Background
Sparse data exist regarding the manifestations and optimal management of H1N1 infection in immunocompromised patients (1, 2).
Objective
To report a case of H1N1-associated pneumonia in a hematopoietic stem cell transplantation (HCT) recipient with extrapulmonary H1N1 RNA detection who was treated with IV peramivir.
Case Report
A woman in her forties (BMI 31.8 kg/m2) with multiple myeloma underwent outpatient autologous HCT with myeloablative conditioning in May 2009. Two days after transplantation, she was hospitalized with fever, nausea, diarrhea, and minimally productive cough. Chest x-ray was normal; neutrophil count was 1420/mm3, and no lymphocytes were present. Despite empiric broad-spectrum antibiotics, she developed increased tachypnea and hypoxia. Chest computed tomography (CT) on hospital day (HD) #3 showed bilateral ground-glass opacities with central nodules. Nasal swab was positive for influenza A/H1N1 by a real-time RT-PCR assay developed at our center using primers and probe targeting a 102-base pair fragment of the hemagglutinin gene.
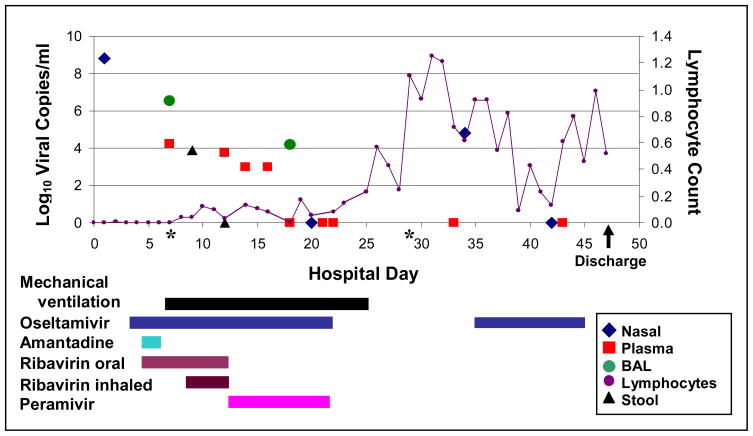
High-dose oseltamivir (150 mg twice daily), our institution’s standard practice for HCT recipients with lower respiratory tract disease (2), was initiated on HD#3. The following day, the patient started open-label combination therapy consisting of standard-dose oseltamivir, amantadine, and oral ribavirin as part of a clinical trial. Despite this, she developed respiratory failure requiring intubation on HD#6. Study drugs were discontinued and high-dose oseltamivir was restarted via nasogastric tube, with inhaled ribavirin (2g every 8 hours), oral ribavirin (10 mg/kg every 8 hours), and intravenous immunoglobulin (500 mg/kg every other day) (Figure).
Figure.
Time course of novel influenza A/H1N1 infection in hematopoietic stem cell transplantation recipient. Limit of RT-PCR assay detection equals 3 log10 viral copies/ml.
*BAL on HD#7 and blood culture on HD#29 was also positive for Saccharomyces cerevisiae. The patient received a short course of intravenous micafungin initially; positive blood culture was treated with line removal and intravenous micafungin followed by oral voriconazole.
Influenza A RNA was first detected by RT-PCR in plasma and bronchoalveolar lavage fluid on HD#7 and stool on HD#9. Given her lack of improvement, detection of influenza RNA in plasma, and possible malabsorption of oral drugs, intravenous peramivir was initiated on HD#13 at 600 mg daily for 10 days. Inhaled and oral ribavirin were discontinued but oseltamivir was continued. The patient improved slowly, was extubated on HD#24, and discharged on HD#47.
She had sustained detection of H1N1 RNA in plasma and respiratory secretions for at least 9 and 11 days, respectively, which resolved with lymphocyte recovery and antiviral therapy (Figure). H1N1 recurred in a nasal wash on HD#34, prompting a second 10-day course of oral oseltamivir (150 mg twice daily). There was no oseltamivir resistance (H275Y mutation) in viral RNA from BAL on HD#7 or nasal wash on HD#34 (IntelligentMDx, Cambridge, MA).
Discussion
Novel influenza A/H1N1 may cause rapidly progressive disease after HCT, as in this obese patient with respiratory failure with influenza viral RNA detected in extrapulmonary samples, including plasma and stool. Fatal cases of avian influenza A/H5N1 and, rarely, of seasonal human influenza among immunocompromised patients, have had viral RNA detected in blood and extra-respiratory tissues (3, 4). Our finding of viral RNA in the plasma of this patient is novel and may indicate hematologic dissemination of the virus. We suggest that intravenous influenza antivirals may be useful or required for critically ill patients with severe novel influenza A/H1N1 infections, particularly if viral RNA is detectable in plasma. Whether peramivir was responsible for ultimate recovery of our patient cannot be determined with certainty; lymphocyte reconstitution in addition to aggressive antiviral treatment may be required for successful management of influenza pneumonia in this setting (5).
Conclusion
Although the significance of extrapulmonary influenza viral RNA detection is not clear and the benefit of intravenous antiviral therapy in such patients is speculative, we hypothesize that viral RNA in plasma may be associated with severe influenza disease. Further investigation is needed to replicate this finding since this easy-to-measure biomarker could be used to determine patients who will most benefit from aggressive intravenous and/or combination approaches to antiviral therapy.
Acknowledgments
We thank Christi Dahlgren and Deborah Reyes for assistance compiling clinical and laboratory data; Anne Cent, Nancy Wright, Patricia Nguyen for specimen processing, testing, and laboratory expertise; and Debra Mattson for critical review of the manuscript. We also acknowledge the assistance of Biocryst Pharmaceuticals for provision of peramivir under emergency investigational new drug status.
Grant support: National Institutes of Health (CA18029, HL081595, K23HL091059, L40AI071572, K24AI071113, K24HL093294)
Footnotes
Potential Conflicts of Interest: A.P.C. received grant support from MedImmune, Inc. S.T.J. received grant support from Pfizer, Inc. A.W. received grant support from Roche Pharmaceuticals and GlaxoSmithKline. J.A.E. has received grant support from MedImmune, Inc., Adamas Pharmaceuticals, and Novartis. M.B. received grant support from Adamas Pharmaceuticals and Roche Pharmaceuticals, is a consultant for Roche and Novartis, and has received speaking fees from Roche.
References
- 1.Lapinsky SE. H1N1 novel influenza A in pregnant and immunocompromised patients. Crit Care Med. 2009 doi: 10.1097/CCM.0b013e3181c85d5f. [DOI] [PubMed] [Google Scholar]
- 2.Casper C, Englund J, Boeckh M. How we treat influenza in patients with hematologic malignancies. Blood. 2009 doi: 10.1182/blood-2009-11-255455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell AP, Chien JW, Kuypers J, et al. Respiratory virus pneumonia after hematopoietic cell transplantation: associations between viral load in bronchoalveolar lavage, viral RNA detection in serum, and transplant outcomes. J Infect Dis. doi: 10.1086/651662. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuiken T, Taubenberger JK. Pathology of human influenza revisited. Vaccine. 2008;26 (Suppl 4):D59–66. doi: 10.1016/j.vaccine.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gooskens J, Jonges M, Claas EC, Meijer A, Kroes AC. Prolonged influenza virus infection during lymphocytopenia and frequent detection of drug-resistant viruses. J Infect Dis. 2009;199(10):1435–41. doi: 10.1086/598684. [DOI] [PubMed] [Google Scholar]