cholesterol content.35 We borrowed methodology from the angiogenesis field by using Matrigel, a protein mixture that is liquid at 4°C and gels at >22°C. By implanting [3H]cholesterol loaded macrophages in matrigel s.c into mice, we were able to measure the [3H]cholesterol over time as it goes into the plasma, liver, and feces; and, we were also able to retrieve the cells from the matrigel plug 3 to 5 days later for assay of cellular cholesterol content. In this way we could determine if cellular cholesterol decreases, indicative of foam cell regression, increases, indicative of foam cell progression, or remains stable. We validated this novel assay using apoAI-deficient, wild type, and apoAI transgenic mice. We found that RCT and foam cell cholesterol content were apoAI gene dosage dependent. The cholesterol content of the cells recovered from apoAI-deficient mice had not changed, while there was a small net decrease in the cells recovered from the wild type mice. The cells recovered from the mice over expressing apoAI had the largest reduction in cellular cholesterol, demonstrating the robust foam cell regression. We are now using this assay to discover more features of dysfunctional HDL and RCT. This 3 to 5 day assay might be useful in preclinical studies during drug development, and serve as a surrogate for atherosclerosis studies that take many weeks to complete.
Conclusions and Future Directions
In summary, apoAI and HDL are susceptible to modification during inflammation and diabetes that is accompanied by a loss of their protective activities (Figure 1). There are many steps in which inflammation and diabetes can impair HDL function and RCT, including the inhibition of macrophage cholesterol transporters, the loss of apoAI/HDL cholesterol acceptor activity, impaired hepatic sterol metabolism and excretion, and impaired HDL endothelial protective activities. Presently, assays for dysfunctional HDL are only available in research laboratories. The development of clinical assays for HDL dysfunction, perhaps based upon antibody mediated recognition of apoAI covalent modification or HDL proteomics would be very useful in order to further pin down the role of HDL dysfunction in CAD risk and in the development of specific therapeutic interventions to improve not only HDL abundance but also its protective activities.
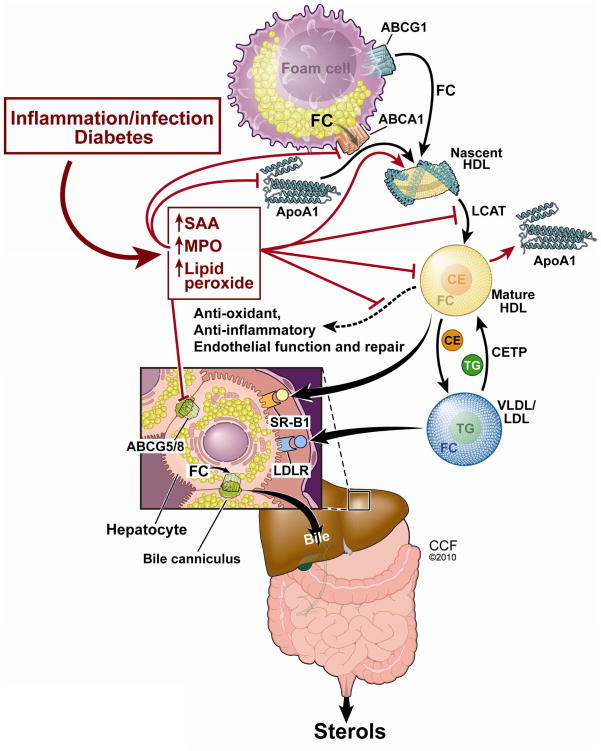
Figure 1.
Mechanisms associated with the production of dysfunctional HDL by inflammation, infection, or diabetes are shown in red. FC, free cholesterol; CE, cholesterol esters; TG, triglycerides. Illustration by David Schumick, BS, CMI. Reprinted with the permission of the Cleveland Clinic Center for Medical Art & Photography © 2010. All Rights Reserved
List of Abbreviations
- CAD
coronary artery disease
- RCT
reverse cholesterol transport
- apoAI
apolipoprotein A-I
- ABCA1
ATP binding cassette protein A1
- ABCG1
ATP binding cassette protein G1
- LCAT
lecithin cholesterol acyl transferase
- SR-BI
scavenger receptor B-I
- CETP
cholesterol ester transfer protein
- apoAII
apolipoprotein A-II
- LPS
lipopolysaccharide
- SAA
serum amyloid A
- sPLA2
secretory phospholipase A2
- MPO
myeloperoxidase
- CRP
C-reactive protein
- rHDL
reconstituted HDL
Footnotes
Conflict of Interest: The author is a consultant for and receives research support from Esperion Therapeutics.
