Abstract
The antigen-rich environment of the gut interacts with a highly integrated and specialized mucosal immune system that has the challenging task of preventing invasion and the systemic spread of microbes, while avoiding excessive or unnecessary immune responses to innocuous antigens. Disruption of the mucosal barrier and/or defects in gut immune regulatory networks may lead to chronic intestinal inflammation as seen in inflammatory bowel disease. The T-cell populations of the intestine play a critical role in controlling intestinal homeostasis, and their unique phenotypes and diversities reflect the sophisticated mechanisms that have evolved to maintain the delicate balance between immune activation and tolerance at mucosal sites. In this article, we will discuss the specialized properties of mucosal T cells in the context of immune homeostasis and inflammation.
Keywords: effector memory T cells, inflammatory bowel disease, intraepithelial lymphocytes, lamina propria, mucosal barrier, regulatory T cells
In the intestines, a single layer of epithelium separates the body from the outside world. This physical barrier also represents a dynamic interface between the host immune system and the luminal environment, which is loaded with a plethora of foreign antigens and (potentially harmful) microbes. The immune system of the gut has evolved to protect the mucosal barrier by maintaining a delicate balance between effective immunity against invading pathogens and immune regulation to avoid extensive tissue damage. The importance of the mucosal barrier integrity is exemplified in inflammatory bowel diseases (IBDs) where a compromised barrier function associates with excessive immune responses to commensals and leads to chronic intestinal inflammation.
The intestinal immune system is separate from, yet connected to, the peripheral immune system and is regionally specialized and compartmentalized, in large part by conditioning effects of the microenvironment. Adaptations of the intestinal immune system as a consequence of its intimate contact with the exterior environment are reflected in its complex organization and by the presence of unique lymphocyte populations. The lymphoid compartments or the gut-associated lymphoid tissues (GALT) closely resemble other secondary lymphoid tissues of the body and consist of mesenteric lymph nodes (mLNs), Peyer’s patches (PPs), the appendix and multiple permanent or transient smaller isolated lymphoid follicles distributed throughout the intestinal wall. A second level of organization is formed by isolated immune cells, including IgA-secreting plasma cells and T cells in the lamina propria (LP), and an almost exclusive population of antigen-experienced T cells scattered throughout the epithelium compartment. Together with the innate branch of the gut immune system, these effector lymphocytes form the first line of defense against invading pathogens and play a crucial role in maintaining barrier integrity.
T-cell subsets in the gut
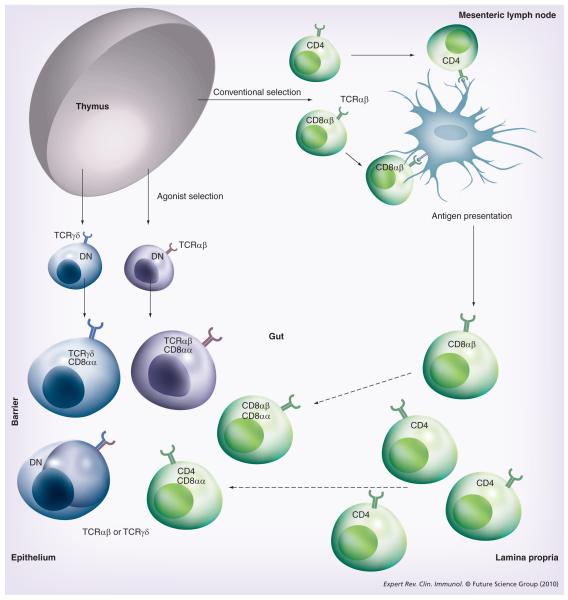
Although mucosal T cells are notably heterogeneous in phenotype and function, they have been classified into two major subsets based on T-cell receptor (TCR) and coreceptor expression [1]: the conventional or ‘type a’ mucosal T cells express TCRαβ together with CD4 or CD8αβ as TCR coreceptors, whereas non conventional or ‘type b’ mucosal T cells express either TCRαβ or TCRγδ and typically also CD8αα homodimers, and lack expression of the conventional TCR coreceptors CD4 and CD8αβ. The phenotypic differences between the two subsets (partially) overlap with their major functional differences and location. In general, the LP is largely populated by type a T cells, whereas type b T cells are far more prevalent in the mucosal epithelium (Figure 1).
Figure 1. Intestinal T-cell subsets.
