Abstract
The Adenoma Prevention with Celecoxib (APC) Trial examined the efficacy and safety of the Cox-2 inhibitor, celecoxib, for sporadic colorectal adenoma prevention in patients at high risk for colorectal cancer (CRC). The trial randomized 2035 subjects to receive either placebo, celecoxib 200mg twice daily, or celecoxib 400mg twice daily. The primary study safety and efficacy analyses involved three years of treatment. The results showed significant anti-tumor effect, but also indicated increased cardiovascular adverse events in patients treated with celecoxib compared to placebo. A total of 933 patients participated in an extension of the APC Trial, with a planned total treatment and surveillance duration of 5 years. Study medication was stopped early, resulting in a median treatment duration of 3.1 years. Patients treated on the placebo arm had a cumulative adenoma incidence of 68.4% over 5 years of observation. This figure was 59.0% (p<0.0001) for those receiving low dose celecoxib, and 60.1% (p<0.0001) for those receiving high dose celecoxib. The cumulative incidence of advanced adenomas over 5 years was 21.3% of those taking placebo, 12.5% (p<0.0001) of those taking low dose celecoxib and 15.8% (p<0.0001) of those taking high dose celecoxib. Investigator reported treatment emergent adverse events were similar across all treatment groups for categories including renal and hypertensive events and gastrointestinal ulceration and hemorrhage events. For a category composed of cardiovascular and thrombotic events, the risk relative to placebo was 1.6 (95%CI 1.0, 2.5) for those using 200mg BID celecoxib and 1.9 (95%CI 1.2, 3.1) for those using 400mg BID celecoxib. Secondary analysis showed an interaction between a baseline history of atherosclerotic heart disease and study drug use with respect to cardiovascular and thrombotic adverse events (p=0.004). These results confirm the inhibitory effect of celecoxib on colorectal adenoma formation, and provide additional safety data indicating an elevated risk for cardiovascular and thrombotic adverse events, particularly for patients with pre-existing atherosclerotic heart disease.
Introduction
Although colorectal cancer (CRC) is a common cause of cancer mortality, the risk for this disease is reduced by as much as 90% following removal of pre-malignant adenomas by endoscopic polypectomy (1). Unfortunately, this procedure is uncomfortable and expensive, resulting in an unacceptably low level of utilization even among populations for whom health care access is not an issue (2). Chemoprevention using non-steroidal antiiflammatory drugs (NSAIDs) is a possible adjunct to endoscopic polypectomy. Prospective randomized trials in patients with familial adenomatous polyposis (FAP) found that both sulindac and celecoxib produced regression of existing adenomas (3, 4). NSAIDs have also been tested in large studies of patients at high risk of sporadic CRC because of a history of previously resected adenomas. These clinical trials documented significant reductions in adenoma recurrence for patients treated with aspirin (5, 6), sulindac (7), celecoxib (8), and rofecoxib (9). Important data for the field of chemoprevention were also obtained from large placebo-controlled trials of aspirin for cardiovascular protection. These studies, conducted in the UK, showed that CRC incidence was decreased by 40% among those randomized to aspirin, with a timepoint determined at 10–19 years from study randomization (10). This result is significant because these studies of preventive health care were conducted in a patient population with access to colonoscopy, suggesting that chemoprevention would have a major impact on cancer mortality for patients at risk who do not adhere to colon cancer screening guidelines.
Although recent randomized controlled trials showed that NSAIDs effectively prevented colorectal adenomas, they also raised significant safety concerns. Long term aspirin use is associated with a 1.6–3.1 fold increased risk of serious gastrointestinal adverse events, including gastroduodenal ulcers and major gastrointestinal bleeding (11, 12). These events are even more common for other non-selective NSAIDs such as sulindac (12). Selective cyclooxygenase-2 (Cox-2) inhibitors, such as celecoxib and rofecoxib, are associated with fewer gastrointestinal toxicities due to their relative inactivity against Cox-1, the cyclooxygenase isoform responsible for protecting the gastric mucosa. Evidence for this comes from trials for arthritis patients, where selective Cox-2 inhibitors demonstrated a better safety profile than non-selective NSAIDs (13–16). However, one arthritis study, the VIGOR trial, also found that patients treated with rofecoxib had greater numbers of serious cardiovascular adverse events than those receiving naproxen (14). An association with cardiovascular toxicity was also identified in two randomized colorectal adenoma prevention trials that compared rofecoxib to placebo (APPROVe Trial) and celecoxib to placebo (The APC Trial) (17, 18).
The Adenoma Prevention with Celecoxib (APC) Trial was a randomized placebo-controlled trial of celecoxib for prevention of colorectal adenomas in patients at high risk for CRC because of a history of colorectal adenomas that were either large (≥6 mm diameter) or multiple. A total of 2035 patients were randomized to receive placebo (679 patients), 200mg celecoxib twice daily (685 patients), or 400mg celecoxib twice daily (671 patients). The primary aim of the APC Trial was to assess the efficacy and safety of celecoxib for preventing colorectal adenomas over a 3 year treatment and surveillance interval. After completing the 3 year initial study period, patients were offered participation in a double-blind extension of the APC Trial allowing them to either remain on study medication for an additional 2 years or to participate in an off-treatment surveillance extension of the trial, with a final colonoscopy at study exit 5 years after randomization. Accrual to the APC Trial was completed in March, 2002. In late 2004, rofecoxib was withdrawn from the market due to recognition of its association with increased risk of serious cardiovascular adverse events. This led APC Trial investigators to conduct an independent adjudicated review of selected cardiovascular safety endpoints at a time when the trial was still in progress. This analysis showed 2.6- and 3.4-fold increases in selected cardiovascular events compared to placebo in patients receiving celecoxib at doses of 200mg bid and 400mg bid, respectively (18). In response, all study medication use was discontinued on December 17, 2004, a date approximately 3 months before the final randomized patients were scheduled to complete the 3 year treatment interval. Although use of study medication was terminated, patients enrolled in the APC Trial extension study remained on study for the full 5 years, with continued collection of safety information and year 5 colonoscopic surveillance.
The primary efficacy and safety endpoints of the 3 year APC Trial have been reported previously (8, 18). Significant anti-tumor efficacy was identified. For patients on placebo, the incidence of newly detected adenomas by year 3 was 60.7%, confirming that the APC Trial included a high adenoma risk cohort. New adenoma detection during 3-year surveillance interval was reduced 33% for those on 200mg celecoxib bid, and 45% for those on 400mg celecoxib bid. Here, we report the results of the 5 year extension of the APC Trial.
Study Design
The APC Trial tested whether celecoxib would reduce the occurrence of endoscopically-detected colorectal adenomas. Treatment consisted of either placebo, celecoxib 200mg bid, or celecoxib 400mg bid, with randomization stratified based upon low dose aspirin use (defined as doses of ≤ 325 mg po qod or 162.5 mg po qd) and clinical site. The trial involved 91 clinical sites; 72 in the United States, 8 in Australia, 10 in Canada, and 1 in Great Britain. Each site received human subjects committee approval of the study protocol, and all patients provided written consent prior to study enrollment. During the treatment portion of the trial, an independent data and safety monitoring board reviewed safety data monthly and efficacy data semianually.
Recruitment and Randomization
Details of participant recruitment and randomization for the APC trial cohort have been published previously (8). APC Trial participants ranged from 31 to 88 years of age at enrollment and were considered to have a high risk of recurrent colorectal adenomas based upon either multiple adenomas or removal of a single adenoma ≥6 mm in diameter. Within 3 months before enrollment, eligible patients had had a complete colonoscopy to the cecum with removal of all polyps, one or more of which was a histologically confirmed adenoma. Subjects were required to be willing to abstain from chronic use of all NSAIDs or Cox-2 inhibitors for the duration of the study. Chronic use of NSAIDs was defined as more than 21 days of use per year. Those using low dose aspirin at baseline were required to continue using this medication, and those not using aspirin at baseline were required to abstain from aspirin use during the trial. Exclusion criteria included a history of FAP, hereditary nonpolyposis colon cancer, inflammatory bowel disease, or large bowel resection other than appendectomy. Other exclusions included a history of a renal or hepatic disorder, a significant bleeding disorder, or treatment for a gastrointestinal ulcer in the month prior to study entry. Patients were also ineligible if they used NSAIDs or high dose aspirin at a frequency ≥ three times per week during the two months prior to randomization, or if they used oral or intravenous corticosteroids for more than 2 weeks in the 6 months prior to randomization.
