Abstract
The objective of this study was to identify and quantify the heteromeric neuronal nicotinic receptors (nAChRs) in the rat hippocampus. The density of nAChR subtypes was assessed by labeling them with [3H]epibatidine followed by immunoprecipitation with subunit-selective antibodies. Sequential immunoprecipitation assays were used to establish associations between two different subunits, which then allowed the full subunit composition of the receptors to be deduced. Our results show that most of the hippocampal heteromeric nAChRs contain α4 and β2 subunits. In fact, we identified two populations containing these two predominant subunits, the α4β2 and α4β2α5 subtypes which account for ~40% and ~35%, respectively, of the total [3H]epibatidine-labeled receptors. An additional heteromeric subtype with the subunit composition of α4β2α3 represented ~10% of the total nAChRs, and another 10% of the immunoprecipitated receptors contained α4 and β4 subunits, with or without the α3 subunit. To determine if α4β2 and α4β2α5 nAChR subtypes differ in their ligand binding affinities, the α3- and β4-containing receptors were first removed by immunoprecipitation and then, competition studies with acetylcholine, nicotine, cytisine and sazetidine-A against [3H]epibatidine were carried out on the remaining α4β2 and α4β2α5 subtypes. Results suggested these subtypes have comparable binding affinities for the nicotinic ligands used here.
Keywords: Neuronal nicotinic receptor, heteromeric subtypes, α5 subunit, hippocampus, immunoprecipitation, binding affinity
Neuronal nicotinic receptors (nAChRs) are channel proteins involved in the modulation of crucial neuronal processes in the brain and autonomic nervous system. The receptors are comprised of two different types of subunits, α and β, which assemble as pentamers to form different receptor subtypes. Nine α and three β neuronal nAChR subunits are known, and these associate either as simple heteromeric receptors composed of only one type of α and one type of β subunit, or mixed heteromeric composed of more than one type of α or β subunit. These different nAChR subtypes have different pharmacological and biophysical characteristics, as well as different brain region distributions.
The hippocampus is a major target of basal forebrain cholinergic neurons (Mesulam et al. 1983), which are implicated in Alzheimer’s disease pathology (Whitehouse et al. 1982). In fact, in Alzheimer’s disease there is a major loss of nAChRs in the hippocampus (Perry et al. 1987), with presumed consequences for cholinergic neurotransmission. The hippocampus contains α4β2 and α4β2α5 nAChRs (Mao et al. 2008), as well as a relatively high density of homomeric α7 nAChRs (Pauly et al. 1991; Alkondon and Albuquerque, 1993; Jones and Yakel 1997). However, the presence of mRNA for most other nAChR subunits (Son and Winzer-Serhan 2008), as well as pharmacological, physiological and molecular biological studies (Wada et al., 1989; Alkondon and Albuquerque, 1993; McQuiston and Madison 1999) strongly suggest that additional, unidentified, heteromeric nAChR subtypes are present in the hippocampus and play potentially important roles in nicotinic cholinergic physiology and pharmacology (Clarke and Reuben 1996; Alkondon and Albuquerque 2004; Jia et al. 2009). Consequently, we carried out studies to identify and quantify the subunit composition of nAChR subtypes in the hippocampus. In addition, the hippocampus expresses the highest percentage of α5-containing nAChRs in the brain, all of which are a component of the α4β2α5 subtype (Mao et al. 2008). Although the α5 subunit does not form a component of the receptor binding site (Abramson et al. 1989; Cohen et al. 1991; Tomaselli et al. 1991; Conroy et al. 1992) its presence appears to influence the physiological characteristics of nAChRs, such as calcium conduction (Tapia et al. 2007) and desensitization (Ramirez-Latorre et al. 1996; Gerzanich et al. 1998). Therefore, in this study we have also tried to determine whether the incorporation of the α5 subunit allosterically influences the binding affinity of several common nicotinic ligands.
Materials and methods
Materials
Frozen whole brains from adult male Sprague-Dawley rats were purchased from Zivic Miller laboratories (Portersville, PA) and kept frozen at −80 °C until use. [3H]epibatidine ([3H]EB; specific activity ~ 55 Ci/mmol) and [125I]α-bungarotoxin ([125I]α-BTX; specific activity ~ 88 Ci/mmoles) were obtained from Perkin Elmer Life Science (Boston, MA). Nicotine tartrate, acetylcholine and cytisine were purchased from Sigma-Aldrich (St. Louis, MO). Sazetidine-A was synthesized as described previously (Xiao et al. 2006) and was a kind gift from Drs. Milton Brown and Mikell Paige (Georgetown University). Protein G Sepharose beads were purchased from GE Healthcare (Little Chalfont, Buckinghamshire, UK). Normal Rabbit Serum (NRS) was purchased from Calbiochem (San Diego, CA).
Antisera and antibodies
Rabbit antisera directed at bacterially expressed fusion proteins containing partial sequences of the cytoplasmic domains of nAChR α2, α3, α4, α5, and β4 subunits were kind gifts from Drs. Scott Rogers and Lorise Gahring (University of Utah, Salt Lake City). These antisera have been described previously (Flores et al. 1992; Rogers et al. 1992). An antibody directed at the C-terminal peptide sequence of the rat nAChR α3 subunit was affinity purified from rabbit serum. This antibody has been described previously (Yeh et al. 2001); we will refer to this antibody as α32 in the results section. A monoclonal antibody (mAb 270) to the chick β2 subunit was made from hybridoma stocks (American Type Culture Collection, Manassas, VA). This mAb has been previously developed and characterized (Whiting and Lindstrom 1987). The specificity of all of these antibodies for immunoprecipitation of nAChRs has been previously reported (Hernandez et al. 2004; Marritt et al. 2005; Perry et al. 2007). For simplicity, in this paper, we use the term antibody to refer to unpurified antiserum, as well as to affinity purified antiserum and monoclonal antibody.
Binding Assays
Hippocampi were dissected and homogenized in Tris buffer (50mM Tris HCl, pH 7.0) and centrifuged at 35,000 × g for 10 minutes at 4°C. The pellet was re-homogenized, centrifuged and re-suspended in Tris HCl buffer. Tissue was solubilized in 2% Triton X-100 with gentle rotation at 4°C for 2 hours, followed by centrifugation at 35,000 × g for 10 minutes. Aliquots of supernatant equivalent to 20 mg original weight were distributed in binding tubes containing 2 nM [3H]EB. Non-specific binding was defined in the presence of 300µM nicotine. In some experiments, receptors were labeled with 0.5 nM [125I]α-BTX, and non-specific binding was defined with 1.5 mM nicotine. Assay tubes were incubated for 2 hours at 24°C and bound receptors were separated from the free ligand by vacuum filtration over Brandel GF/C glass-fiber filters (Gaithersburg, MD), pre-wet with 0.5% polyethyleneimine. Filters were counted in a liquid scintillation counter. Specific binding to nAChRs in these solubilized fractions, which was defined as total binding minus non-specific binding, was equivalent to the specific binding in membrane homogenates before solubilization.
Immunoprecipitation Assays
The tissue was prepared as described above and distributed into tubes containing radiolabeled ligand and subunit-specific antibodies (Ab) or normal rabbit serum (NRS), as a control. The samples were gently rotated overnight at 4°C, and then a 50% slurry of Protein G Sepharose beads (50µl) was added. After 2 hours incubation at 4°C, the tubes were centrifuged at 7,000 × g for 1 minute at 4°C to precipitate the radiolabeled antibody-receptor complex. Supernatants were filtered over Brandel GF/B glass-fiber filters pre-wet with 0.5% polyethyleneimine and then counted in a liquid scintillation counter. The radiolabeled nAChRs remaining in the supernatant represent the receptors not precipitated by the antibody used (i.e., receptors that did not contain that specific subunit recognized by the antibody). The remaining pellets, containing radiolabeled receptors immunoprecipitated by the antibody and protein G, were washed twice with fresh Tris HCl buffer (pH 7.0) and centrifugation at 7,000 × g for 1 minute at 4°C. The final pellet was re-suspended in 200µl of 0.1 N NaOH for 1 h and then counted. The radioactivity measured in these pellets thus represented the radiolabeled nAChRs containing the specific subunit recognized by the antibody used. Values obtained from tissues incubated with NRS were used as control for non-specific precipitation and were subtracted from the values obtained with subunit-specific antibody. The amount of radiolabeled nAChRs immunoprecipitated with a particular antibody was then compared to the total number of nAChRs measured with the radioligand to determine the percentage of nAChRs that contain a particular subunit.