The lamina propria and epithelium of the intestine harbor diverse populations of T cells. Conventional or ‘type a’ mucosal T cells that have matured in the thymus along the conventional selection pathway migrate, after antigen priming in the mesenteric lymph nodes, mainly to the lamina propria but also the epithelium. Upon entry into the epithelium, these cells often coexpress the CD8αα homodimer. Most intraepithelial lymphocytes (IELs), however, belong to two subsets of unconventional or ‘type b’ mucosal T-cell populations: the TCRγδ+ CD8αα+ IELs that are thymus derived and develop along the double-negative pathway and the TCRαβ+ CD8αα+ IELs that have matured and differentiated in the thymus along the agonist-selection pathway. Both subsets migrate as antigen-experienced directly to the intestine where the majority of cells upregulate CD8αα, while some remain double negative.
DN: Double negative; TCR: T-cell receptor.
Type a mucosal T cells
The presence of GALT allows for tissue-restricted priming to intestinal antigens and localization of the immune response. Dendritic cells (DCs) that populate the LP take up antigens from the gut lumen and migrate to the T-cell areas of PP and mLN where they prime conventional naive CD4 and CD8αβ T cells. During activation, the T cells acquire gut homing properties through the upregulation of several adhesion receptors including α4β7 and CCR9. The imprinting of T-cell trafficking is regulated by the local mucosal lymphoid environment [2] and mucosal DCs play a key role by releasing retinoic acid (a vitamin A metabolite), which induces α4β7 and CCR9 expression on activated T cells [3]. After priming, the antigen-experienced T cells migrate to the LP and epithelial compartments where they reside as long-lived effector memory T cells and accumulate over time (Figure 1). Although these type a mucosal memory T cells have much in common with other acquired memory T cells, they also have unique properties that are related to their functions and location. For instance, the LP lymphocytes (LPLs) and intraepithelial lymphocytes (IELs) display an oligoclonal repertoire, which indicates that re-encounter with specific antigens in the gut may lead to selective expansion of certain clones [4]. Furthermore, upon (repeated) assault by enteric pathogens, the effector memory CD8αβ LPLs and IELs are able to respond quickly to exert rapid and potent cytotoxicity against pathogen-infected or damaged target cells, thereby preventing systemic spread of the pathogen [5,6]. Although most CD8αβ mucosal memory T cells maintain an activated phenotype, they concurrently display reduced proliferation and inflammatory cytokine production [7]. These unique features might have evolved in order to support immunological protection without compromising organ integrity.
Type a mucosal CD4 T cells are mainly found in the LP where they provide ‘help’ to plasma cells and CD8αβ memory T cells. Interestingly, although all classical CD4 T helper (Th) subsets are present, the LP is under steady-state conditions enriched for both Foxp3-expressing regulatory T cells (Tregs) and proinflammatory, IL-17-producing Th17 cells. Thymus-derived natural Tregs as well as peripherally induced Tregs preferentially accumulate in the gut and are crucial for maintaining GI tract integrity by preventing excessive responses against the gut flora [8]. Th17 cells, on the other hand, play a critical role in host defense against a variety of fungus and bacterial infections [9,10]. The favorable induction of both Treg and Th17 cells in the gut mucosa is another paradigm illustrating the necessary equilibrium between effective immunity and preserving tissue integrity. How is this balance between pro- and anti-inflammatory responses regulated? TGF-β, abundantly present in the intestine, has been shown to promote Treg conversion [11]. However, in the presence of the proinflammatory cytokine IL-6, TGF-β induces Th17 differentiation [12], which underscores an important but nonexclusive role for TGF-β. We recently identified retinoic acid (RA) as a key regulator in controlling the TGF-β-driven immune balance [13]. RA secreted by mucosal DCs enhances Treg induction while inhibiting Th17 development [13,14], thereby strengthening the mutually exclusive development pathways of Treg versus Th17 (Figure 2). Although under steady-state conditions mLN and LP-derived DCs (especially the CD103+ subset) are highly effective in inducing antigen-specific Tregs [15], specific LP DC subsets have been described that promote Th17 differentiation [16,17]. In addition, commensal bacteria have been shown to be required for ‘spontaneous’ IL-17 production in the LP [18,19], and recently it was shown that a single commensal microbe, the segmented filamentous bacterium, appears to be sufficient for the induction of Th17 cells in the LP of the small intestine [20]. By contrast, the presence of commensal bacterial DNA was shown to limit Treg conversion [21].
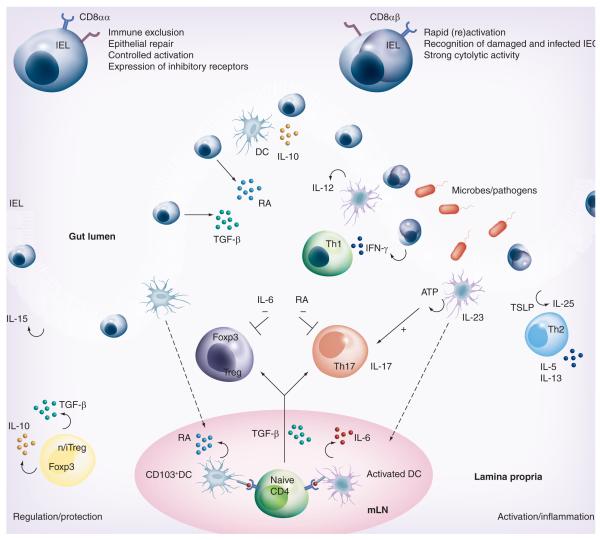
Figure 2. Mucosal T-cell regulation and activation.