Participants randomized to the APC trial who successfully completed the 3 year study interval were offered the option of continuing on an extension study, allowing them to continue taking study medication in a blinded manner for an additional 2 years. In addition, participants not wishing to remain on study medication were allowed to continue in an additional 2 year surveillance arm. At 5 years after initial randomization, participants in the extension study underwent a colonoscopy for identification and removal of colorectal polyps.
Study Treatment
Study medication was distributed in gelatin capsules containing 100 mg celecoxib for the 200mg bid arm, 200mg celecoxib for the 400mg bid arm, or placebo, each identical in appearance. Subjects were provided medication at 6 month intervals, and they were instructed to take two capsules with food in the morning and in the evening each day. Low dose aspirin was supplied for subjects already taking this medication, and acetaminophen was supplied for the treatment of minor pain and febrile illnesses. Study medication use was discontinued for all APC Trial participants on December 17, 2004. Patients then returned to usual care, which entailed colonoscopic surveillance without chemoprevention. After this point, all participants wishing to do so remained on study for continued collection of safety data and completion of the year 5 colonoscopy.
Endpoint Assessment and Follow-up
A complete physical examination, with determination of vital signs, complete blood count, serum chemistry studies and urinalysis was performed at baseline and 1, 3 and 5 years after randomization. Subjects on treatment were contacted every 2 months by researchers at their study site to record concomitant medication use and report any adverse events. Subjects who were no longer using study medication were contacted by telephone every 6–12 months for retrospective reporting of serious adverse events. During these discussions, patients were also counseled to avoid non-protocol use of aspirin and NSAIDs.
A study investigator performed a complete colonoscopy with visualization of the cecum and endoscopic removal of all polyps 1 year, 3 years, and 5 years after randomization. All polyps removed during these colonoscopies were reviewed by a central study pathologist. If the central study pathologist and institutional pathologist disagreed, polyps were reviewed by an adjudicating pathologist who was blinded to the previous histological diagnosis and whose independent opinion resolved the discrepancy. Investigator-reported adverse events were classified according to MedRA 8.1 criteria.
Statistical Analysis
All primary efficacy analyses were performed on an intention-to-treat basis, with primary endpoints determined for all patients with follow-up colonoscopies regardless of whether the patient complied with study drug use. The primary efficacy endpoint was detection of an adenoma during a post-randomization colonoscopy. Secondary endpoints included detection of advanced adenomas, defined as those having any of the following characteristics: size ≥ 1 cm by endoscopic measurement, villous or tubulovillous histology, high grade dysplasia, intramucosal carcinoma or invasive cancer.
The trial was designed with a statistical power of 96% to detect a 35% relative reduction in the proportion of subjects with adenomas detected over 3 years for celecoxib compared to placebo, independent of aspirin use. Power estimates assumed up to a 40% dropout rate, and adjusted for multiple comparisons (placebo vs celecoxib 200mg bid and celecoxib 400mg bid). Primary and secondary endpoints of adenoma and advanced adenomas, respectively, over time were compared for each treatment group using the Mantel-Cox test based on a life-table extension of the Mantel-Haenszel statistic with stratification for aspirin use at baseline (19–21). Subjects without follow-up colonoscopies were excluded from the analysis at both time points. A patient with a year 3 colonoscopy but with no colonoscopy at year 1 was included in the analysis at year 1 with the assumption that the patient had no adenoma at year 1, then included in the analysis at year 3 according to the findings of the year 3 colonoscopy. A patient with an adenoma at the year 1 colonoscopy was not included in the year 3 analysis. A similar strategy was employed for the year 5 analysis to determine the cumulative percent with adenomas or advanced adenomas over the five years. In addition the percentage of patients in the extension study with any adenoma detected at the year 5 colonoscopy were compared by treatment assignment and the relative risk for celecoxib compared to placebo was obtained.
Investigator-reported adverse events were analyzed in total and according to pre-specified categories to describe renal/hypertensive disorders, gastrointestinal ulceration/hemorrhage and cardiovascular and thromboembolic disorders. All events occurring following the first dose of study medication use up to 30 days after the last dose of study medication were included in these analyses. Homogeneity of treatment effect on the cardiovascular and thromboembolic disorders safety endpoint defined above was tested in subgroups defined by baseline aspirin use and cardiovascular risk factors: smoking, hyperlipidemia, diabetes, hypertension, age, artherosclerosis, and cerebrovascular disease, as well as number of baseline risk factors (0,1, 2 or more).
Results
Cohort charateristics and treatment
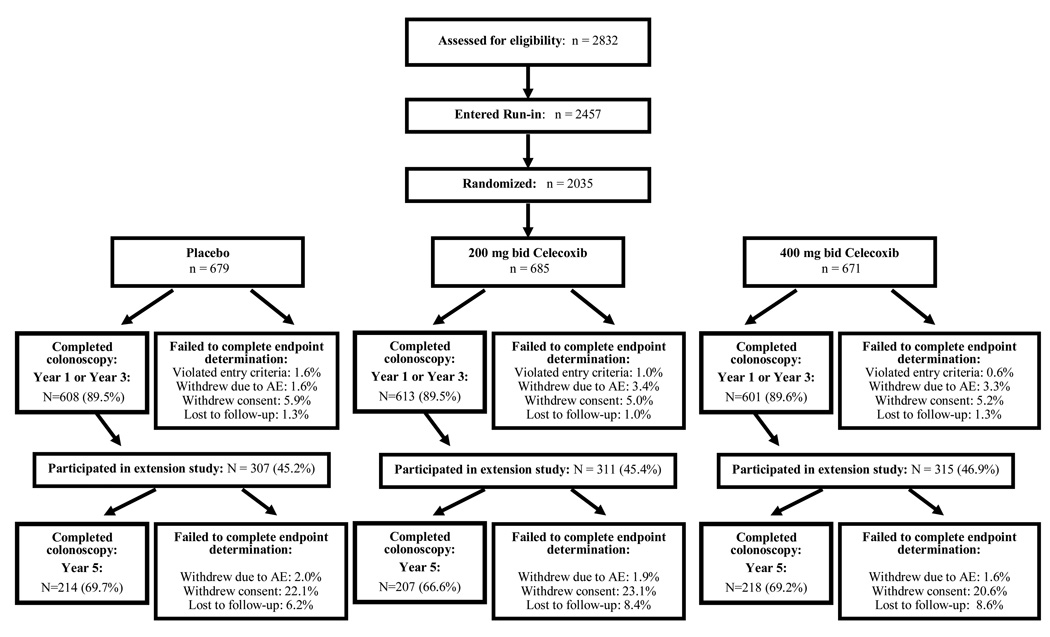
Baseline variables were similar across all treatment groups, and there were no significant differences for these values when the set of all patients randomized to the APC Trial were compared to those completing the year 5 colonoscopy (Table 1). Variables associated with risk of developing colorectal adenomas, such as age, CRC family history, and adenoma size and number were also balanced among treatment groups. Colonoscopic endpoints were assessed in 1822 (89.5%) randomized subjects; 1541 (75.7%) completed the year 3 examination and 639 (31.4%) completed the year 5 examination (Figure 1). Reasons for patient withdrawal from the study before endpoint determination included withdrawal of consent for participation, withdrawal following an adverse event, and failure to respond to investigator's requests for follow-up (Figure 1). Subjects who failed to complete the study were evenly distributed among the treatment groups. During the treatment interval, compliance with study medication use, as determined by pill count, was > 80% in two-thirds of participants, with no significant difference among the treatment groups (8).
Table 1.