Sequential Immunoprecipitation Assays
To determine if two specific subunits are associated in the same receptor, we carried out sequential immunoprecipitation assays of [3H]EB-labeled receptors with two different antibodies. Hippocampal extracts were prepared and after overnight incubation with the first antibody (“clearing Ab”) and the addition of protein-G, the resulting pellets were washed and counted. The supernatants, which contained the remaining [3H]EB-labeled receptors, were incubated with a second subunit-selective antibody (“capturing Ab”) directed against a different subunit. Samples were incubated and gently rotated overnight at 4°C. Pellets from the second incubation were then processed as above.
The rational for this procedure is, briefly, that if two subunits are entirely associated, the clearing antibody directed against the first subunit will precipitate nAChRs containing this subunit as well as the second subunit because they are associated in the same receptor. On the other hand, if the two subunits are not associated in the same receptor, the second antibody will precipitate all of the receptors containing the second subunit. Similarly, the extent of association between the two subunits will be reflected by the degree of immunoprecipitation with the second (capturing) antibody. In control studies, the number of solubilized nAChRs measured with [3H]EB was stable when incubated in the absence of an antibody over the time-course of the sequential immunoprecipitation procedure.
Comparison of ligand binding affinities at α4β2 and α4β2α5 nAChRs
Tissues were solubilized and nAChRs containing α3 and β4 subunits were removed by immunoprecipitation. The supernatant, containing α4β2 and α4β2α5 nAChRs, was used to carry out competition binding assays with various ligands against 0.5 nM [3H]EB and then analyzed for one or two sites.
Data Analysis
For immunoprecipitation studies, mean and standard error (SEM) for the difference between groups was calculated by the propagation of error method (Bevington 1969). Statistical analyses of the difference between group means were assessed using Student’s t-test or one-way analysis of variance followed by Bonferroni’s Multiple Comparison Test. A one-sample t-test was used to determine if values in immunoprecipitation assays were statistically different from zero. For competition studies, data were fit to one- and two-site models using the GRAPHPAD Prism 4.0 software package (GraphPad Software, San Diego, CA).
Results
nAChRs and their subunit composition in Rat Hippocampus
The density of heteromeric nAChRs in the hippocampus was measured with [3H]EB. Immunoprecipitations of [3H]EB-labeled receptors were then carried out using subunit-selective antibodies. Following determination of the subunit composition in the nAChRs, sequential immunoprecipitation assays allowed assessment of the subunit associations comprising the receptors, thus providing critical information about the receptor subtypes.
Single Immunoprecipitation
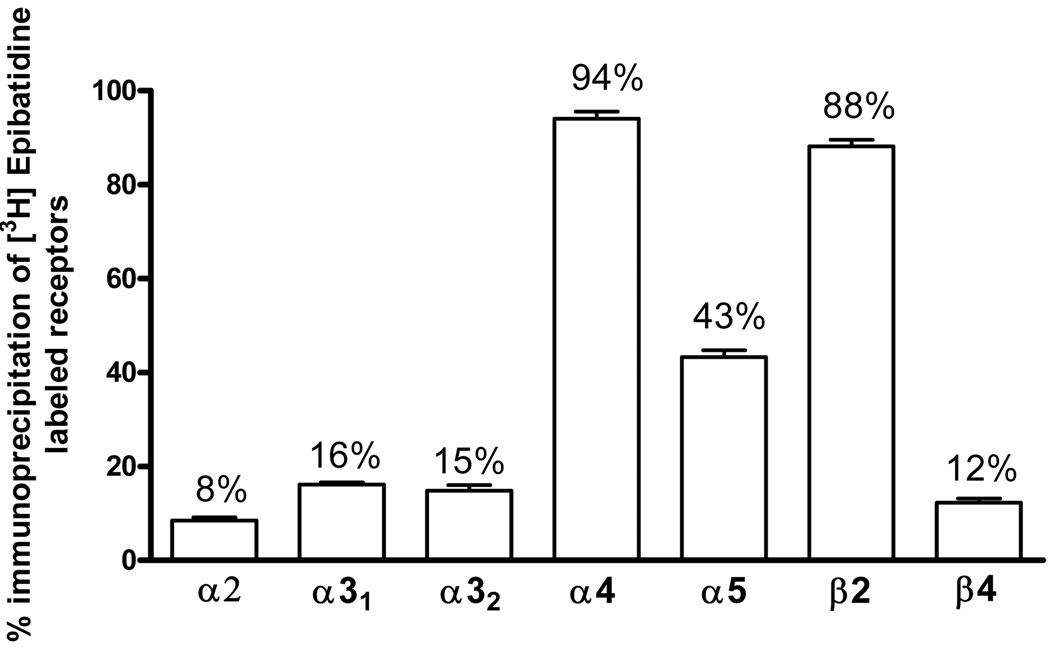
Specific antibodies directed against the α2, α3, α4, α5, β2 and β4 nAChR subunits were used in these studies. Previously, Mao et al. (2008) showed that α6 and β3 subunits are not expressed in hippocampus, therefore measurements of these subunits were not included in this study. The amount of receptors immunoprecipitated by each subunit-selective antibody is expressed as the percentage of total [3H]EB-labeled receptors. Single immunoprecipitation results confirmed that receptors containing the α4 and β2 subunits predominate in hippocampus, representing 94% and 88% of the total [3H]EB-labeled nAChRs, respectively (Fig. 1). nAChRs containing α5 subunits are also relatively abundant, accounting for about 43% of the receptors (Fig. 1). In addition, receptors containing the α3 (16%), β4 (12%) and α2 (8%) subunits were detected in smaller amounts (Fig. 1). The α3-containing nAChRs were measured with two different antibodies (here called α31 and α32) with similar results. The α32 antibody was used only in the immunoprecipitation studies shown in figure 1; all of the other experiments used the α31 antibody.
Fig. 1. Single Subunit Immunoprecipitation.
Rat hippocampi were solubilized, labeled with [3H]EB and immunoprecipitated with each of the subunit-selective antibodies shown. Two different α3-directed antibodies (indicated as α31 and α32) were used. Non-specific immunoprecipitations were measured with normal rabbit serum (NRS) and subtracted. Numeric values on each bar indicate the percent of total labeled nAChRs. Data are means ± SEM of 3 independent experiments. Student's t-test indicates that all results are statistically different from zero (p<0.05).