The functionally diverse T-cell populations of the intestine that are shaped by the gut environment are important players in sustaining the delicate immune balance between activation and regulation. The CD8αα+ IELs of the intestine play a crucial role in protecting the mucosal barrier. They are involved in maintaining and restoring barrier homeostasis by stimulating IEC turnover. Upon pathogen entry, rapid activation and high cytolytic activity of the CD8αβ+ IELs contribute to the prevention of pathogen spreading by killing infected IECs. The activation of IELs is highly controlled through the expression of inhibitory receptors that may alter the threshold for activation. In some conditions, such as celiac disease, activation of CD8αβ+ cytotoxic T lymphocytes is associated with epithelial damage. In the lamina propria (LP), all classical CD4+ Th subtypes are present. Under the influence of IECs and IELs, LP DCs acquire the ability to produce RA, thereby inducing gut-homing receptors during CD4 T-cell priming in the mLNs. The LP of the gut is enriched with both Foxp3+ Tregs and Th17 cells. Gut-derived CD103+ DCs favor the conversion of Foxp3+ iTregs in a RA- and TGF-β-mediated fashion, whereas activated DCs promote the differentiation of IL-17-producing Th17 cells via a combination of IL-6 and TGF-β. This pro- and anti-inflammatory immune deviation of iTreg and Th17 is reciprocally controlled by RA and IL-6. Specific bacteria in the small intestine have been shown to be crucial for Th17 induction, possibly through the induction of ATP release by DC. Finally, the LP is also home to agonist-selected Foxp3-expressing nTregs that, like iTregs, can produce the suppressive cytokines IL-10 and TGF-β. T helper subsets can also contribute to immune pathogenesis and damage under inflammatory conditions. Th1 and Th17 cells are implicated in Crohn’s disease, Th1 cells in celiac disease and Th2 cells in ulcerative colitis. Under these proinflammatory conditions, cytokines secreted by IECs and DCs such as IL-12, IL-23, TSLP and IL-25 promote differentiation of inflammatory Th1, Th17 and Th2 subsets, respectively.
DC: Dendritic cell; IEC: Intestinal epithelial cell; IEL: Intraepithelial lymphocyte; iTreg: Induced regulatory T cell; mLN: Mesenteric lymph node; nTreg: Naturally occurring regulatory T cell; RA: Retinoic acid; TSLP: Thymic stromal lymphopoietin.
Type b mucosal T cells
Type b mucosal T cells are almost exclusively located in the epithelium where they represent the vast majority of IELs. IELs are in close contact with intestinal epithelial cells (IECs) – among others, through adhesion between αEβ7 expressed on IELs and E-cadherin on IECs – and it has been estimated that there are 10–20 IELs per 100 IECs in the human gut [22]. Type b IELs express either TCRαβ or TCRγδ, and unconventional characteristics that distinguish them from type a IELs include their enrichment for self-antigen specificity, expression of CD8αα homodimers in the absence of the TCR coreceptors CD4 or CD8αβ and lack of expression of some ‘typical’ T-cell markers such as CD2, CD28 and Thy-1. They are already present in the gut at birth and IL-15 produced by IECs is crucial for their maturation and homeostasis in the intestine [23,24]. TCRαβ CD8αα+ IELs are believed to be selected in the thymus in response to agonist self-antigens (Figure 1) and, as a consequence, preferentially respond to self-antigens in the periphery [25]. The repertoire of type b IELs is limited and although neither the identity nor the nature of the antigens are known, nonclassical MHC class I antigens have been strongly implicated in their activation [26].
One of the most prominent features of type b IELs is their ‘activated yet resting’ phenotype. Like type a IELs, they are highly cytolytic but, in the absence of strong stimulation, their presence often correlates with immune quiescence instead of productive immunity. Gene microarray data have confirmed this twofold identity, showing high levels of expression of both effector and regulatory molecules [27,28]. In addition, the characteristic CD8αα receptor, which recognizes the nonclassical MHC class I molecule thymic leukemia antigen (TL) expressed on epithelial cells of the small intestine in mice, has been shown to restrain proliferation and control reactivity of CD8αα IELs [29,30]. Although no homologue of TL has been identified in humans, the activation status and action of IELs in humans is also controlled by signals (including natural killer cell receptor–ligand interactions) provided by the epithelial cells [31]. Together, these observations indicate that type b IELs are well-prepared for an immediate first-line defense response but, at the same time, their activation is fine-tuned and highly regulated to avoid uncontrolled immune responses and tissue destruction (Figure 2).