Baseline Patient Characteristics
| Characteristic | Placebo twice daily |
200mg celecoxib twice daily |
400mg celecoxib twice daily |
|---|---|---|---|
| All APC Trial Participants | n=679 | n=685 | n=671 |
| Age – yr mean (range) | 59 (31–88) | 59 (35–83) | 59 (34–87) |
| Male sex – no. (%) | 473 (69.7) | 460 (67.2) | 454 (67.7) |
| Mean Body-Mass Index – kg/m2 | 28.8(M) 29.3 (F) | 28.9 (M) 28.5 (F) | 28.6 (M) 29.0 (F) |
| Race or ethnic group — no. (%) | |||
| Non-Hispanic white | 624 (91.9) | 632 (92.3) | 607 (90.5) |
| Non-Hispanic black | 37 (5.4) | 30 (4.4) | 45 (6.7) |
| Hispanic | 11 (1.6) | 16 (2.3) | 10 (1.5) |
| Asian, Pacific Islander, or other | 7 (1.0) | 7 (1.0) | 9 (1.3) |
| Colorectal cancer in a parent — no. (%) | 140 (20.6) | 147 (21.5) | 142 (21.2) |
| No. of reported adenomas | 2.0±0.1 | 2.1±0.1 | 2.1±0.1 |
| At least one adenoma ≥1 cm — no. (%) | 288 (42.4) | 303 (44.2) | 291 (43.4) |
| Multiple adenomas — no. of patients (%) | 374 (55.1) | 375 (54.7) | 363 (54.1) |
| Adenoma burden — cm1 | 1.48±0.05 | 1.50±0.05 | 1.47±0.04 |
| Use of low-dose aspirin — no. (%)2 | 212 (31.2) | 211 (30.8) | 204 (30.4) |
| History of cardiovascular events — no.(%)3 | 99 (14.6) | 94 (13.7) | 99 (14.8) |
| History of hypertension — no. (%) | 280 (41.2) | 290 (42.3) | 264 (39.3) |
| History of diabetes — no. (%) | 61 (9.0) | 67 (9.8) | 66 (9.8) |
| Current cigarette smoker — no. (%) | 122 (18.0) | 119 (17.4) | 96 (14.3) |
| All APC Trial Extension Study Participants | n=307 | n=311 | n=315 |
| Age – yr mean (range) | 59 (31–88) | 59 (35–83) | 59 (34–87) |
| Male sex – no. (%) | 222 (72.3) | 221 (71.1) | 215 (68.3) |
| Mean Body-Mass Index – kg/m2 | 28.7 (M) 28.6 (F) | 28.3 (M) 27.8 (F) | 28.6 (M) 29.0 (F) |
| Race or ethnic group – no. (%) | |||
| Non-Hispanic White | 289 (94.1) | 291 (93.6) | 287 (91.1) |
| Non-Hispanic Black | 10 (3.3) | 10 (3.2) | 21 (6.6) |
| Hispanic | 4 (1.3) | 8 (2.6) | 3 (1.0) |
| Asian, Pacific Islander, or other | 4 (1.3) | 2 (0.6) | 4 (1.3) |
| APC Trial Extension Study Participants With Year 5 Colonoscopy Results | n=214 | n=207 | n=218 |
| Age – yr mean (range) | 59 (35–88) | 59 (35–79) | 59 (38–79) |
| Male sex – no. (%) | 148 (69.2) | 152 (73.4) | 155 (71.1) |
| Mean Body-Mass Index – kg/m2 | 28.7 (M) 28.6 (F) | 28.3 (M) 28.2 (F) | 28.9 (M) 28.9 (F) |
| Colorectal cancer in a parent – no. (%) | 57 (26.6) | 45 (21.7) | 48 (22.0) |
| No. of reported adenomas at baseline - mean (SE) | 2.2 (0.1) | 2.1 (0.1) | 2.1 (0.1) |
| At least one adenoma ≥ 1 cm at baseline – no. (%) | 82 (38.3) | 88 (42.5) | 74 (43.1) |
| Multiple adenomas at baseline – no. (%) | 120 (56.1) | 115 (55.6) | 119 (55.3) |
| Adenoma burden at baseline1 - mean (SE) | 1.51 (0.09) | 1.51 (0.10) | 1.47 (0.08) |
defined as sum of diameter of all adenomas, in cm
low dose aspirin = ≤100 mg per day or 325 mg every other day
defined as a history of angina, myocardial infarction, coronary artery disease, congestive heart failure, or cerebrovascular disease
Figure 1. APC trial CONSORT-E flowchart.
Reasons for withdrawal from the study prior to efficacy endpoint determination were classified as follows: 1) Violation of study entry criteria, e.g., unable to confirm the presence of an adenoma on baseline colonoscopy; 2) Withdrew following development of an adverse event or other medical reason; 3) Withdrew consent for study participation, including all study withdrawal for non-medical reasons, failed to complete a post-randomization colonoscopy for non-medical reasons, or other protocol non-compliance; 4) Lost to follow-up, i.e., investigators were unable to contact patient following randomization despite repeated attempts. Study sites reported the first and last days of medication use for each participant. Compliance with study medication use was calculated as the duration of study medication use in days, divided by 1095 days.
Because of the discontinuation of study medication in December 2004, the colonoscopies conducted at 1 and 3 years after randomization reflected a period of maximal treatment exposure. For those who received a year 5 colonoscopy, the median duration of treatment exposure was just over 3 years, with an even distribution among treatment groups (Table 2). Approximately 5% of those having a year 5 colonoscopy received more than 4 years of study medication. Therefore, >90% of those receiving a year 5 colonoscopy had been off study medication for a year or more at the time of the final colonoscopy. Safety was assessed using investigator reported, treatment emergent data, including all patients receiving at least one dose of study drug. For this population, treatment duration was also balanced across the study arms, with a median treatment duration of approximately 2.95 years (range 0.01–4.38 years) (Table 2).
Table 2.
Duration of treatment exposure
| Placebo twice daily |
200mg celecoxib twice daily |
400mg celecoxib twice daily |
|
|---|---|---|---|
| Efficacy Population1 | |||
| All Randomized in both APC core and extension study – no. (% out of total randomized) | 679 (33.4) | 685 (33.7) | 671 (32.9) |
| Subjects (no. - % of arm randomized) whose duration of treatment lasted: | |||
| 0–2 years | 203 (29.9) | 199 (29.0) | 197 (29.4) |
| >2–3 years | 220 (32.4) | 220 (32.1) | 176 (26.2) |
| >3–4 years | 246 (36.2) | 243 (35.5) | 282 (42.0) |
| >4 years | 10 (1.5) | 23 (3.4) | 16 (2.4) |
| median (range) - years | 2.95 (0.002–4.22) | 2.94 (0.01–4.34) | 2.99 (0.02–0.439) |
| APC Trial Extension Study Participants with year 5 colonoscopy results – no. (%) | 214 (31.5) | 207 (30.0) | 218 (32.5) |
| Subjects (no. - % with Year 5 colonoscopy results) whose duration of treatment lasted: | |||
| 0–2 years | 7 (3.3) | 8 (3.9) | 8 (3.7) |
| >2–3 years | 74 (34.6) | 64 (30.9) | 60 (27.5) |
| >3–4 years | 125 (58.4) | 121 (58.5) | 138 (63.3) |
| >4 years | 8 (3.7) | 14 (6.8) | 12 (5.5) |
| median (range) - years | 3.02 (0.76–4.22) | 3.05 (0.14–4.33) | 3.07 (0.02–4.38) |
| Safety Population2 | |||