α4β2 Subunit Associations
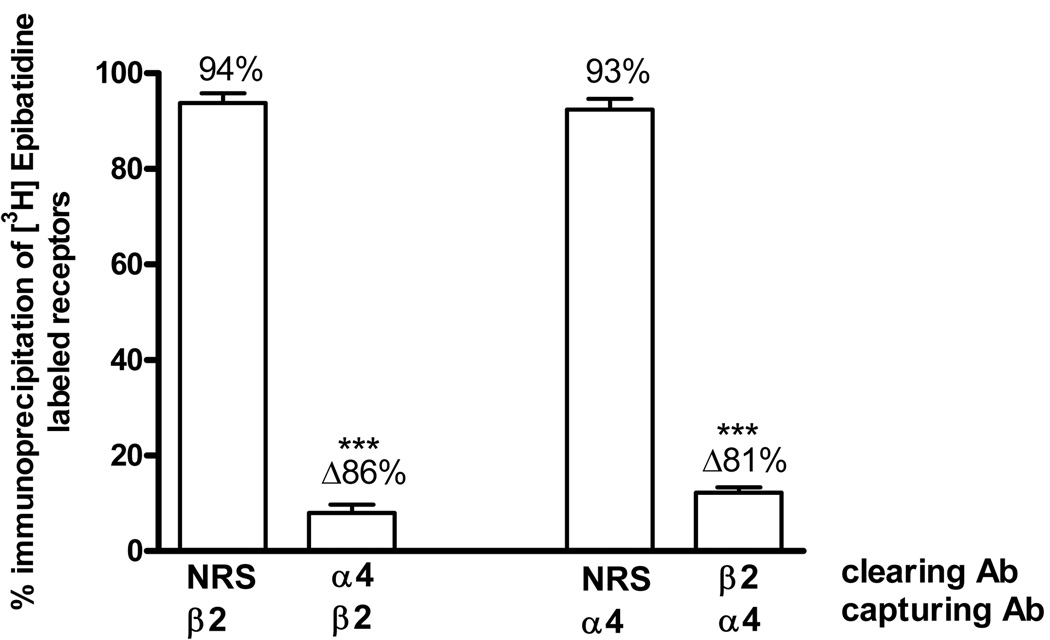
Sequential immunoprecipitation assays were carried out to quantify the possible nAChR subunit associations present in the rat hippocampus. For these experiments associations were evaluated in a bidirectional manner; for example, to assess the association between α4 and β2 subunits, tissues were first cleared by immunoprecipitation with the α4 antibody and this was followed by the second immunoprecipitation (the capturing step) with the β2 antibody. The order of antibodies was then reversed and the results were compared. In each case, NRS was used as a control for nonspecific immunoprecipitation. As shown in figure 2, initial immunoprecipitation with the α4 antibody decreased the number of receptors subsequently immunoprecipitated by the β2 antibody by 86%; similarly, initial immunoprecipitation with the β2 antibody decreased the number of receptors immunoprecipitated by the α4 antibody by 81%. Thus, these data indicate that the α4 and β2 subunits are associated in about 84% of the total [3H]EB-labeled receptors, indicating that the α4β2* subtype is the predominant heteromeric nAChR in the rat hippocampus.
Fig. 2. α4β2 Subunit Associations.
[3H]EB-labeled hippocampus extracts were first immunoprecipitated with normal rabbit serum (NRS) as a control or with α4 or β2 antibodies, indicated as clearing Ab. The resulting supernatants were then immunoprecipitated with β2 or α4 antibodies, indicated as capturing Ab. Each bar indicates the percent of the total labeled nAChRs immunoprecipitated by the capturing antibody. Δ represents the difference between the percent captured after clearing with NRS and the indicated antibody. Data are means ± SEM of 5 independent experiments. The value for the α4β2 association is statistically different compared to the corresponding NRS control, ***p<0.001, one-way ANOVA followed by Bonferroni's multiple comparison test.
a3β4 Subunit Associations
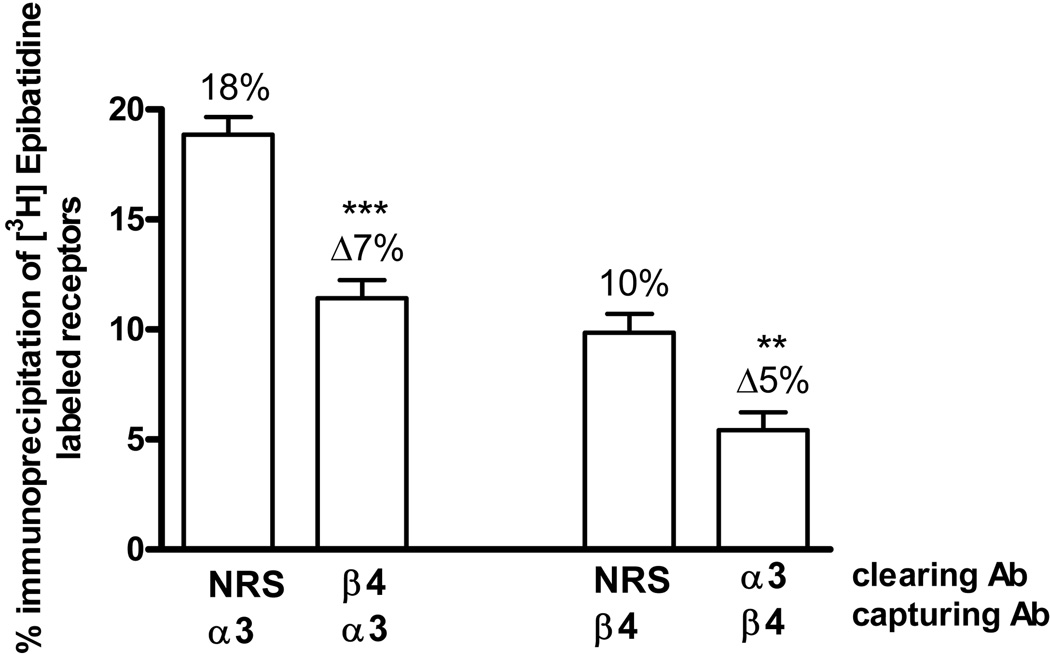
As shown in figure 1, the α3 and β4 subunits are present in ~16% and ~12%, respectively, of nAChRs in the hippocampus. We examined the association between these subunits by sequential immunoprecipitation. As shown in figure 3, clearing with the β4 antibody reduced the number of nAChRs captured by the α3 antibody by ~7%, and clearing with the α3 antibody reduced the number of nAChRs captured by the β4 antibody by ~5%. Although these percentages are low, the results were consistent and statistically significant. These results indicate that the α3 and β4 subunits are associated in ~6% of the nAChRs in the hippocampus. This suggests that 5 to 10% of the α3 and β4 subunits are not associated with each other, but are instead in other nAChR subtypes.
Fig. 3. α3β4 Subunit Associations.
[3H]EB-labeled hippocampus extracts were first immunoprecipitated with normal rabbit serum (NRS) as a control or with β4 or α3 antibodies, indicated as clearing Ab. The resulting supernatants were then immunoprecipitated with either α3 or β4 antibodies, indicated as capturing Ab. Each bar indicates the percent of the total labeled nAChRs immunoprecipitated by the capturing antibody. Δ represents the difference between the percent captured after clearing with NRS and the indicated antibody. Data are means ± SEM of 7 independent experiments. The value for the α3β4 association is statistically different compared to the corresponding NRS control, ***p<0.001, **p<0.01 one-way ANOVA followed by Bonferroni's multiple comparison test.
α3α4 and α3β2 Subunit Associations
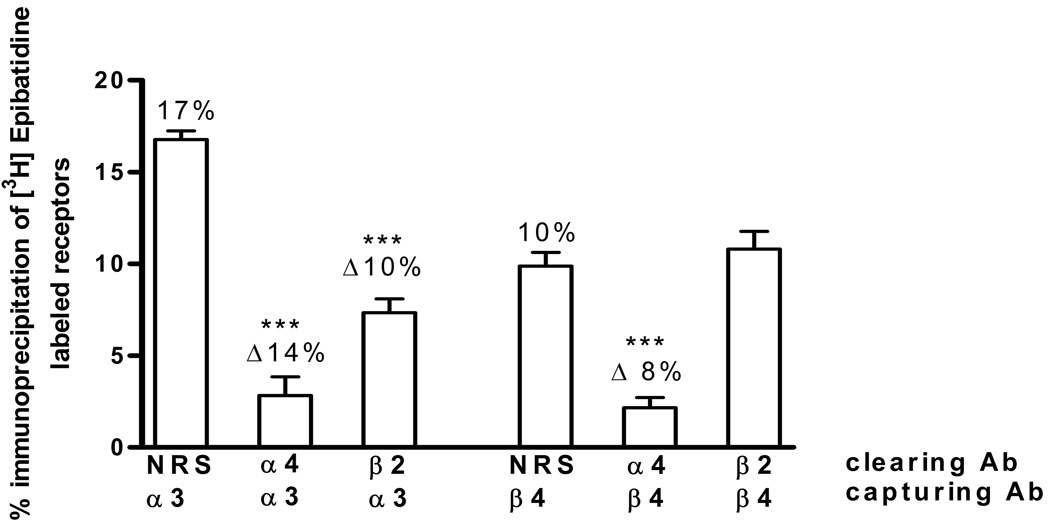
Approximately 17% of the heteromeric nAChRs in the hippocampus contain α3 subunits. Pre-clearing with the α4 antibody decreased the α3-containing receptors to ~ 3% (Fig. 4), indicating that nearly all of the hippocampal receptors containing α3 subunits also contain the α4 subunit. We next analyzed the associations between α3 and β2 subunits and found that about 10% of total receptors contained α3 subunits in association with β2 subunits (Fig. 4). Reversing the order of immunoprecipitations (i.e., using α3 as the clearing antibody before capturing with the α4 or β2 antibody) gave similar results (data not shown).