Although the exact functions and behavior of type b IELs remain elusive, their primary role seems to be ensuring the integrity of the intestinal epithelium. In particular, TCRγδ IELs play a central role in regulating epithelial turnover and repair [32,33], thereby sustaining the barrier function of the epithelium (Figure 2). As discussed previously, type b IELs have minimal pathogen specificity and do not seem to provide sterilizing protective immunity against a variety of infections [34,35]. However, similar to their type a counterparts, they may allow for restriction of microbial invasion by killing infected or damaged IECs through TCR- or natural killer cell receptor-mediated recognition of stress-induced (self)-antigens and receptors [31,36]. Finally, several findings have demonstrated contributions of type b IELs toward maintaining local immune quiescence and controlling inflammatory responses (Figure 2) [34,37,38].
T cells & chronic intestinal inflammation
The differentiation, activation and function of intestinal T-cell subsets are regulated by their interaction with other cell types and soluble factors in the gut environment and are strongly influenced by microbial colonization of the gut. The dynamic interactions between the gut flora and the mucosal adaptive immune system normally initiate a stable equilibrium and sustained barrier function. A disrupted balance, caused for example by an infection, can induce excessive immune responses and tissue damage, which may fuel chronic intestinal inflammation and lead to inflammatory bowel disease (IBD) or even gastrointestinal cancer [39]. Ulcerative colitis (UC) and Crohn’s disease (CD) represent the two major forms of IBD and both are generally believed to be driven by aberrant T-cell responses to the intestinal flora. For extensive reviews on IBD pathogenesis and mechanisms see Abraham and Cho [40] and Xavier and Podolsky [41].
Mucosal T cells as aggressors
Whereas disruptions in the protective barrier and aberrant innate immune responses may initiate IBD, effector T cells of the adaptive immune system play an essential role in disease progression and persistence. However, although both CD and UC share some important end-stage pathways of tissue damage, they represent immunologically different diseases with a specific spectrum of effector T cells involved. There are at present three established subsets of effector CD4 T cells – Th1, Th2 and Th17 – each of which are suggested to play a role in one or another variant of IBD (Figure 2). CD has always been considered a classical Th1-mediated inflammatory disorder with elevated levels of IFN-γ and IL-12, but the identification of proinflammatory Th17 cells and the finding that inflamed colons in both mice and men show considerable infiltrates of Th17 cells has challenged this belief. In addition, experimental data in mouse models identified the Th17-polarizing cytokine IL-23 as a major player in IBD pathogenesis [42,43] and concomitant genome-wide association studies in humans defined IL-23R as one of the major IBD susceptibility genes [44]. Whether CD is the result of dysregulated Th1 cells, Th17 cells or both remains a subject of debate and continuous research. In addition to CD4 T cells, auto-aggressive CD8 T cells in the gut have also been implicated in the progression of IBD [45]. Naive CD8 T cells have also been shown to be able to induce colitis in an IL-17-dependent fashion [46].
In contrast to CD, UC has been associated with Th2 responses and elevated levels of IL-5 and IL-13. The underlying mechanisms remain elusive, however, partly due to the lack of a suitable animal model for UC. Recently, studies have pointed to a possible role of thymic stromal lymphopoietin and the Th17-family cytokine IL-25 in the induction of Th2-type intestinal inflammation and are subject to further exploration in the context of UC.
Another common inflammatory condition of the small intestine is celiac disease, which is triggered and maintained in genetically predisposed individuals by gluten proteins [47]. Although both adaptive and innate immune responses are thought to initiate and propagate the inflammation, CD4 T cells have been shown to play a central role in celiac disease pathogenesis. Virtually all patients with celiac disease share the MHC class II molecules HLA-DQ2 and HLA-DQ8, which bind gluten peptides with high affinity and stimulate gluten-specific CD4 effector T cells in the gut mucosa. Both IFN-γ/IL-21-producing Th1 [48] and, recently, Th17 cells [49] have been implicated in promoting mucosal inflammation in celiac disease. The favored differentiation of pro inflammatory CD4 T cells in celiac disease is thought to be supported by high levels of proinflammatory cytokines including IL-15 and IFN-α that are present in the intestinal mucosa from celiac disease patients and induce DC activation [47]. IL-15 produced by IECs also activates intraepithelial CD8αβ cytotoxic T lymphocytes [50], which leads to IEC destruction and villus atrophy.