| All Participants treated in both APC core and extension study – no. (% out of total participants treated) | 676 (33.3) | 683 (33.7) | 669 (33.0) |
| Subjects (no. - %) whose duration of treatment lasted: | |||
| 0–2 years | 200 (29.6) | 197 (28.8) | 195 (29.1) |
| >2–3 years | 220 (32.5) | 220 (32.2) | 176 (26.3) |
| >3–4 years | 246 (36.4) | 243 (35.6) | 282 (42.2) |
| >4 years | 10 (1.5) | 23 (3.4) | 16 (2.4) |
| median (range) - years | 2.95 (0.002–4.22) | 2.94 (0.01–4.34) | 2.99 (0.02–0.439) |
Efficacy was determined by comparing the cumulative incidence of adenoma detection over 5 years of surveillance
Safety analyses included all investigator-reported events occurring in the interval from first study drug use to 30 days after last study drug use
Adenoma prevention
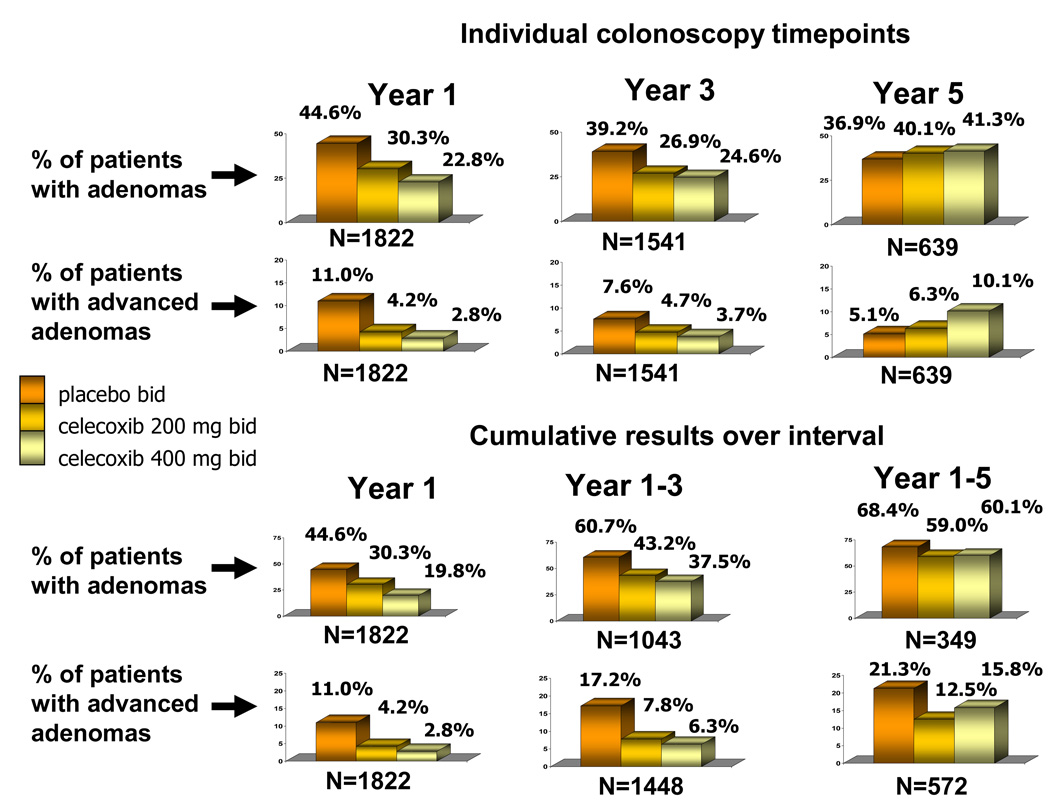
The primary efficacy analysis considered adenomas detected at any time after randomization (Table 3). For the initial 3 year study period, the estimated cumulative incidence for one or more adenomas detected were 60.7%, 43.2%, and 37.5% for patients taking placebo, 200mg celecoxib bid and 400mg of celecoxib bid, respectively (8). This corresponds to a risk ratio (RR) of 0.67 (95%CI 0.59, 0.77) in the 200mg bid users and 0.55 (95%CI 0.48, 0.64) in the 400mg bid users. For the interval from randomization through the 5 year colonoscopy, the cumulative incidence of adenomas detected was 68.4% for placebo users, and 59.0% (RR 0.71; 95%CI 0.62, 0.80), and 60.1% (RR 0.62; 95%CI 0.54, 0.71) for patients taking 200mg celecoxib bid and 400mg of celecoxib bid, respectively. This represents a significant reduction in adenoma incidence over 5 years compared to placebo for both treatment groups with 29% reduction for 200mg BID celecoxib relative to placebo (p<0.0001) and 38% reduction for 400mg BID celecoxib relative to placebo (p<0.0001). The rates of adenoma detection over 5 years for those without and with concomitant low-dose aspirin use were 58.6% and 59.7%, respectively, for the 200mg celecoxib bid group and 60.4% and 59.7%, respectively, for the 400mg celecoxib bid group. These values were also significantly lower than those of the placebo group, which were 69.2% and 66.9% for aspirin non-users and users, respectively (p≤0.01 for all comparisons).
Table 3.
Risk of Adenomas
| Variable | Placebo twice daily |
200mg celecoxib twice daily |
400mg celecoxib twice daily |
|---|---|---|---|
| All subjects – detection of any adenoma | n=679 | n=685 | n=671 |
| Year 1 colonoscopy – no. with any adenoma/total no. at risk (%) | 271/608 (44.6) | 186/613 (30.3) | 137/601 (22.8) |
| Year 3 colonoscopy – no. with any adenoma/total no. at risk (%) | 83/286 (29.0) | 66/357 (18.5) | 76/400 (19.0) |
| Year 5 colonoscopy – no. with any adenoma/total no. at risk (%) | 16/81 (19.8) | 33/119 (27.7) | 54/149 (36.2) |
| Cumulative incidence of adenoma detection through year 3 - % (SE) | 60.7 (2.1) | 43.2 (2.1) | 37.5 (2.1) |
| Risk Ratio (95% CI) | 0.67 (0.59, 0.77) | 0.55 (0.48, 0.64) | |
| p-value vs placebo | <0.0001 | <0.0001 | |
| Cumulative incidence of adenoma detection through year 5 - % (SE) | 68.4 (2.4) | 59.0 (2.8) | 60.1 (2.8) |
| Risk Ratio (95% CI) | 0.71 (0.62, 0.80) | 0.62 (0.54, 0.71) | |
| p-value vs placebo | <0.0001 | <0.0001 | |
| Year 5 colonoscopy – no. with any adenoma at year 5 colonoscopy/total no. with year 5 colonoscopy(%) | 79/214 (36.9) | 83/207 (40.1) | 90/218 (41.3) |
| Risk Ratio (95% CI) | 1.09 (0.85,1.38) | 1.11 (0.89. 1.41) | |
| p-value vs placebo | 0.51 | 0.38 | |
| Subjects using aspirin | n=212 | n=211 | n=204 |
| Cumulative incidence of adenoma detection through year 5 - % (SE) | 66.9 (4.2) | 59.7 (5.1) | 59.7 (5.0) |
| Risk Ratio (95% CI) | 0.75 (0.60, 0.94) | 0.63 (0.49, 0.81) | |
| p-value, vs placebo | 0.01 | 0.0002 | |
| Year 5 colonoscopy – no. with any adenoma at year 5 colonoscopy/total no. with year 5 colonoscopy(%) | 21/71 (29.6) | 30/67 (44.8) | 32/71 (45.1) |
| Risk Ratio (95% CI) | 1.514 (.97, 2.37) | 1.524 (.98, 2.37) | |
| p-value vs placebo | 0.066 | 0.057 | |
| Subjects not using aspirin | n=467 | n=474 | n=467 |
| Cumulative incidence of adenoma detection through year 5 - % (SE) | 69.2 (3.0) | 58.6 (3.3) | 60.4 (3.4) |
| Risk Ratio (95% CI) | 0.69 (0.59, 0.80) | 0.61 (0.52, 0.72) | |
| p-value vs placebo | <0.0001 | <0.0001 | |
| Year 5 colonoscopy – no. with any adenoma at year 5 colonoscopy/total no. with year 5 colonoscopy(%) | 58/143 (40.6) | 53/140 (37.9) | 58/147 (39.5) |
| Risk Ratio (95% CI) | .93 (.7, 1.3) | .97 (.73, 1.28) | |
| p-value vs placebo | .64 | .81 | |
| All subjects – detection of advanced adenomas 1 | n=679 | n=685 | n=671 |
| Year 1 colonoscopy – no. with advanced adenoma/total no. at risk (%) |
68/608 (11.2) | 26/613 (4.2) | 17/601 (2.8) |
| Year 3 colonoscopy – no. with advanced adenoma/total no. at risk (%) |
32/458 (7.0) | 18/487 (3.7) | 18/503 (3.6) |
| Year 5 colonoscopy – no. with advanced adenoma/total no. at risk (%) |
8/171 (4.7) | 10/194 (5.2) | 21/207 (10.1) |
| Cumulative incidence of advanced adenoma through year 3 - % (SE) | 17.4 (1.6) | 7.8 (1.1) | 6.3 (1.0) |
| Risk Ratio (95% CI) | 0.43 (0.31, 0.61) | 0.34 (0.24, 0.50) | |
| p-value vs placebo | <0.001 | <0.001 | |
| Cumulative incidence of advanced adenoma through year 5 - % (SE) | 21.3 (2.0) | 12.5 (1.8) | 15.8 (2.2) |
| Risk Ratio (95% CI) | 0.48 (0.35, 0.66) | 0.49 (0.35, 0.67) | |
| p-value vs placebo | <0.0001 | <0.0001 | |
| Year 5 colonoscopy – no. with any adenoma at year 5 colonoscopy/total no. with year 5 colonoscopy (%) | 11/214 (5.1) | 13/207 (6.3) | 22/219 (10.1) |
| Risk Ratio (95% CI) | 1.21(0.56, 2.65) | 1.95 (0.97, 3.92) | |
| p-value vs placebo | 0.621 | 0.055 | |
defined as adenomas ≥1 cm in diameter, containing villous or tubulovillous histology, high grade dysplasia, carcinoma-in-situ, or invasive carcinoma
A a previous report of year 3 data failed to include one patient in the placebo group. The values provided here reflect this correction.