Fig. 4. α3α4, α3β2, α4β4 and β2β4 Subunit Associations.
[3H]EB-labeled hippocampus extracts were first immunoprecipitated with normal rabbit serum (NRS) as a control or with α4 or β2 antibodies, indicated as clearing Ab. The resulting supernatants were then sequentially immunoprecipitated with either α3 or β4 antibodies, indicated as capturing Ab. Each bar indicates the percent of the total labeled nAChRs immunoprecipitated by the capturing antibody. Δ represents the difference between the percent captured after clearing with NRS and the indicated antibody. Data are means ± SEM of 6–7 independent experiments. The values for the α3α4, α3β2 and α4β4 associations are statistically different compared to the corresponding NRS control, ***p<0.001, one-way ANOVA followed by Bonferroni's multiple comparison test. The value for the β2β4 association is not statistically different from the NRS control.
α4β4 and β2β4 Subunit Associations
Examination of the α4/β4 association indicated that pre-clearing with the α4 antibody, decreased the β4-containing nAChR receptors to ~ 2% of their total (Fig. 4), indicating that nearly all of the β4 subunits are associated with α4 subunits. In contrast, the β2/β4 subunit combination was not detected in these studies (Fig. 4). Again, reversing the order of immunoprecipitations (i.e., using β4 as the clearing antibody before capturing with the α4 or β2 antibody) yielded similar results (data not shown).
Receptors containing the α5 subunit
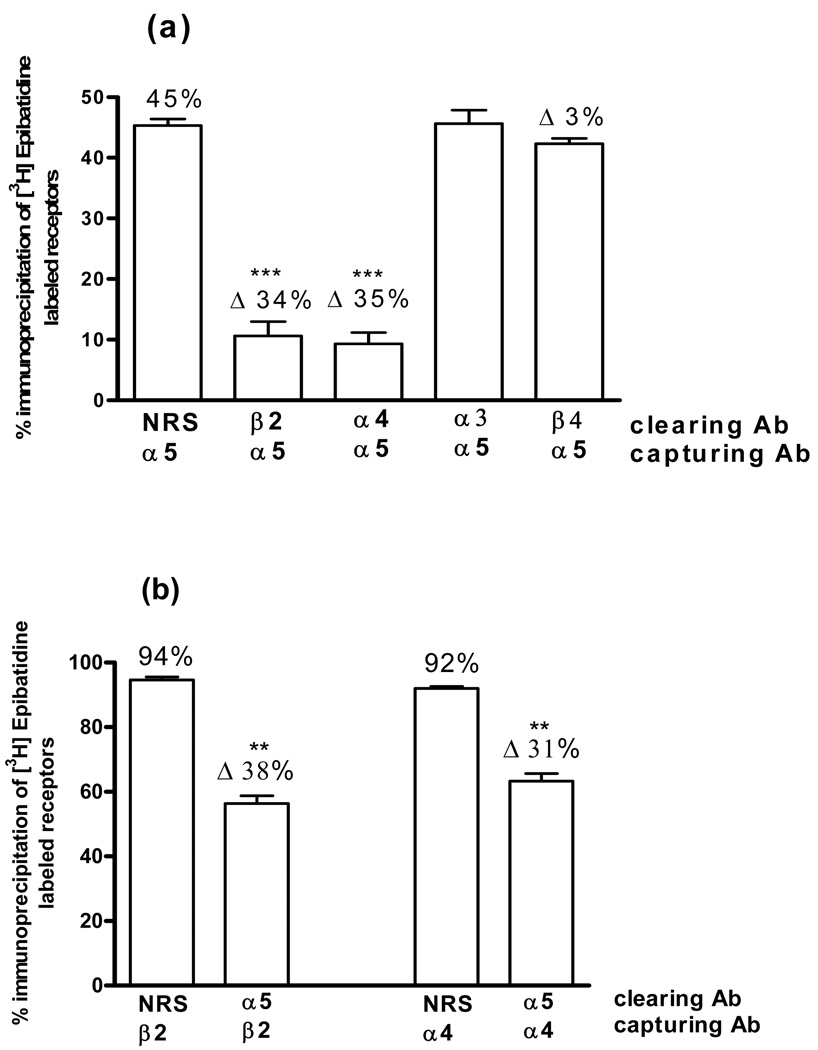
In these studies, single immunoprecipitation assays indicated that ~ 45% of the nAChRs contain the α5 subunit (Fig. 1). Pre-clearing with the β2 antibody in sequential immunoprecipitation assays decreased the α5-containing receptors by ~ 34% (Fig. 5a). Similarly, pre-clearing with the α4 antibody decreased the α5-containing receptors by ~ 35% (Fig. 5a). Studies where the order of immunoprecipitations was reversed yielded similar results (Fig. 5b). In contrast, no decrease in α5-containing receptors was found after pre-clearing with the α3 or β4 antibody (Fig. 5a), indicating that these hippocampal receptors are not associated with either α3 or β4 subunits.
Fig. 5. α5 Subunit Associations.
(a) [3H]EB-labeled hippocampus extracts were first immunoprecipitated with normal rabbit serum (NRS) as a control or with β2, α4, α3 or β4 antibodies, indicated as clearing Ab. The resulting supernatants were then sequentially immunoprecipitated with the α5 antibody, indicated as capturing Ab. (b) Experiments were repeated by reversing the order of the antibodies, thus the α5 antibody was used as the clearing antibody and the β2 and α4 antibodies were used as capturing antibodies.
Each bar indicates the percent of the total labeled nAChRs immunoprecipitated by the capturing antibody. Δ represents the difference between the percent captured after clearing with NRS and the indicated antibody. Data are means ± SEM of 3 independent experiments. The values for both the α5α4 and α5β2 associations are statistically different from the corresponding NRS control, ***p<0.001, one-way ANOVA followed by Bonferroni's multiple comparison test. The values for the α5α3 and α5β4 associations are not statistically different from the NRS control.
nAChR subtypes deduced from the immunoprecipitation results
Our results indicate that the α4β2* nAChR subtypes account for ~ 84% of the total [3H]EB labeled receptors in the rat hippocampus (Fig. 2). The α4 and β2 subunits occur in ~ 94% and 88% of the total receptors, respectively; therefore, about 10% of the receptors contain α4 subunits without a β2 subunit— i.e., an α4β4* subtype. (It’s also possible that a small percentage of receptors contain β2 subunits without an α4 subunit but are below our limits of detection.) The α3 and β2 subunits are associated in approximately 10% of the total receptors (Fig. 4); but, interestingly, the α3 subunit, which was found in ~ 16% of the nAChRs, also seems to be nearly completely associated with the α4 subunit (Fig. 4). To reconcile these data, we propose the existence of an α4β2α3* mixed heteromeric subtype, which accounts for ~10% of the total nAChRs in the hippocampus (Table 1). The remaining α4α3 associations, which do not contain the β2 subunit, are presumed to include the β4 subunit to form an agonist binding site. In fact, our results indicate that the β4 subunits, which are found in ~10% of the receptors, are nearly totally associated with the α4 subunit (Fig. 4). Moreover, as shown in figure 3, the α3 and β4 subunits are combined in only ~5% of total nAChRs, indicating that not all α3 and β4 subunits are associated in the same receptor. This suggests that some fraction of the 10% of the receptors that contain the α4β4 subunit combination also contain the α3 subunit, forming an α4β4α3* subtype. Together, these data suggest that ~10% of the nAChRs in the hippocampus are an α4β4 subtype with or without an α3 subunit (Table 1).