Financial Disclosure
The author’s research in this area is supported by grants RO1 HL066082 and PO1 HL098055 from the NIH. The author is a consultant for and receives research support from Esperion Therapeutics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DJ, Bangdiwala S, Tyroler HA. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Tall AR. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J Intern Med. 2008;263:256–273. doi: 10.1111/j.1365-2796.2007.01898.x. [DOI] [PubMed] [Google Scholar]
- 3.Navab M, Imes SS, Hama SY, Hough GP, Ross LA, Bork RW, Valente AJ, Berliner JA, Drinkwater DC, Laks H. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J Clin Invest. 1991;88:2039–2046. doi: 10.1172/JCI115532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navab M, Hama SY, Hough GP, Subbanagounder G, Reddy ST, Fogelman AM. A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. J Lipid Res. 2001;42:1308–1317. [PubMed] [Google Scholar]
- 5.Van Lenten BJ, Hama SY, de Beer FC, Stafforini DM, McIntyre TM, Prescott SM, La Du BN, Fogelman AM, Navab M. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest. 1995;96:2758–2767. doi: 10.1172/JCI118345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ansell BJ, Navab M, Hama S, Kamranpour N, Fonarow G, Hough G, Rahmani S, Mottahedeh R, Dave R, Reddy ST, Fogelman AM. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation. 2003;108:2751–2756. doi: 10.1161/01.CIR.0000103624.14436.4B. [DOI] [PubMed] [Google Scholar]
- 7.de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol. 2010;30:796–801. doi: 10.1161/ATVBAHA.109.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Annema W, Nijstad N, Tolle M, de Boer JF, Buijs RV, Heeringa P, van der Giet M, Tietge UJ. Myeloperoxidase and serum amyloid A contribute to impaired in vivo reverse cholesterol transport during the acute phase response but not group IIA secretory phospholipase A(2) J Lipid Res. 2010;51:743–754. doi: 10.1194/jlr.M000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Undurti A, Huang Y, Lupica JA, Smith JD, Didonato JA, Hazen SL. Modification of high density lipoprotein by myeloperoxidase generates a pro-inflammatory particle. J Biol Chem. 2009 doi: 10.1074/jbc.M109.047605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorrentino SA, Besler C, Rohrer L, Meyer M, Heinrich K, Bahlmann FH, Mueller M, Horvath T, Doerries C, Heinemann M, Flemmer S, Markowski A, Manes C, Bahr MJ, Haller H, von EA, Drexler H, Landmesser U. Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation. 2010;121:110–122. doi: 10.1161/CIRCULATIONAHA.108.836346. [DOI] [PubMed] [Google Scholar]
- 11.van der Westhuyzen DR, de Beer FC, Webb NR. HDL cholesterol transport during inflammation. Curr Opin Lipidol. 2007;18:147–151. doi: 10.1097/MOL.0b013e328051b4fe. [DOI] [PubMed] [Google Scholar]
- 12.Davidson WS, Silva RA, Chantepie S, Lagor WR, Chapman MJ, Kontush A. Proteomic Analysis of Defined HDL Subpopulations Reveals Particle-Specific Protein Clusters. Relevance to Antioxidative Function. Arterioscler Thromb Vasc Biol. 2009 doi: 10.1161/ATVBAHA.109.186031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castellani LW, Navab M, Van Lenten BJ, Hedrick CC, Hama SY, Goto AM, Fogelman AM, Lusis AJ. Overexpression of apolipoprotein AII in transgenic mice converts high density lipoproteins to proinflammatory particles. J Clin Invest. 1997;100:464–474. doi: 10.1172/JCI119554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Lenten BJ, Wagner AC, Nayak DP, Hama S, Navab M, Fogelman AM. High-density lipoprotein loses its anti-inflammatory properties during acute influenza a infection. Circulation. 2001;103:2283–2288. doi: 10.1161/01.cir.103.18.2283. [DOI] [PubMed] [Google Scholar]
- 15.Pirillo A, Uboldi P, Bolego C, Kuhn H, Catapano AL. The 15-lipoxygenase-modified high density lipoproteins 3 fail to inhibit the TNF-alpha-induced inflammatory response in human endothelial cells. J Immunol. 2008;181:2821–2830. doi: 10.4049/jimmunol.181.4.2821. [DOI] [PubMed] [Google Scholar]
- 16.Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, Schmitt D, Fu X, Thomson L, Fox PL, Ischiropoulos H, Smith JD, Kinter M, Hazen SL. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94:437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brennan ML, Penn MS, Van Lente F, Nambi V, Shishehbor MH, Aviles RJ, Goormastic M, Pepoy ML, McErlean ES, Topol EJ, Nissen SE, Hazen SL. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349:1595–1604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 19.Peng DQ, Wu Z, Brubaker G, Zheng L, Settle M, Gross E, Kinter M, Hazen SL, Smith JD. Tyrosine modification is not required for myeloperoxidase-induced loss of apolipoprotein A-I functional activities. J Biol Chem. 2005;280:33775–33784. doi: 10.1074/jbc.M504092200. [DOI] [PubMed] [Google Scholar]