Protective role for mucosal T cells
During homeostasis, T-cell-mediated immune suppression appears to be the default pathway in the intestine. The major regulatory populations in the intestine are the Foxp3+ Tregs and IL-10- producing CD4 T cells [51]. The enriched Foxp3+ Treg population in the LP of the intestine contributes to tolerance against the gut flora and dietary antigens. These cells have also been shown to be able to prevent and even cure colitis in mouse models [52,53], among others, by secretion of the anti-inflammatory cytokine IL-10 [54]. Other intestinal CD4+ Foxp3− IL-10-producing T cells in the intestine are thought to serve a similar function. These so-called Tr1 cells are present in the intestine under homeostatic conditions but are also induced in large numbers during chronic immune activation in an antigen-specific manner. Other T-cell subsets that have been implicated in restraining IBD inflammation include CD8αα IELs [38] and TCRγδ T cells [55].
An emerging concept is that virtually all T-cell subtypes can display direct and/or indirect regulatory properties depending on the conditions. For example, IL-17-deficient T cells have been shown to induce accelerated disease in a transfer model of colitis [56] and both inflammatory and anti-inflammatory properties have also been attributed to IL-22, another Th17-related cytokine [57]. In SIV/HIV infection, a critical preferential loss of Th17 memory cells is observed [58] and this has been associated with a loss of mucosal barrier integrity, bacterial translocation and infection [59]. There is also evidence that most CD4 Th subsets (including Th1 and Th17) can produce IL-10 under certain conditions, especially during chronic stimulation [57,60], which can present these cells with regulatory properties (Figure 2). Finally, recent evidence has raised the intriguing phenomenon of lineage plasticity of CD4+ effector and Tregs in the intestine [61,62]. However, a possible role for these lineage conversions in the control of or contribution to intestinal inflammation remains to be investigated.
These findings stress the complex roles of T cells and their products in local immune responses and represent an important issue for novel treatment strategies targeting inflammatory cytokines.
Expert commentary
In the past decade, knowledge on mucosal immunology has expanded rapidly and new concepts have emerged that address the complex interactions in this specialized and compartmentalized branch of the immune system. At the intestinal host–microbial interface, the innate and adaptive immune system have been shown to collaborate to detect and regulate microbial populations, while at the same time dietary factors and the composition of the gut flora influence the characteristics and function of the different arms of the immune system. A good example of the latter is the recent identification of one individual bacteria species, the segmented filamentous bacteria, which is responsible for the accumulation of Th17 cells in the LP of the intestine [20]. DCs that shuttle between the intestinal surface and the draining mLNs have also been shown to be ‘educated’ by the gut environment, providing them with unique T-cell-inducing capabilities related to homing and regulation (Figure 2). Interestingly, the latest insights also suggest that the differentiated T-cell subsets that are generated may even change fate and function depending on the immune environment of the effector tissues. This extreme display of T-cell plasticity seems to occur particularly in the gut where T cells adapt to the antigen-rich environment and play a crucial role in the balance between antimicrobial defense and avoidance of immune-mediated tissue damage (Figure 2). Together, these findings have advanced our understanding on how homeostatic conditions in the intestines are maintained and regulated and how disruption of this homeostasis may lead to chronic immune inflammation. They also provide new challenges and opportunities for therapeutic strategies.
Five-year view
The coming years will shed more light on the intense communication between the environment and the heterogeneous populations of frontline T cells that protect the interface between the body and the outside world. One of the most important challenges for the future is the translation of our basic knowledge on mucosal T-cell behavior and regulation into novel therapeutic applications, including the development of mucosal vaccines and a more sophisticated control of inflammatory diseases.
The presence of large numbers of highly effective and long-lived effector memory T cells in the gut may create opportunities for mucosal vaccine strategies. Although the GI tract is one of the major entry points for human pathogens and it is believed that the route of vaccination is important in influencing immune responses at the initial site of pathogen invasion (where protection is most effective), only a few mucosal vaccines have been approved for human application [63]. Identifying the mechanisms that underlie the induction of effector memory cytotoxic T lymphocytes but also the activation, differentiation and plasticity of Th subsets at mucosal sites will contribute to the development of tailored mucosal vaccines.
For the treatment of IBD, research should focus on combination therapies that target local and specific components of the mucosal immune system, such as T cells or their products, rather than general immune suppression.
To really speed up the progress of translational medicine, improvement of appropriate animal models that can be used for human preclinical immune research is crucial. One big step has been the development of humanized mouse models and ongoing efforts may achieve a fully functional human immune system in mice [64]. This powerful new tool will provide tremendous opportunities to further illuminate the features and potentials of the complex, elusive and yet critical mucosal immune system.