Table 1.
Summary of the five heteromeric nAChRs identified in the rat hippocampus.
| Subtype | Percent of Total |
|---|---|
| α4β2α3 | ~10% |
| α4β4 ± α3 | ~10% |
| α4β2α5 | ~35% |
| α4β2 | ~40% |
Consistent with previous results (Mao et al. 2008), we found that the α5-containing receptors exist predominantly and possibly exclusively as the α4β2α5 subtype and represent 35% of the total receptors in the hippocampus (Fig 5 and Table 1). As shown above, the α4β2 associations represent ~84% of the nAChR subtypes in the hippocampus. Subtracting the fraction of the mixed heteromeric subtypes, α4β2α3 (10%) and α4β2α5 (35%) from the total α4β2 association leads to the conclusion that ~39% of the total nAChR populations in the rat hippocampus are the simple heteromeric α4β2 subtype (Table 1).
We also found that ~8% of nAChRs in the hippocampus express the α2 subunit (Fig. 1), but our methods did not allow us to establish with confidence which other subunits are associated with the α2 subunit. Although we found that in some experiments, a fraction of the α2 subunit appeared to be associated with the β2 subunit, but not with the β4 subunit, (data not shown), this result was not consistent and did not reach statistical significance. In our assays, the limit of reliably detected subunit associations measured directly is usually ~ 10%, but this depends on the affinity of the antibody. However, in some cases, a smaller fraction of subunit associations could be deduced from the total number of receptors containing that subunit and its other associations. This was not the case for the receptors containing the α2 subunit.
Although this study was focused on the heteromeric nAChRs containing α2 to α5 subunits, there are reports of association between α7 and β2 subunits (Khirouh et al. 2002; Azam et al. 2003; Liu et al. 2009); therefore we also attempted to determine whether the α7 subunit was associated with the β2 or β4 subunits in the hippocampus. Because antibodies to the α7 subunit suitable for immunoprecipitation are not available, we immunoprecipitated [125I]α-BTX binding sites with the β2 and β4 antibodies. These studies did not find evidence of association between the [125I]α-BTX binding site and either β subunit (data not shown).
Pharmacology of α4β2 and α4β2α5 nAChR binding sites
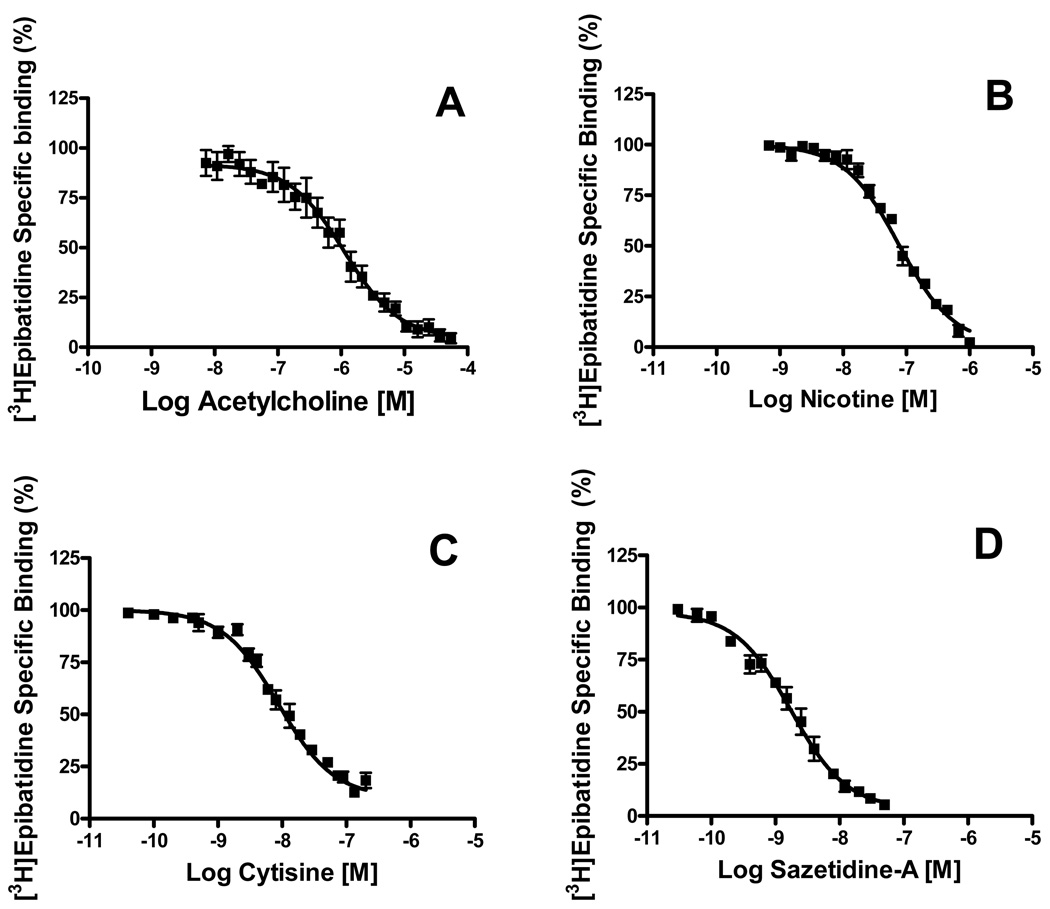
To determine if there are differences in the binding site pharmacology of α4β2 and α4β2α5 nAChRs, the two major subtypes in the hippocampus, we carried out competition assays on these two receptor subtypes. Solubilized hippocampus membranes were first cleared of α3- and β4-containing nAChRs by immunoprecipitation with both α3 and β4 antibodies. This isolated the two major nAChRs subtypes, α4β2 and α4β2α5, which together account for ~75% of the nAChRs in the hippocampus. Ligand binding competition studies were then carried out with acetylcholine, nicotine, cytisine and sazetidine-A against [3H]EB to determine if any of these ligands distinguish between the binding sites for these two α4β2 subtypes. As shown in figure 6, all of these ligands competed for all the binding sites with high affinity. In each case, the data fit a model for a single class of binding site with a Hill coefficient close to 1 and Ki values expected at an α4β2 nAChR binding site (Table 2).
Fig. 6. Pharmacological profiles of α4β2 and α4β2α5 nAChR binding sites.
Tissue was first immunoprecipitated with α3 and β4 antibodies to remove the α3- and β4-containing nAChRs. Ligand binding competition studies were then carried out on the remaining α4β2 and α4β2α5 subtypes. (A) Acetylcholine, (B) nicotine, (C) cytisine and (D) sazetidine-A were used at the indicated concentrations against 0.5 nM of [3H]EB. Data are expressed as percent of binding remaining and are means ± SEM of 3 to 4 experiments. Data were fit to a one or two-site binding model using GRAPHPAD Prism 4.0 software package. In all cases the one site model provided the preferred statistical fit.
Table 2.
Ki values of nAChR ligand binding to isolated α4β2 and α4β2α5 subtypes in Rat Hippocampus.
| Ligand | Ki (nM) ± S.E.M. | Hill Coefficient |
|---|---|---|
| Acetylcholine | 102 ± 0.16 | 0.80 ± 0.09 |
| Nicotine | 7.5 ± 0.75 | 1.01 ± 0.03 |
| Cytisine | 0.8 ± 0.49 | 0.87 ± 0.04 |
| Sazetidine-A | 0.2 ± 0.33 | 0.93 ± 0.06 |
Discussion
Neuronal heteromeric nicotinic receptors subtypes were identified in the rat hippocampus by using [3H]EB and immunoprecipitation with subunit-selective antibodies. Our results show the presence of several heteromeric nAChRs in the hippocampus (Table 1). The α4β2 and α4β2α5 nAChRs represent the predominant subtypes (~40% and 35%, respectively). The remaining 20% of the nAChRs identified here appear to be comprised of a mixture of α4β2α3 and α4β4 ± α3 subtypes.