As a method of estimating the effect of drug withdrawal on adenoma incidence, we examined treatment-associated differences in adenomas detected for all patients undergoing a year 5 colonoscopy (Table 3, Figure 2). At this individual timepoint for those on placebo, 36.9% had adenomas detected at year 5. This figure was 40.1% for those using 200mg bid celecoxib (RR 1.09; 95%CI 0.85, 1.38) and 41.3% for those using 400mg bid celecoxib (RR 1.11; 95%CI 0.89, 1.41).
Figure 2. Adenomas detected during study observation period.
The top six figures represent colonoscopy results for individual timepoints. The year 1 and year 3 results include only patients who did not have an adenoma detected on previous colonoscopies. The year 5 result depicts the results for all individuals receiving a year 5 colonoscopy, irrespective of whether or not one or more adenomas had been detected at previous colonoscopies. The bottom six figures report the cumulative results over 5 year observation period. The year 1 and year 3 colonoscopy results correspond to the time of planned study drug use. By the year 5 colonoscopy, approximately 94% of subjects had been off study drug for at least one year.
The development of advanced adenomas was an important secondary efficacy analysis for this trial. Advanced adenomas are those ≥1 cm in diameter, containing villous or tubulovillous histology, high grade dysplasia, carcinoma-in-situ, or invasive carcinoma. A total of 8 patients developed invasive colorectal cancer during the APC trial; three on the placebo arm and 5 on the 400mg bid celecoxib arm. These cases were included in this analysis as having developed an advanced adenoma. During the initial 3 year treatment interval, the estimated cumulative incidence of advanced adenomas was 17.4% for patients taking placebo, 7.8% for those treated with celecoxib 200mg bid and 6.3% for those treated with celecoxib 400mg bid, corresponding to risk ratios of 0.43 (95%CI 0.30,0.60) and 0.34 (95%CI 0.23,0.50), respectively. The cumulative incidence of advanced adenomas over 5 years was 21.3% in the placebo users, compared to 12.5% of those taking celecoxib 200mg bid and 15.8% of those taking celecoxib 400mg bid (p<0.0001). These values correspond to risk ratios of 0.48 (95%CI 0.35, 0.66) and 0.49 (95%CI 0.35, 0.67), respectively. When the results of the individual colonoscopy timepoint at year 5 were examined, not considering adenomas found at earlier timepoints, we found a trend toward more advanced adenomas in the high dose treatment group, with a rate of 5.1% in placebo users and 10.1% in those on 400mg celecoxib bid (RR 1.95; 95%CI 0.97, 3.92) (Table 3, Figure 2).
Adverse effects associated with treatment
Previous reports described safety results from the initial 3 year treatment interval, providing data on an intention-to-treat basis (8, 18). The results of a separate adjudicated analysis of cardiovascular serious adverse events were also reported for the 3 year study interval (18). For this final 5 year safety analysis, we examined investigator reported, treatment emergent data, including any adverse event that occurred during the time from first study medication dose to 30 days after the last study medication dose. Because the majority of patients with year 5 study data had been off study medication for more than a year, we considered this treatment emergent safety analysis to be a more accurate reflection of drug-associated toxicity.
At least one adverse event was reported in 89.9% (n=608) of placebo users, 93.9% (n=641) of 200mg bid celecoxib users, and 94.0% (n=629) of 400mg bid celecoxib users (Table 4). At least one serious adverse event was reported in 17.2% of those taking placebo, 18.3% of the 200mg bid celecoxib users (RR 1.1, 95%CI 0.8,1.3, p=0.5), and 21.2% of the 400mg celecoxib bid users (RR 1.2, 95%CI 1.0,1.5, p=0.06). Only one subject experienced a serious complication as a result of a study colonoscopy. This patient on the control arm was successfully treated for a grade 3 post-polypectomy bleed. Pre-specified analyses separately examined non-adjudicated investigator reports of renal/hypertensive disorders and gastrointestinal ulceration/hemorrhage events. No consistent dose-related trends toward increased incidence of renal/hypertensive disorders or gastrointestinal ulceration/hemorrhage events were observed.
Table 4.
Incidence of Adverse Events Following Randomization
| Adverse Event1 | Placebo twice daily |
200mg celecoxib twice daily |
400mg celecoxib twice daily |
|---|---|---|---|
| All serious adverse events - no. subjects experiencing event (%) | 116 (17.2) | 125 (18.3) | 142 (21.2) |
| Risk ratio (95% CI) | 1.1 (0.8,1.3) | 1.2 (1.0,1.5) | |
| Deaths | 7 (1.04) | 9 (1.32) | 13 (1.94) |
| Risk ratio (95% CI) | 1.3 (0.5,3.4) | 1.9 (0.8,4.7) | |
| Any adverse event – no. subjects with AE/no. subjects in cohort (%) | 608/676 (89.9) | 641/683 (93.9) | 629/669 (94.0) |
| Risk ratio (95% CI) | 1.0 (1.0,1.1) | 1.0 (1.0,1.1) | |
| Renal/Hypertensive disorders2 | 119/676 (17.6) | 149/683 (21.8) | 122/669 (18.2) |
| Risk ratio (95% CI) | 1.2 (1.0,1.5) | 1.0 (0.8,1.3) | |
| Gastrointestinal ulceration and hemorrhage3 | 70/676 (10.4) | 73/683 (10.7) | 63/669 (9.4) |
| Risk ratio (95% CI) | 1.0 (0.8,1.4) | 0.9 (0.7,1.3) | |
| Cardiovascular and thromboembolic disorders4 | 26/676 (3.8) | 41/683 (6.0) | 50/669 (7.5) |
| Risk ratio (95% CI) | 1.6 (1.0,2.5) | 1.9 (1.2,3.1) | |
| Aspirin users – Any adverse event – no. subjects with AE/no. subjects using aspirin (%) | 201/219 (91.8) | 193/205 (94.1) | 198/211 (93.8) |
| Risk ratio (95% CI) | 1.0 (1.0,1.1) | 1.0 (1.0,1.1) | |
| Renal/Hypertensive disorders | 39/219 (17.8) | 54/205 (26.3) | 33/211 (15.6) |
| Risk ratio (95% CI) | 1.5 (1.0,2.1) | 0.9 (0.6,1.3) | |
| Gastrointestinal ulceration and hemorrhage | 20/219 (9.1) | 27/205 (13.2) | 26/211 (12.3) |
| Risk ratio (95% CI) | 1.4 (0.8,2.5) | 1.3 (0.8,2.3) | |
| Cardiovascular and thromboembolic disorders | 15/219 (6.8) | 20/205 (9.8) | 18/211 (8.5) |
| Risk ratio (95% CI) | 1.4 (0.7,2.7) | 1.2 (0.6,2.4) | |
| Non-aspirin users – Any adverse event – no. subjects with AE/no. subjects not using aspirin (%) | 409/457 (89.5) | 450/478 (94.1) | 436/458 (95.2) |
| Risk ratio (95% CI) | 1.0 (1.0,1.1) | 1.1 (1.0,1.1) | |
| Renal/Hypertensive disorders | 80/457 (17.5) | 95/478 (19.9) | 89/458 (19.4) |
| Risk ratio (95% CI) | 1.1 (0.9,1.5) | 1.1 (0.8,1.5) | |
| Gastrointestinal ulceration and hemorrhage | 50/457 (10.9) | 46/478 (9.6) | 37/458 (8.1) |
| Risk ratio (95% CI) | 0.9 (0.6,1.3) | 0.7 (0.5,1.1) | |
| Cardiovascular and thromboembolic disorders | 11/457 (2.4) | 21/478 (4.4) | 32/458 (7.0) |