Key issues.
The intestinal immune system is regionally specialized and compartmentalized.
Maintenance of the protective barrier by avoiding collateral damage is a key issue in mucosal immunity.
Intestinal T cells are notably heterogeneous in phenotype and function and encompass both conventional (type a) and unconventional (type b) subpopulations.
Intraepithelial lymphocytes play an important role in maintaining barrier function.
Effector memory T cells residing in the gut mucosa are able to mount rapid and highly efficient immune responses to pathogenic insults but are concurrently tightly regulated.
Aberrant effector T-cell responses to commensal antigens are relatively rare but can lead to chronic organ-specific inflammation.
Virtually all T-cell subtypes may display direct and/or indirect regulatory properties, depending on the conditions.
Although classically viewed as different lineages, CD4 Th cell subsets are flexible and can change function under the influence of the immune environment.
Acknowledgments
Financial & competing interests disclosure This work was supported by a Ter Meulen Fonds fellowship from the Royal Netherlands Academy of Arts and Sciences (Femke van Wijk) and by Grant RO1AI050265 from the National Institute of Allergy and Infectious Diseases (Hilde Cheroutre and Femke van Wijk).
No writing assistance was utilized in the production of this manuscript.
Footnotes
Disclosure The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat. Immunol. 2001;2(11):997–1003. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- 2.Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4+ T cells activated in cutaneous or mucosal lymphoid tissues. J. Exp. Med. 2002;195(1):135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwata M, Hirakiyama A, Eshima Y, et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21(4):527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Probert CS, Saubermann LJ, Balk S, Blumberg RS. Repertoire of the αβ T-cell receptor in the intestine. Immunol. Rev. 2007;215:215–225. doi: 10.1111/j.1600-065X.2006.00480.x. [DOI] [PubMed] [Google Scholar]
- 5.Cheroutre H, Madakamutil L. Mucosal effector memory T cells: the other side of the coin. Cell. Mol. Life Sci. 2005;62(23):2853–2866. doi: 10.1007/s00018-005-5232-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen SG, Vieville C, Whizin N, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med. 2009;15(3):293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J. Immunol. 2006;176(4):2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- 8.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol. Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 9.Ishigame H, Kakuta S, Nagai T, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30(1):108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Conti HR, Shen F, Nayyar N, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 2009;206(2):299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 2003;198(12):1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. • First paper showing a crucial role for TGF-β in the induction of Th17 cells. Along with [13–17], shows a reciprocal regulation of regulatory T cells (Tregs) and Th17 cells by the vitamin A metabolite retinoic acid (RA) and the role of mucosal dendritic cells (DCs) in regulating this balance.
- 13.Mucida D, Park Y, Kim G, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317(5835):256–260. doi: 10.1126/science.1145697. • Along with [12,14–17], shows a reciprocal regulation of Treg and Th17 cells by the vitamin A metabolite RA and the role of mucosal DCs in regulating this balance.
- 14.Sun CM, Hall JA, Blank RB, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 Treg cells via retinoic acid. J. Exp. Med. 2007;204(8):1775–1785. doi: 10.1084/jem.20070602. • Along with [12,13,15–17], shows a reciprocal regulation of Treg and Th17 cells by the vitamin A metabolite RA and the role of mucosal DCs in regulating this balance.
- 15.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204(8):1757–1764. doi: 10.1084/jem.20070590. • Along with [12–14,16,17] shows a reciprocal regulation of Treg and Th17 cells by the vitamin A metabolite RA and the role of mucosal DCs in regulating this balance.
- 16.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat. Immunol. 2007;8(10):1086–1094. doi: 10.1038/ni1511. • Along with [12–15,17], shows a reciprocal regulation of Treg and Th17 cells by the vitamin A metabolite RA and the role of mucosal DCs in regulating this balance.
- 17.Uematsu S, Fujimoto K, Jang MH, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat. Immunol. 2008;9(7):769–776. doi: 10.1038/ni.1622. • Along with [12–16], shows a reciprocal regulation of Treg and Th17 cells by the vitamin A metabolite RA and the role of mucosal DCs in regulating this balance.
- 18.Ivanov II, Frutos Rde L, Manel N, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4(4):337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atarashi K, Nishimura J, Shima T, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455(7214):808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 20.Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498. doi: 10.1016/j.cell.2009.09.033. • One individual commensal microbe, the segmented filamentous bacterium, is shown to promote the generation of Th17 cells in the gut.