The finding that all of the α3- and the β4-containing receptors are associated with the α4 subunit in the hippocampus indicates that the simple heteromeric α3β4 subtype, which is a predominant receptor in the superior cervical and probably the nodose sympathetic ganglia (Mao et al. 2006), does not exist in the hippocampus. Interestingly, an α3β4* receptor subtype has been proposed to be involved in nicotine-stimulated norepinephrine (NE) release in hippocampus (Clarke and Reuben 1996; Luo et al. 1998; Kulak et al. 2001; Dajas-Bailador and Wonnacott 2004). This is supported by studies showing that cytisine, a full agonist at nAChRs containing β4 subunits but a weak partial agonist at the receptors containing β2 subunits (Luetje and Patrick 1991; Papke and Heinemann 1994), fully stimulates the release of NE in the rat hippocampus (Clarke and Reuben 1996). Consistent with this, dihydro-β-erythroidine, a relatively selective β2 nAChR antagonist does not block the nicotine-evoked NE release from rat hippocampal synaptosomes (Clarke and Reuben 1996). Our results suggest that the previously reported nicotine- and cytisine-stimulated release of NE in the hippocampus is mediated by α4β4α3 and/or α4β4 nAChRs, rather than simple α3β4 receptors. Interestingly, both nicotine and cytisine are more potent in eliciting responses at α4β4 than at α3β4 nAChRs expressed in oocytes (Luetje and Patrick 1991). Similarly, most agonists that have been examined, including acetylcholine, nicotine and cytisine, have binding affinities 10- to 100- times higher at α4β4 than at α3β4 nAChRs (Parker et al. 1998; Xiao and Kellar 2004). Depending on the subunit order and stoichiometry, the small number of α4β4α3 nAChR subtypes that we found here may have characteristics of an α3β4 or an α4β4 receptor, or even intermediate characteristics; therefore, they might differentially affect agonist-stimulated hippocampal NE release.
We also found that ~10% of the hippocampal nAChRs were a subtype with a subunit composition of α4β2α3. A similar percentage of this subtype was also found in rat cerebellum (Turner and Kellar 2005) but has not been reported in any other brain tissue (Gotti et al. 2006). As with the α4β4α3 mixed receptor subtype, the functional characteristics of the α4β2α3 subtype might reflect both α4β2 and α3β2 interfaces. The pharmacology of α4β2 and α3β2 nAChR responses measured in oocytes differs, with the potency of acetylcholine being 40-fold higher and the potency of nicotine being 17-fold higher at α4β2 than at α3β2 receptors (Luetje and Patrick 1991; Papke and Heinemann 1994; Rush et al. 2002). Similarly, the binding characteristics of ligands at these interfaces differ, with nicotine having ~5-fold higher affinity at α4β2 than at α3β2 subtypes (Parker et al. 1998; Xiao and Kellar 2004). Whether the α4β2α3 nAChRs in the hippocampus have characteristics closer to the α4β2 or α3β2 subtype may be important; in particular, in the case of chronic exposure to nicotine, as in smokers, nicotine may exert a greater effect via the α4β2 interface because of its higher potency.
We also found a relatively small percentage of α2-containing receptors in the hippocampus. This is consistent with previous reports that found the α2 subunit mRNA in the stratum oriens of CA1 by RT-PCR (Sudweeks and Yakel, 2000) and also by situ hybridization histochemistry (Son and Winzer-Serhan, 2006). Studies in heterologous expression systems found that α2-containing receptors have certain distinguishing characteristics related to channel open time (Papke et al., 1989), extent of desensitization (Chavez-Noriega et al, 1997) and pharmacology (Harvey et al, 1996; Chavez-Noriega et al, 1997; Parker et al, 1998; Khiroug et al, 2004; Xiao and Kellar, 2004). Unfortunately, our sequential immunoprecipitation methods did not allow us to determine which β subunit is associated with the α2 subunit in the hippocampus or whether it is associated with other α subunits.
This study also confirmed that in the hippocampus the α5 subunit is associated predominantly and possibly exclusively with the α4 and β2 subunits (Mao et al. 2008). This α4β2α5 subtype, which represents ~35% of the receptors, is one of the two major heteromeric nAChRs in the hippocampus. In fact, the hippocampus has the highest percentage of α5-containing nAChRs in the adult rat brain (Mao et al. 2008). The α5 subunit differs structurally from other nAChR α subunits and does not form an agonist binding interface (Abramson et al. 1989; Boulter et al. 1990; Cohen et al. 1991; Tomaselli et al. 1991; Conroy et al. 1992); therefore, it must form a complex with other α and β subunits (Ramirez-Latorre et al. 1996; Fucile et al. 1997). Nevertheless, the presence of the α5 subunit is reported to markedly influence the properties of nAChRs, increasing their Ca2+ conductance and rate of desensitization (Ramirez-Latorre et al. 1996; Wang et al. 1996; Fucile et al. 1997; Sivilotti et al. 1997; Nelson and Lindstrom 1999). Moreover, although the α5 subunit does not participate directly in ligand binding, it may still allosterically influence the binding interfaces and consequently affect the binding affinity and potency of nicotinic ligands (Gerzanich et al. 1998). However, we detected no differences in the affinities of several nicotinic ligands, as measured by their competition for the α4β2 and α4β2α5 subtypes. Therefore, it appears that in the rat hippocampus, α4β2 and α4β2α5 nAChRs, despite possible differences in their physiological characteristics, have a similar binding site pharmacology.
Another characteristic of α5-containing receptors is that they do not up-regulate during chronic nicotine treatment (Mao et al. 2008). Up-regulation of nAChRs is believed to be important in the mechanisms underlying nicotine addiction; therefore, the absence of nicotine-stimulated α4β2α5 up-regulation suggests that these receptors may be directly involved in the mechanisms leading to nicotine dependence.
Recently, a single nucleotide polymorphism in the CHRNA5 gene that translates into a single amino acid change (D398N) in the α5 subunit was discovered (Saccone et al. 2007; Bierut et al. 2008; Sherva et al. 2008). This genetic variant of the α5 subunit appears to increase the susceptibility to nicotine addiction (Saccone et al. 2007; Berettini et al. 2008; Bierut et al. 2008), heavy smoking (Berettini et al. 2008; Stevers et al. 2008) and even lung cancer risk (Falvella et al. 2009). This supports the hypothesis of a genetic basis for nicotine dependence and that α5 nAChRs are involved in this process.
In conclusion, the rat hippocampus expresses several subtypes of heteromeric nAChRs, including an α4β4 ± α3 subtype, which might be related to the previously reported nAChR that modulates NE release. In addition, we confirm that the hippocampus expresses the highest percentage of α5-containing receptors, all of which are part of the α4β2α5 subtype. The high percentage of α5-containing nAChRs in the rat hippocampus makes this brain tissue a suitable target to study the potential effects that this subunit exerts on signaling in the brain.
Acknowledgements
This work was supported by the National Institute of Health Grant DA 012976.
We thank Drs. Milton Brown and Mikell Paige for the synthesis of Sazetidine-A, and Drs. Scott Rogers and Lorise Gahring (University of Utah, Salt Lake City, USA) for kindly providing us with the antisera to several nAChR subunits. We thank Dr. Yingxian Xiao (Georgetown University, Washington, DC, USA) for helpful discussions.