| Risk ratio (95% CI) | 1.8 (0.9,3.7) | 2.9 (1.5,5.7) | |
Treatment-emergent adverse events included were those that occurred during the time after the first dose of study mediation to 30 days after the last dose of study medication
Category includes reports of elevated creatinine, fluid retention/edema, hypertension, proteinuria, and renal failure
Category includes anemia, gastrointestinal bleeding, gastritis/duodenitis, upper or lower gastrointestinal ulceration, and other hemorrhage
Category includes angina, myocardial infarction, cardiovascular therapeutic procedure, cerebrovascular disease, peripheral vascular disease, peripheral vascular therapeutic procedure, venous thrombosis or thromboembolism, and death or circulatory collapse due to cardiovascular causes
Cardiovascular adverse events in APC Trial participants have been reported previously, using a pre-specified analysis of adjudicated serious adverse events (18), and indicated increased risk of serious cardiovascular complications - defined as cardiovascular death, non-fatal myocardial infarction, stroke, or heart failure - among those using celecoxib, with risk ratios of 2.6 (95%CI 1.1,6.1) and 3.4 (95%CI 1.5,7.9) for the low and high dose cohorts, respectively. Adjudicated analyses of safety data were not planned or conducted for timepoints beyond the 3-year initial treatment trial. For the year 5 analysis, we examined investigator reported, treatment emergent cardiovascular and thrombotic adverse events, including in this category all events defined as myocardial infarction, cardiovascular therapeutic procedure, cerebrovascular disease, peripheral vascular disease, peripheral vascular therapeutic procedure, venous thrombosis or thromboembolism, and death or circulatory collapse due to cardiovascular causes (see Supplementary data online for more details). In the two years off treatment, very few serious cardiovascular or thrombotic events occurred (1.3% placebo; 2.6% 200 mg bid celecoxib; 1.9% 400mg bid celecoxib). In the treatment-emergent analysis, 3.8% of placebo users experienced one or more cardiovascular and thrombotic adverse events, compared to 6.0% (RR 1.6; 95%CI 1.0, 2.5) and 7.5% (RR 1.9; 95%CI 1.2,3.1) for those using celecoxib 200mg bid and celecoxib 400mg bid, respectively (Table 4). These values were higher in aspirin users than in aspirin non-users. The absolute magnitude of risk was greatest for patients with cardiovascular risk factors at baseline, defined as those reporting a history of atherosclerotic heart disease, age > 65 years, smoking, hypertension, or hyperlipidemia. Patients with no baseline risk factors comprised only 15.4% of those randomized, and had a cardiovascular and thrombotic event rate per 100 of 0.9 if using placebo, 3.9 if using celecoxib 200mg bid, and 1.9 if using celecoxib 400mg bid (Table 5). These rates were 2.2, 3.7, and 4.9, respectively for the 35% of patients with one cardiovascular risk factor at baseline, and 5.9, 8.2, and 11.2, respectively, for those with two or more baseline risk factors (p value for interaction 0.51). When individual risk factors were examined, the only factor significantly associated with celecoxib dose and event risk was a baseline history of atherosclerotic heart disease (p value for interaction 0.004).
Table 5.
Selected Cardiovascular and Thrombotic Events According to Baseline Risk Factors1
| Placebo twice daily |
200mg celecoxib twice daily |
400mg celecoxib twice daily |
P value for interaction |
|
|---|---|---|---|---|
| No risk factors at baseline | ||||
| Patients with no baseline risk factors – no. (%)2 | 108 (15.9) | 102 (14.9) | 103 (15.4) | |
| Patients experiencing event – no. (%) | 1 (0.9) | 4 (3.9) | 2 (1.9) | |
| One risk factor at baseline | ||||
| Patients with one baseline risk factor – no. (%) | 228 (33.7) | 241 (35.3) | 244 (36.5) | |
| Patients experiencing event – no. (%) | 5 (2.2) | 9 (3.7) | 12 (4.9) | |
| Two or more risk factors at baseline | ||||
| Patients with ≥2 baseline risk factors – no. (%) | 340 (50.3) | 340 (49.8) | 322 (48.1) | |
| Patients experiencing event – no. (%) | 20 (5.9) | 28 (8.2) | 36 (11.2) | |
| Association between number of risk factors and events | 0.515 | |||
| Association between events and individual baseline risk factors | ||||
| Smoking – no. (%) | 20 (4.9) | 28 (6.5) | 32 (7.9) | 0.514 |
| Hyperlipidemia – no. (%) | 15 (5.8) | 17 (6.6) | 29 (11.5) | 0.210 |
| Diabetes – no. (%) | 5 (8.2) | 7 (10.4) | 10 (15.2) | 0.385 |
| Hypertension – no. (%) | 16 (5.7) | 21 (7.3) | 24 (9.2) | 0.864 |
| Atherosclerotic heart disease – no. (%) | 7 (8.2) | 14 (18.4) | 18 (21.4) | 0.004 |
| Cerebrovascular disease – no. (%) | 2 (13.3) | 4 (16.7) | 3 (18.8) | 0.828 |
| Age > 65 years – no. (%) | 9 (2.0) | 18 (3.8) | 26 (5.5) | 0.779 |
Risk factors = cigarette smoking, hypertension, hyperlipidemia, atherosclerotic heart disease, cerebrovascular disease, diabetes.
Percent of those with the risk factor who experienced an event in the time interval from first dose of study medication to 30 days after last dose of study medication.
Discussion
The APC Trial was designed to identify the relationship between celecoxib dose, anti-tumor efficacy, and treatment safety for patients at risk for CRC because of a history of adenomas. In the initial 3 year study interval, we found that a celecoxib dose of 200mg bid was associated with a 33% reduction in adenoma detection, with a 57% reduction in advanced adenomas. For this dose, analysis of a selected category of treatment-emergent, investigator reported cardiovascular and thrombotic events indicated a 1.5-fold increase in risk, from a rate of 4.4% to 6.6% of those treated. In comparison, the 400mg bid celecoxib dose achieved somewhat higher benefit (45% reduction in adenomas, 66% reduction in advanced adenomas), but also at a higher cardiovascular risk of 1.8-fold (8.1% incidence) (8). A second randomized trial, the PreSAP Trial, showed that 400mg of celecoxib once daily for three years produced a 36% reduction in adenoma detection, a 51% decrease in advanced adenoma detection, and no statistically significant increase in cardiovascular serious adverse events (7). These data led us to conclude that celecoxib at 400mg bid is not an acceptable adenoma chemoprevention regimen for sporadic disease, whereas the lower, and once daily dose, may be considered in patients at high risk for sporadic CRC and low risk for cardiovascular complications. In addition, for patients with FAP whose high cancer risk may justify a 400mg bid regimen, those with a history of cardiovascular disease should avoid celecoxib use and consider surgery or a non-selective NSAID such as sulindac.