- 20.Zheng L, Settle M, Brubaker G, Schmitt D, Hazen SL, Smith JD, Kinter M. Localization of nitration and chlorination sites on apolipoprotein A-I catalyzed by myeloperoxidase in human atheroma and associated oxidative impairment in ABCA1-dependent cholesterol efflux from macrophages. J Biol Chem. 2005;280:38–47. doi: 10.1074/jbc.M407019200. [DOI] [PubMed] [Google Scholar]
- 21.Peng DQ, Brubaker G, Wu Z, Zheng L, Willard B, Kinter M, Hazen SL, Smith JD. Apolipoprotein A-I Tryptophan Substitution Leads to Resistance to Myeloperoxidase-Mediated Loss of Function. Arterioscler Thromb Vasc Biol. 2008;28:2063–2070. doi: 10.1161/ATVBAHA.108.173815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergt C, Oettl K, Keller W, Andreae F, Leis HJ, Malle E, Sattler W. Reagent or myeloperoxidase-generated hypochlorite affects discrete regions in lipid-free and lipid-associated human apolipoprotein A-I. Biochem J. 2000;346(Pt 2):345–354. [PMC free article] [PubMed] [Google Scholar]
- 23.Hazen SL, Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest. 1997;99:2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pennathur S, Bergt C, Shao B, Byun J, Kassim SY, Singh P, Green PS, McDonald TO, Brunzell J, Chait A, Oram JF, O’brien K, Geary RL, Heinecke JW. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J Biol Chem. 2004;279:42977–42983. doi: 10.1074/jbc.M406762200. [DOI] [PubMed] [Google Scholar]
- 25.Bergt C, Pennathur S, Fu X, Byun J, O’brien K, McDonald TO, Singh P, Anantharamaiah GM, Chait A, Brunzell J, Geary RL, Oram JF, Heinecke JW. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci U S A. 2004;101:13032–13037. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao B, Oda MN, Bergt C, Fu X, Green PS, Brot N, Oram JF, Heinecke JW. Myeloperoxidase impairs ABCA1-dependent cholesterol efflux through methionine oxidation and site-specific tyrosine chlorination of apolipoprotein A-I. J Biol Chem. 2006;281:9001–9004. doi: 10.1074/jbc.C600011200. [DOI] [PubMed] [Google Scholar]
- 27.Panzenbock U, Kritharides L, Raftery M, Rye KA, Stocker R. Oxidation of methionine residues to methionine sulfoxides does not decrease potential antiatherogenic properties of apolipoprotein A-I. J Biol Chem. 2000;275:19536–19544. doi: 10.1074/jbc.M000458200. [DOI] [PubMed] [Google Scholar]
- 28.Brubaker G, Peng DQ, Somerlot B, Abdollahian DJ, Smith JD. Apolipoprotein A-I lysine modification: effects on helical content, lipid binding and cholesterol acceptor activity. Biochim Biophys Acta. 2006;1761:64–72. doi: 10.1016/j.bbalip.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Wu Z, Wagner MA, Zheng L, Parks JS, Shy JM, III, Smith JD, Gogonea V, Hazen SL. The refined structure of nascent HDL reveals a key functional domain for particle maturation and dysfunction. Nat Struct Mol Biol. 2007;14:861–868. doi: 10.1038/nsmb1284. [DOI] [PubMed] [Google Scholar]
- 30.Shao B, Cavigiolio G, Brot N, Oda MN, Heinecke JW. Methionine oxidation impairs reverse cholesterol transport by apolipoprotein A-I. Proc Natl Acad Sci U S A. 2008;105:12224–12229. doi: 10.1073/pnas.0802025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGillicuddy FC, de la Llera MM, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, Rothblat GH, Reilly MP. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119:1135–1145. doi: 10.1161/CIRCULATIONAHA.108.810721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khovidhunkit W, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. Endotoxin down-regulates ABCG5 and ABCG8 in mouse liver and ABCA1 and ABCG1 in J774 murine macrophages: differential role of LXR. J Lipid Res. 2003;44:1728–1736. doi: 10.1194/jlr.M300100-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Zanotti I, Reilly MP, Glick JM, Rothblat GH, Rader DJ. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 2003;108:661–663. doi: 10.1161/01.CIR.0000086981.09834.E0. [DOI] [PubMed] [Google Scholar]
- 34.Smith JD. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2008;118:S453. doi: 10.1161/CIRCULATIONAHA.108.810721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malik P, Smith JD. A novel in vivo assay for reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2009;29:e46. [Google Scholar]