Abbreviations used
- [3H]EB
[3H]Epibatidine
- nAChR
neuronal nicotinic acetylcholine receptor
- mAb
monoclonal antibody
- AB
antibody
- NRS
normal rabbit serum
- NE
norepinephrine
- [125I]α-BTX
[125I]α-bungarotoxin.
Footnotes
Portions of this work were previously presented as an abstract at the Society for Neuroscience annual conference in 2008 (328.5) and 2009 (226.2).
References
- Abramson SN, Li Y, Culver P, Taylor P. An analog of lophotoxin reacts covalently with Tyr190 in the alpha-subunit of the nicotinic acetylcholine receptor. J Biol Chem. 1989;264:12666–12672. [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J Pharmacol Exp Ther. 1993;265:1455–1473. [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. The nicotinic acetylcholine receptor subtypes and their function in the hippocampus and cerebral cortex. Prog Brain Res. 2004;145:109–120. doi: 10.1016/S0079-6123(03)45007-3. [DOI] [PubMed] [Google Scholar]
- Azam L, Winzer-Serhan U, Leslie FM. Co-expression of alpha7 and beta2 nicotinic acetylcholine receptor subunit mRNAs within rat brain cholinergic neurons. Neuroscience. 2003;119:965–977. doi: 10.1016/s0306-4522(03)00220-3. [DOI] [PubMed] [Google Scholar]
- Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, Waterworth D, Muglia P, Mooser V. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry. 2008;13:368–373. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevington PR. Data reduction and error analysis for the physical sciences. New York: McGraw-Hill Companies; 1969. pp. 58–64. [Google Scholar]
- Bierut LJ, Stitzel JA, Wang JC, et al. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J, O'Shea-Greenfield A, Duvoisin RM, Connolly JG, Wada E, Jensen A, Gardner PD, Ballivet M, Deneris ES, McKinnon D. Alpha 3, alpha 5, and beta 4: three members of the rat neuronal nicotinic acetylcholine receptor-related gene family form a gene cluster. J Biol Chem. 1990;265:4472–4482. [PubMed] [Google Scholar]
- Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, Johnson EC. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors h alpha 2 beta 2, h alpha 2 beta 4, h alpha 3 beta 2, h alpha 3 beta 4, h alpha 4 beta 2, h alpha 4 beta 4 and h alpha 7 expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1997;280:346–356. [PubMed] [Google Scholar]
- Clarke PB, Reuben M. Release of [3H]-noradrenaline from rat hippocampal synaptosomes by nicotine: mediation by different nicotinic receptor subtypes from striatal [3H]-dopamine release. Br J Pharmacol. 1996;117:595–606. doi: 10.1111/j.1476-5381.1996.tb15232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JB, Sharp SD, Liu WS. Structure of the agonist-binding site of the nicotinic acetylcholine receptor. [3H]acetylcholine mustard identifies residues in the cation-binding subsite. J Biol Chem. 1991;266:23354–23364. [PubMed] [Google Scholar]
- Conroy WG, Vernallis AB, Berg DK. The alpha 5 gene product assembles with multiple acetylcholine receptor subunits to form distinctive receptor subtypes in brain. Neuron. 1992;9:679–691. doi: 10.1016/0896-6273(92)90031-8. [DOI] [PubMed] [Google Scholar]
- Dajas-Bailador F, Wonnacott S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol Sci. 2004;25:317–324. doi: 10.1016/j.tips.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Falvella FS, Galvan A, Frullanti E, Spinola M, Calabrò E, Carbone A, Incarbone M, Santambrogio L, Pastorino U, Dragani TA. Transcription deregulation at the 15q25 locus in association with lung adenocarcinoma risk. Clin Cancer Res. 2009;15:1837–1842. doi: 10.1158/1078-0432.CCR-08-2107. [DOI] [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol. 1992;41:31–37. [PubMed] [Google Scholar]
- Fucile S, Barabino B, Palma E, Grassi F, Limatola C, Mileo AM, Alemà S, Ballivet M, Eusebi F. Alpha 5 subunit forms functional alpha 3 beta 4 alpha 5 nAChRs in transfected human cells. Neuroreport. 1997;8:2433–2436. doi: 10.1097/00001756-199707280-00005. [DOI] [PubMed] [Google Scholar]
- Gerzanich V, Wang F, Kuryatov A, Lindstrom J. alpha5 Subunit alters desensitization, pharmacology, Ca++ permeability and Ca++ modulation of human neuronal alpha 3 nicotinic receptors. J Pharmacol Exp Ther. 1998;286:311–320. [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Harvey SC, Maddox FN, Luetje CW. Multiple determinants of dihydro-beta-erythroidine sensitivity on rat neuronal nicotinic receptor alpha subunits. J Neurochem. 1996;67:1953–1959. doi: 10.1046/j.1471-4159.1996.67051953.x. [DOI] [PubMed] [Google Scholar]
- Hernandez SC, Vicini S, Xiao Y, Dávila-García MI, Yasuda RP, Wolfe BB, Kellar KJ. The nicotinic receptor in the rat pineal gland is an alpha3beta4 subtype. Mol Pharmacol. 2004;66:978–987. doi: 10.1124/mol.104.002345. [DOI] [PubMed] [Google Scholar]
- Jia Y, Yamazaki Y, Nakauchi S, Sumikawa K. Alpha2 nicotine receptors function as a molecular switch to continuously excite a subset of interneurons in rat hippocampal circuits. Eur J Neurosci. 2009;29:1588–1603. doi: 10.1111/j.1460-9568.2009.06706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Yakel JL. Functional nicotinic ACh receptors on interneurones in the rat hippocampus. J Physiol. 1997;504:603–610. doi: 10.1111/j.1469-7793.1997.603bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khiroug SS, Harkness PC, Lamb PW, Sudweeks SN, Khiroug L, Millar NS, Yakel JL. Rat nicotinic ACh receptor alpha7 and beta2 subunits co-assemble to form functional heteromeric nicotinic receptor channels. J Physiol. 2002;540(Pt 2):425–434. doi: 10.1113/jphysiol.2001.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khiroug SS, Khiroug L, Yakel J. Rat nicotinic acetylcholine receptor alpha2beta2 channels: comparison of functional properties with alpha4beta2 channels in Xenopus oocytes. Neuroscience. 2004;124:817–822. doi: 10.1016/j.neuroscience.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Kulak JM, McIntosh JM, Yoshikami D, Olivera BM. Nicotine-evoked transmitter release from synaptosomes: functional association of specific presynaptic acetylcholine receptors and voltage-gated calcium channels. J Neurochem. 2001;77:1581–1589. doi: 10.1046/j.1471-4159.2001.00357.x. [DOI] [PubMed] [Google Scholar]
- Liu Q, Huang Y, Xue F, Simard A, DeChon J, Li G, Zhang J, Lucero L, Wang M, Sierks M, Hu G, Chang Y, Lukas RJ, Wu J. A novel nicotinic acetylcholine receptor subtype in basal forebrain cholinergic neurons with high sensitivity to amyloid peptides. J Neurosci. 2009;29:918–929. doi: 10.1523/JNEUROSCI.3952-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetje CW, Patrick J. Both alpha- and beta-subunits contribute to the agonist sensitivity of neuronal nicotinic acetylcholine receptors. J Neurosci. 1991;11:837–845. doi: 10.1523/JNEUROSCI.11-03-00837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Kulak JM, Cartier GE, Jacobsen RB, Yoshikami D, Olivera BM, McIntosh JM. alpha-conotoxin AuIB selectively blocks alpha3 beta4 nicotinic acetylcholine receptors and nicotine-evoked norepinephrine release. J Neurosci. 1998;18:8571–8579. doi: 10.1523/JNEUROSCI.18-21-08571.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao D, Yasuda RP, Fan H, Wolfe BB, Kellar KJ. Heterogeneity of nicotinic cholinergic receptors in rat superior cervical and nodose Ganglia. Mol Pharmacol. 2006;70:1693–1699. doi: 10.1124/mol.106.027458. [DOI] [PubMed] [Google Scholar]