The current analysis expands the results of the APC Trial to include in two important ways. First, it provides treatment-emergent safety data for the patients who continued on treatment beyond 3 years, extending the median treatment duration to approximately 2.4 years across all study arms. Consistent with the 3-year data, this extended analysis showed no consistent treatment-associated increase in adverse event categorized as renal/hypertensive disorders or gastrointestinal ulceration and hemorrhage (Table 4). The cardiovascular and thrombotic risks continued to be evident, with a 1.6-fold increased risk for the 200mg bid cohort and 1.9-fold risk for the 400mg bid cohort. This expanded data set also allowed us to explore baseline factors that indicate an increased risk of cardiovascular complications. We found a significant interaction between celecoxib treatment and cardiovascular and thrombotic events for those reporting a baseline history of atherosclerotic heart disease. From these results, we conclude that celecoxib at the doses studied should not be used for adenoma prevention in patients with a history of atherosclerosis.
The second important contribution of this study is that it provides data concerning the effect on disease incidence and severity associated with withdrawal of an effective chemopreventive agent. At the time of the year 5 colonoscopy, >90% of the participants had been off study medication for a year or more. Adenomas develop over time, with an estimated rate of progression to malignant disease of from 10–20 years (22). Studies of repeated colonoscopy with polypectomy show that the rate of new adenoma detection decreases over time as patients with a lower risk of adenoma development drop out of the denominator (1). One of the concerns over use of chemoprevention is that, rather than preventing disease progression, the drug would merely inhibit development of low grade lesions, allowing those with higher malignant potential to continue to progress. A manifestation of this could be drug-associated reduction in adenoma size but not severity, therefore preventing colonoscopic detection and removal of the more significant disease. If so, it follows that withdrawal of drug would result in re-emergence of disease at an accelerated rate compared to those without drug. However, the year 5 colonoscopy results for the APC Trial indicate that this was not the case for celecoxib chemoprevention. The rates of adenoma detection at the year 5 colonoscopy were similar across all treatment groups (Figure 2). At the low celecoxib dose, we observed a comparable result when disease severity was examined, with advanced adenomas detected in 5.1% and 6.3% of participants using placebo and 200mg bid celecoxib, respectively. Interestingly, we did find a trend toward more advanced adenomas at year 5 in patients using celecoxib 400mg bid (Figure 2, Table 3).
Aspirin prevents colorectal adenomas and reduces the incidence of CRC (6, 10, 23). A significant number of patients randomized on the APC trial (31%) were using low dose aspirin at the time of study entry, and were required to remain on this treatment for the duration of the trial. Entry into the study required recent detection and removal of adenomas, therefore the aspirin user subset provided an important cohort of patients who developed colorectal adenomas while using low dose aspirin. Comparison of year 5 efficacy results between users and non-users of aspirin showed similar rates of disease detected during surveillance for the placebo arm (66.9% vs 69.2%, respectively, Table 2). The effect of celecoxib was also comparable for both groups, with a 37% reduction in adenomas for aspirin users and a 39% reduction in aspirin non-users with the 400mg bid celecoxib dose. This relationship was observed for all surveillance intervals, and indicates that celecoxib mediates adenoma suppression in individuals resistant to chemoprevention with low dose aspirin.
In summary, the APC Trial showed that celecoxib prevents sporadic colorectal adenomas in patients at high risk for CRC. This effect was particularly strong with respect to the detection of advanced adenomas, and significant benefit was achieved even in patients who entered the study having developed adenomas while routinely using low dose aspirin. Because the APC Trial was initiated before the cardiovascular risks of NSAIDs were appreciated, the study was not designed to test cardiovascular endpoints. As a result, the event numbers in the selected cardiovascular and thrombotic category are small, and the confidence intervals are wide. The extended safety data presented here continue to show an increased risk for cardiovascular toxicity, particularly for the 400mg bid celecoxib dose and for patients with a history of atherosclerotic heart disease. We conclude that chemoprevention efficacy of the magnitude seen in the APC and Pre-SAP Trials would have a major impact on CRC incidence and mortality, but this regimen cannot be recommended for routine use in sporadic patients due to safety concerns. Additional Cox-2 inhibitor trials should therefore be conducted so that treatment risks and benefits can be adequately quantified for patients at high CRC risk.
Supplementary Material
Acknowledgments
Grant support: National Cancer Institute (N01-CN-95015) and Pfizer
Abbreviations
- APC Trial
Adenoma Prevention with Celecoxib Trial
- CRC
colorectal cancer
- Cox-2
cyclooxygenase 2
- CI
confidence interval
- NSAIDs
nonsteroidal anti-inflammatory drugs
- Pre-SAP Trial
Prevention of Colorectal Sporadic Adenomatous Polyps Trial
APPENDIX
The following authors have financial disclosures to make:
| M.M. Bertagnolli, M. Redston: | Grant support for the APC Trial from Pfizer, Inc. under a Clinical Trials Agreement with Brigham & Women’s Hospital |
| C.J. Eagle, A. Breazna, R.B. Rosenstein, J. Tang, N. Collins, N. Dengler, J. Sanocki: | Employees of Pfizer, Inc. |
| C.J. Eagle, A. Breazna, R.B. Rosenstein, J. Tang, N. Collins: | Owner of shares in Pfizer, Inc. |
| F. Macrae: | Grant support for clinical trials from Pfizer, Inc. |
The following persons participated in the APC Study: Steering Committee: M.M. Bertagnolli, E.T. Hawk, C.J. Eagle, G.B. Gordon; Statistical Team: A.G. Zauber, A. Brezna, KM.Kim, D. Corle, R.B. Rosenstein, J. Tang, T. Hess, A. Wilton, J. Sanocki; Medical Monitors: W. Anderson, L. Doody; Central Pathology Review: M. Redston; K. Geisinger, C. Compton; Project Directors: N. Collins, G.M. Woloj, D. Bagheri, A. Crawford, M. Schietrum, V. Ladouceur, N. Dengler; Data and Safety Monitoring Board: S. Rosen (chair), L. Friedman, R. Makuch, R. Phillips, P. Taylor; Principal Investigators, United States: S. Auerbach (California Professional Research, Newport Beach), C.F. Barish (Wake Research Associates, Raleigh, NC), T. Barringer (Carolinas Medical Center, Charlotte, NC), R.W. Bennetts (Northwest Gastroenterology Clinic, Portland, OR), M. Blitstein (Associates in Gastroenterology and Liver Disease, Lake Forest, IL), J. Bruggen (Wake Forest University Baptist Medical Center, Winston Salem, NC), P Carricaburu (Veterans Affairs Hospital, Sheridan, WY), D. Chung (Massachusetts General Hospital, Boston, MA), F. Colizzo (Pentucket Medical Associates, Haverhill, MA), R. Curtis (Newton-Wellesley Hospital, Newton, MA), T. Dewar (Harris Methodist Hospital Fort Worth, Ft. Worth, TX), R. DuBois (Vanderbilt University Medical Center, Nashville, TN), T. Feinstat (Gastroenterology Consultants of Sacramento, Roseville, CA), T.R. Foley (Regional Gastroenterology Associates of Lancaster, Lancaster, PA, D. Gabbaizadeh (Huntington Research Group, Huntington Station, NY), J. Geenen (Wisconsin Center for Advanced Research, Milwaukee, WI), F. Giardiello (Johns Hopkins Hospital, Baltimore, MD), A. Goetsch (nTouch Research, Huntsville, AL), M. Goldberg (Regional Gastroenterology Associates of Lancaster, Evanston, IL), J.L. Goldstein (University of Illinois at Chicago, Chicago, IL), W. Harlan, III (Asheville Gastroenterology Associates, Asheville, NC), R. Hogan (Gastrointestinal Associates, Jackson, MS), M. Kamionkowski (Gastroenterology Associates of Cleveland, Mayfield