- 21.Hall JA, Bouladoux N, Sun CM, et al. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29(4):637–649. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crowe PT, Marsh MN. Morphometric analysis of intestinal mucosa. VI –Principles in enumerating intra-epithelial lymphocytes. Virchows Arch. 1994;424(3):301–306. doi: 10.1007/BF00194615. [DOI] [PubMed] [Google Scholar]
- 23.Ma LJ, Acero LF, Zal T, Schluns KS. Trans-presentation of IL-15 by intestinal epithelial cells drives development of CD8αα IELs. J. Immunol. 2009;183(2):1044–1054. doi: 10.4049/jimmunol.0900420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu Q, Tang C, Xun S, et al. MyD88-dependent signaling for IL-15 production plays an important role in maintenance of CD8 αα TCR αβ and TCR γδ intestinal intraepithelial lymphocytes. J. Immunol. 2006;176(10):6180–6185. doi: 10.4049/jimmunol.176.10.6180. [DOI] [PubMed] [Google Scholar]
- 25.Gangadharan D, Lambolez F, Attinger A, et al. Identification of pre- and postselection TCRαβ+ intraepithelial lymphocyte precursors in the thymus. Immunity. 2006;25(4):631–641. doi: 10.1016/j.immuni.2006.08.018. • Describes the unique thymic selection pathway of unconventional CD8αα intraepithelial lymphocytes.
- 26.Cheroutre H. IELs: enforcing law and order in the court of the intestinal epithelium. Immunol. Rev. 2005;206:114–131. doi: 10.1111/j.0105-2896.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- 27.Denning TL, Granger SW, Mucida D, et al. Mouse TCRαβ+CD8αα intraepithelial lymphocytes express genes that down-regulate their antigen reactivity and suppress immune responses. J. Immunol. 2007;178(7):4230–4239. doi: 10.4049/jimmunol.178.7.4230. [DOI] [PubMed] [Google Scholar]
- 28.Shires J, Theodoridis E, Hayday AC. Biological insights into TCRγδ+ and TCRαβ+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE) Immunity. 2001;15(3):419–434. doi: 10.1016/s1074-7613(01)00192-3. [DOI] [PubMed] [Google Scholar]
- 29.Leishman AJ, Naidenko OV, Attinger A, et al. T cell responses modulated through interaction between CD8αα and the nonclassical MHC class I molecule, TL. Science. 2001;294(5548):1936–1939. doi: 10.1126/science.1063564. [DOI] [PubMed] [Google Scholar]
- 30.Cheroutre H, Lambolez F. Doubting the TCR coreceptor function of CD8αα. Immunity. 2008;28(2):149–159. doi: 10.1016/j.immuni.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Jabri B, Ebert E. Human CD8+ intraepithelial lymphocytes: a unique model to study the regulation of effector cytotoxic T lymphocytes in tissue. Immunol. Rev. 2007;215:202–214. doi: 10.1111/j.1600-065X.2006.00481.x. [DOI] [PubMed] [Google Scholar]
- 32.Komano H, Fujiura Y, Kawaguchi M, et al. Homeostatic regulation of intestinal epithelia by intraepithelial γ δ T cells. Proc. Natl Acad. Sci. USA. 1995;92(13):6147–6151. doi: 10.1073/pnas.92.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boismenu R, Havran WL. Modulation of epithelial cell growth by intraepithelial γ δ T cells. Science. 1994;266(5188):1253–1255. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- 34.Roberts SJ, Smith AL, West AB, et al. T-cell αβ+ and γδ+ deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc. Natl Acad. Sci. USA. 1996;93(21):11774–11779. doi: 10.1073/pnas.93.21.11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayday AC. γ γ cells: a right time and a right place for a conserved third way of protection. Annu. Rev. Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 36.Inagaki-Ohara K, Dewi FN, Hisaeda H, et al. Intestinal intraepithelial lymphocytes sustain the epithelial barrier function against Eimeria vermiformis infection. Infect. Immun. 2006;74(9):5292–5301. doi: 10.1128/IAI.02024-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saurer L, Seibold I, Rihs S, et al. Virus-induced activation of self-specific TCR α β CD8αα intraepithelial lymphocytes does not abolish their self-tolerance in the intestine. J. Immunol. 2004;172(7):4176–4183. doi: 10.4049/jimmunol.172.7.4176. [DOI] [PubMed] [Google Scholar]
- 38.Poussier P, Ning T, Banerjee D, Julius M. A unique subset of self-specific intraintestinal T cells maintains gut integrity. J. Exp. Med. 2002;195(11):1491–1497. doi: 10.1084/jem.20011793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim BG, Li C, Qiao W, et al. Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature. 2006;441(7096):1015–1019. doi: 10.1038/nature04846. [DOI] [PubMed] [Google Scholar]
- 40.Abraham C, Cho JH. Inflammatory bowel disease. N. Engl. J. Med. 2009;361(21):2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 42.Uhlig HH, McKenzie BS, Hue S, et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25(2):309–318. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 43.Hue S, Ahern P, Buonocore S, et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J. Exp. Med. 2006;203(11):2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duerr RH, Taylor KD, Brant SR, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314(5804):1461–1463. doi: 10.1126/science.1135245. • Identification of the IL23R gene as one of the major susceptibility genes for inflammatory bowel disease.