- Mao D, Perry DC, Yasuda RP, Wolfe BB, Kellar KJ. The alpha4beta2alpha5 nicotinic cholinergic receptor in rat brain is resistant to up-regulation by nicotine in vivo. J Neurochem. 2008;104:446–456. doi: 10.1111/j.1471-4159.2007.05011.x. [DOI] [PubMed] [Google Scholar]
- Marritt AM, Cox BC, Yasuda RP, McIntosh JM, Xiao Y, Wolfe BB, Kellar KJ. Nicotinic cholinergic receptors in the rat retina: simple and mixed heteromeric subtypes. Mol Pharmacol. 2005;68:1656–1668. doi: 10.1124/mol.105.012369. [DOI] [PubMed] [Google Scholar]
- McQuiston AR, Madison DV. Nicotinic receptor activation excites distinct subtypes of interneurons in the rat hippocampus. J Neurosci. 1999;19:2887–2896. doi: 10.1523/JNEUROSCI.19-08-02887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1–Ch6) Neuroscience. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Nelson ME, Lindstrom J. Single channel properties of human alpha3 AChRs: impact of beta2, beta4 and alpha5 subunits. J Physiol. 1999;516:657–678. doi: 10.1111/j.1469-7793.1999.0657u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Boulter J, Patrick J, Heinemann S. Single-channel currents of rat neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. Neuron. 1989;3:589–596. doi: 10.1016/0896-6273(89)90269-9. [DOI] [PubMed] [Google Scholar]
- Papke RL, Heinemann SF. Partial agonist properties of cytisine on neuronal nicotinic receptors containing the beta 2 subunit. Mol Pharmacol. 1994;45:142–149. [PubMed] [Google Scholar]
- Parker MJ, Beck A, Luetje CW. Neuronal nicotinic receptor beta2 and beta4 subunits confer large differences in agonist binding affinity. Mol Pharmacol. 1998;54:1132–1139. [PubMed] [Google Scholar]
- Pauly JR, Marks MJ, Gross SD, Collins AC. An autoradiographic analysis of cholinergic receptors in mouse brain after chronic nicotine treatment. J Pharmacol Exp Ther. 1991;258:1127–1136. [PubMed] [Google Scholar]
- Perry DC, Mao D, Gold AB, McIntosh JM, Pezzullo JC, Kellar KJ. Chronic Nicotine Differentially Regulates {alpha}6- and beta3-Containing Nicotinic Cholinergic Receptors in Rat Brain. J Pharmacol Exp Ther. 2007;322:306–315. doi: 10.1124/jpet.107.121228. [DOI] [PubMed] [Google Scholar]
- Perry EK, Perry RH, Smith CJ, Dick DJ, Candy JM, Edwardson JA, Fairbairn A, Blessed G. Nicotinic receptor abnormalities in Alzheimer's and Parkinson's diseases. J Neurol Neurosurg Psychiatry. 1987;50:806–809. doi: 10.1136/jnnp.50.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L. Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380:347–351. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- Rogers SW, Mandelzys A, Deneris ES, Cooper E, Heinemann S. The expression of nicotinic acetylcholine receptors by PC12 cells treated with NGF. J Neurosci. 1992;12:4611–4623. doi: 10.1523/JNEUROSCI.12-12-04611.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush R, Kuryatov A, Nelson ME, Lindstrom J. First and second transmembrane segments of alpha3, alpha4, beta2, and beta4 nicotinic acetylcholine receptor subunits influence the efficacy and potency of nicotine. Mol Pharmacol. 2002;61:1416–1422. doi: 10.1124/mol.61.6.1416. [DOI] [PubMed] [Google Scholar]
- Saccone SF, Hinrichs AL, Saccone NL, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherva R, Wilhelmsen K, Pomerleau CS, Chasse SA, Rice JP, Snedecor SM, Bierut LJ, Neuman RJ, Pomerleau OF. Association of a single nucleotide polymorphism in neuronal acetylcholine receptor subunit alpha 5 (CHRNA5) with smoking status and with 'pleasurable buzz' during early experimentation with smoking. Addiction. 2008;103:1544–1552. doi: 10.1111/j.1360-0443.2008.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivilotti LG, McNeil DK, Lewis TM, Nassar MA, Schoepfer R, Colquhoun D. Recombinant nicotinic receptors, expressed in Xenopus oocytes, do not resemble native rat sympathetic ganglion receptors in single-channel behaviour. J Physiol. 1997;500:123–138. doi: 10.1113/jphysiol.1997.sp022004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son JH, Winzer-Serhan UH. Postnatal expression of alpha2 nicotinic acetylcholine receptor subunit mRNA in developing cortex and hippocampus. J Chem Neuroanat. 2006;32:179–190. doi: 10.1016/j.jchemneu.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son JH, Winzer-Serhan UH. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs in rat hippocampal GABAergic interneurons. J Comp Neurol. 2008;511:286–299. doi: 10.1002/cne.21828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens VL, Bierut LJ, Talbot JT, Wang JC, Sun J, Hinrichs AL, Thun MJ, Goate A, Calle EE. Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiol Biomarkers Prev. 2008;17:3517–3525. doi: 10.1158/1055-9965.EPI-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudweeks SN, Yakel JL. Functional and molecular characterization of neuronal nicotinic ACh receptors in rat CA1 hippocampal neurons. J Physiol. 2000;527(Pt 3):515–528. doi: 10.1111/j.1469-7793.2000.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia L, Kuryatov A, Lindstrom J. Ca2+ permeability of the (alpha4)3(beta2)2stoichiometry greatly exceeds that of (alpha4)2(beta2)3 human acetylcholine receptors. Mol Pharmacol. 2007;71:769–776. doi: 10.1124/mol.106.030445. [DOI] [PubMed] [Google Scholar]
- Tomaselli GF, McLaughlin JT, Jurman ME, Hawrot E, Yellen G. Mutations affecting agonist sensitivity of the nicotinic acetylcholine receptor. Biophys J. 1991;60:721–727. doi: 10.1016/S0006-3495(91)82102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JR, Kellar KJ. Nicotinic cholinergic receptors in the rat cerebellum: multiple heteromeric subtypes. J Neurosci. 2005;25:9258–9265. doi: 10.1523/JNEUROSCI.2112-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Wang F, Gerzanich V, Wells GB, Anand R, Peng X, Keyser K, Lindstrom J. Assembly of human neuronal nicotinic receptor alpha5 subunits with alpha3, beta2, and beta4 subunits. J Biol Chem. 1996;271:17656–17665. doi: 10.1074/jbc.271.30.17656. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, Struble RG, Clark AW, Price DL. Alzheimer disease: plaques, tangles, and the basal forebrain. Ann Neurol. 1982;12:494. doi: 10.1002/ana.410120517. [DOI] [PubMed] [Google Scholar]
- Whiting P, Lindstrom J. Purification and characterization of a nicotinic acetylcholine receptor from rat brain. Proc Natl Acad Sci USA. 1987;84:595–599. doi: 10.1073/pnas.84.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh JJ, Ferreira M, Ebert S, Yasuda RP, Kellar KJ, Wolfe BB. Axotomy and nerve growth factor regulate levels of neuronal nicotinic acetylcholine receptor α3 subunit protein in rat superior cervical ganglion. J Neurochem. 2001;79:258–265. doi: 10.1046/j.1471-4159.2001.00545.x. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Kellar KJ. The comparative pharmacology and up-regulation of rat neuronal nicotinic receptor subtype binding sites stably expressed in transfected mammalian cells. J Pharmacol Exp Ther. 2004;310:98–107. doi: 10.1124/jpet.104.066787. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Fan H, Musachio JL, Wei ZL, Chellappan SK, Kozikowski AP, Kellar KJ. Sazetidine-A, a novel ligand that desensitizes alpha4beta2 nicotinic acetylcholine receptors without activating them. Mol Pharmacol. 2006;70:1454–1460. doi: 10.1124/mol.106.027318. [DOI] [PubMed] [Google Scholar]