Heights, OH), M. Kelfer (Fallon Clinic, West Boylston, MA), B. Kerzner (Health Trends Research, Baltimore, MD), K. Kim (University of Chicago Medical Center, Chicago, IL), I. Klimberg (Gastroenterology Associates of Ocala, Ocala, FL), G. Koval (West Hills Gastroenterology Associates, Portland, OR), C. Krone (Advanced Clinical Therapeutics, Tucson, AZ), S.Krumholz (Waterside Clinical Research, West Palm Beach, FL), M.W. Layton (South Puget Sound Clinical Research Center, Olympia, WA), C. Lightdale (Columbia-Presbyterian Medical Center, New York, NY), P.J. Limburg (Mayo Clinic, Rochester, MN), C. Lind (Vanderbilt University Medical Center, Nashville, TN), D. Lipkis (Institute for Health Care Assessment, San Diego, CA), M. Lloyd (Idaho Gastroenterology, Meridian, ID), D. Maccini (Spokane Digestive Disease Center, Spokane, WA), F. MacMillan, Sr. (Pentucket Medical Associates, Haverhill, MA), R. Madoff (University of Minnesota, Minneapolis, MN), A. Malik (Advanced Clinical Research, North Providence, RI), A. Markowitz (Memorial Sloan Kettering Cancer Center, New York, NY), R. Marks (Alabama Digestive Research Center, Alabaster, AL), C. J. McDougall (Manhattan Associates, New York, NY), P. Miner (Oklahoma Foundation for Digestive Research, Oklahoma City, OK), M. Murphy (Southern Digestive and Liver Disease Institute, Savannah, GA), A. Namais (Gastrointestinal Physicians, Salem, MA), N. Nickl (University of Kentucky Medical Center, Lexington, KY), M. Pochapin (Jay Monahan Center for Gastrointestinal Health, New York, NY), R.E. Pruitt (Nashville Medical Research Institute, Nashville, TN), J Puolos (Cumberland Research Associates, Fayetteville, NC), D.S. Riff (AGMG Clinical Research, Anaheim, CA), R. Roman (South Denver Gastroenterology, Englewood, CO), L. Rubin (New Jersey Physicians, Passaic, NJ), D. Ruff (Healthcare Discoveries, San Antonio, TX), M. Safdi (Consultants for Clinical Research, Cincinnati, OH), J. Saltzman (Brigham and Women’s Hospital, Boston, MA), B. Salzberg (Atlanta Gastroenterology Associates, Atlanta, GA), J.A. Sattler (Western Clinical Research, Torrence, CA), P. Schleinitz (Americas Doctors Research, Medford, OR), J. Schwartz (Northwest Gastroenterologists, Arlington Heights, IL), M. Schwartz (Jupiter Research Association, Jupiter, FL), M. Silpa (Gastroenterology Associates of the East Bay Medical Group, Berkeley, CA), D. Silvers (Drug Research Services, Metairie, LA), D. Smoot (Howard University Cancer Center, Washington, DC), S. Sontag (Veterans Affairs Medical Center, Hines, IL), R.J. Sorrell (Gastroenterology Specialties, Lincoln, NE), D. Stanton (Community Clinical Trials, Orange, CA), J. Sturgeon (Americas Doctors Research, Shawnee Mission, KS), J.P. Tracey (Hawthorne Medical Associates, North Dartmouth, MA), T. Werth (Charlotte Gastroenterology and Hepatology, Charlotte, NC), C.M. Wilcox (University of Alabama at Birmingham, Birmingham, AL), R. Wohlman (Northwest Gastroenterology Associates, Bellevue, WA), S. Woods (Gastroenterology Associates of Fairfield County, Bridgeport, CT); United Kingdom: J. Burn and Gillian Brigham (South Cleveland Hospital, Middlesbrough); Australia: H. Ee (Sir Charles Gairdner Hospital, Nedlands, W.A.), M. Korman (Monash Medical Centre, Clayton, Victoria), A. Lee (Concord Repatriation and General Hospital, Concord, NSW), B. Leggett (Royal Brisbane Hospital, Herston, Queensland), F. Macrae (Royal Melbourne Hospital, Melbourne, Victoria), L. Mollison (Freemantle Hospital, Freemantle, WA), N. Yeomans (Western Hospital, Footscray, Victoria), G. Young (Flinders Medical Center, Bedford, SA); Canada: G. Aumais (Hospital Maisonneuve-Rosemont, Montreal), R. Bailey (Hys Medical Center, Edmonton, Alberta), C. Bernstein (Winnipeg Health Sciences Centre, Winnipeg, Manitoba), L. Cohen (Sunnybrook and Women’s Hospital, Toronto), C. Dallaire, R. Dube (Centre Hospitalier Universitaire de Quebec, Quebec), D. Morgan (McMaster University, Hamilton, Ontario), T. Sylwestrowicz (St. Paul’s Hospital, Saskatoon, Saskatoon), G. Van Rosendaal (University of Calgary, Alberta), S.J. Van Zantan (Queen Elizabeth II Health Sciences Centre, Halifax, NS).
Footnotes
Investigator participants in the APC study are listed in the Appendix
Note: Supplementary data for this article are available at Cancer Prevention Research Online (http://cancerprevres.aacrjournals.org/).
References
- 1.Winawer SJ, Zauber AG, O'Brien JM, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N Engl J Med. 1993;328:901–906. doi: 10.1056/NEJM199304013281301. [DOI] [PubMed] [Google Scholar]
- 2.Seeff LC, Nadel MR, Klabunde CN, et al. Patterns and predictors of colorectal cancer test use in the adult U.S. population. Cancer. 2004;100:2093–2103. doi: 10.1002/cncr.20276. [DOI] [PubMed] [Google Scholar]
- 3.Waddell WR, Ganser GF, Cerise EJ, Loughry RW. Sulindac for polyposis of the colon. The American Journal of Surgery. 1989;157:175–179. doi: 10.1016/0002-9610(89)90442-x. [DOI] [PubMed] [Google Scholar]
- 4.Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenopatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 5.Sandler RS, Halabi S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:1939. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 6.Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 7.Arber N, Kuwada S, Leshno M, Sjodahl R, Hultcrantz R, Rex D. Sporadic adenomatous polyp regression with exisulind is effective but toxic: a randomized, double-blind, placebo controlled, dose-response study. Gut. 2006;55:367–373. doi: 10.1136/gut.2004.061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertagnolli MM, Eagle CJ, Zauber AG, et al. A Randomized Trial of Celecoxib to Prevent Sporadic Colorectal Adenomas. N Engl J Med. 2006;355:873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 9.Baron JA, Sandler RS, Bresalier RS, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131:1674–1682. doi: 10.1053/j.gastro.2006.08.079. [DOI] [PubMed] [Google Scholar]
- 10.Flossman E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomized and observational studies. Lancet. 2007;369:1603–1613. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 11.Henry D, Lim LL, Garcia Rodeiguez LA, et al. Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: results of a collaborative meta-analysis. BMJ. 1996;312:1563–1566. doi: 10.1136/bmj.312.7046.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roderick PJ, Wilkes HC, Meade TW. The gastrointestinal toxicity of aspirin: an overview of randomised controlled trials. Br J Pharmacol. 1993;35:219–226. doi: 10.1111/j.1365-2125.1993.tb05689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silverstain FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. JAMA. 2000;13:1247–1255. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 14.Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med. 2000;343:1520–1528. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- 15.Singh G, Fort JG, Goldstein JL, et al. Celecoxib versus naproxen and diclofenac in osteoarthritis patients: SUCCESS-I study. Am J Med. 2006;119:255–266. doi: 10.1016/j.amjmed.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 16.Schnitzer TJ, Burmester GR, Mysler E, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet. 2004;364:665–674. doi: 10.1016/S0140-6736(04)16893-1. [DOI] [PubMed] [Google Scholar]
- 17.Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 18.Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 19.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemotherapy Report. 1966;50:163–170. [PubMed] [Google Scholar]
- 20.Stokes ME, Davis CS, Koch GG. Categorical data analysis using the SAS system. 2nd ed. Cary, NC: The SAS Institute; 2000. [Google Scholar]
- 21.Agresti A. Categorical Data Analysis. 2nd ed. New York: John Wiley & Sons, Inc.; 2002. [Google Scholar]
- 22.Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer. 1975;36:2251–2270. doi: 10.1002/cncr.2820360944. [DOI] [PubMed] [Google Scholar]
- 23.Benamouzig R, Deyra J, Martin A, et al. Daily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC trial. Gastroenterology. 2003;125:612–614. doi: 10.1016/s0016-5085(03)00887-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.