- 45.Cheroutre H. In IBD eight can come before four. Gastroenterology. 2006;131(2):667–670. doi: 10.1053/j.gastro.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 46.Tajima M, Wakita D, Noguchi D, et al. IL-6-dependent spontaneous proliferation is required for the induction of colitogenic IL-17-producing CD8+ T cells. J. Exp. Med. 2008;205(5):1019–1027. doi: 10.1084/jem.20071133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jabri B, Sollid LM. Tissue-mediated control of immunopathology in coeliac disease. Nat. Rev. Immunol. 2009;9(12):858–870. doi: 10.1038/nri2670. [DOI] [PubMed] [Google Scholar]
- 48.Fina D, Sarra M, Caruso R, et al. Interleukin 21 contributes to the mucosal T helper cell type 1 response in coeliac disease. Gut. 2008;57(7):887–892. doi: 10.1136/gut.2007.129882. [DOI] [PubMed] [Google Scholar]
- 49.Monteleone I, Sarra M, Del Vecchio Blanco G, et al. Characterization of IL-17A-producing cells in celiac disease mucosa. J. Immunol. 2010;184(4):2211–2218. doi: 10.4049/jimmunol.0901919. [DOI] [PubMed] [Google Scholar]
- 50.Yokoyama S, Watanabe N, Sato N, et al. Antibody-mediated blockade of IL-15 reverses the autoimmune intestinal damage in transgenic mice that overexpress IL-15 in enterocytes. Proc. Natl Acad. Sci. USA. 2009;106(37):15849–15854. doi: 10.1073/pnas.0908834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamanaka M, Kim ST, Wan YY, et al. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25(6):941–952. doi: 10.1016/j.immuni.2006.09.013. • Demonstrates a crucial role for Treg- and non-Treg-derived IL-10 in immune regulation in the gut.
- 52.Uhlig HH, Coombes J, Mottet C, et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J. Immunol. 2006;177(9):5852–5860. doi: 10.4049/jimmunol.177.9.5852. • Demonstrates a crucial role for Treg- and non-Treg-derived IL-10 in immune regulation in the gut.
- 53.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J. Immunol. 2003;170(8):3939–3943. doi: 10.4049/jimmunol.170.8.3939. • Demonstrates a crucial role for Treg- and non-Treg-derived IL-10 in immune regulation in the gut.
- 54.Rubtsov YP, Rasmussen JP, Chi EY, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28(4):546–558. doi: 10.1016/j.immuni.2008.02.017. • Demonstrates a crucial role for Treg- and non-Treg-derived IL-10 in immune regulation in the gut.
- 55.Kuhl AA, Pawlowski NN, Grollich K, et al. Aggravation of intestinal inflammation by depletion/deficiency of γδ T cells in different types of IBD animal models. J. Leukoc. Biol. 2007;81(1):168–175. doi: 10.1189/jlb.1105696. [DOI] [PubMed] [Google Scholar]
- 56.O’Connor W, Jr, Kamanaka M, Booth CJ, et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat. Immunol. 2009;10(6):603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sugimoto K, Ogawa A, Mizoguchi E, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Invest. 2008;118(2):534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prendergast A, Prado JG, Kang YH, et al. HIV-1 infection is characterized by profound depletion of CD161+ Th17 cells and gradual decline in regulatory T cells. AIDS. 2010;24(4):491–502. doi: 10.1097/QAD.0b013e3283344895. [DOI] [PubMed] [Google Scholar]
- 59.Raffatellu M, Santos RL, Verhoeven DE, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat. Med. 2008;14(4):421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGeachy MJ, Bak-Jensen KS, Chen Y, et al. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 2007;8(12):1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 61.Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Curr. Opin. Immunol. 2009;21(3):274–280. doi: 10.1016/j.coi.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 62.O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327(5969):1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Belyakov IM, Ahlers JD. What role does the route of immunization play in the generation of protective immunity against mucosal pathogens? J Immunol. 2009;183(11):6883–6892. doi: 10.4049/jimmunol.0901466. [DOI] [PubMed] [Google Scholar]
- 64.Manz MG. Human-hemato-lymphoid-system mice: opportunities and challenges. Immunity. 2007;26(5):537–541. doi: 10.1016/j.immuni.2007.05.001. [DOI] [PubMed] [Google Scholar]