Abstract
Evidence could not be found of immune modulation of liver regeneration. The powerful immunosuppressive drug FK 506, which augments the response after partial hepatectomy in normal rats, had the same effect in T cell–deficient nude rats. The cytotoxicity of natural killer cells in treated nude rats was not significantly changed by FK 506 therapy. However, the serum of FK 506-treated nude rats increased hepatocyte proliferation when added to third-party hepatocyte cultures, suggesting that FK 506 had induced a serum growth factor in the nude rats or had suppressed an inhibitory factor. A hypothesis was advanced that FK 506 (and cyclosporine) affects hepatic growth by nonimmunological pathways.
An unusual quality of two powerful immunosuppressive agents, FK 506 and cyclosporine, is augmentation of the regeneration response after partial hepatic resection (1–4). Two alternate explanations have been advanced for this phenomenon, one of which is the altered immune modulation of the regeneration response by these immunosuppressive agents that inhibit T-lymphocyte activation (4). The alternate hypothesis is that these drugs influence growth control responses by nonimmunological pathways, involving as yet poorly understood second messenger processes, signal transduction processes or both (4–6).
In this study regeneration was studied in nude rats first, to see whether these T-lymphocyte–deficient animals had normal regeneration, and second, to determine whether FK 506, which is a specific inhibitor of T-helper–lymphoeyte activation, augmented the regeneration response. Failure to do so would support the immune modulation hypothesis. In addition, natural killer (NK) lymphocytes recently have been shown to have increased cytotoxicity against regenerating hepatocytes (7). Consequently, the effect of FK 506 on NK lymphocytes was determined in unoperated nude rats. The results of both kinds of experiments provided no evidence that either lymphocyte subpopulation plays a role in the control of regeneration.
METHODS
In Vivo Experiments
Male nude rats (NIH-RNU, National Cancer Institute, Frederick, MD) were kept in pathogen-free temperature-controlled and light-controlled rooms and provided with food and water ad libitum. Before purchase, these rats were tested by the National Cancer Institute and found to have absent T-lymphocytes and T-lymphocyte–mediated function. The nude rats were assigned to the four groups as shown in Table 1.
Table 1.
Experimental groups
| Group | No. rats | Route | FK | Vehicle | Liver resection |
|---|---|---|---|---|---|
| 1 | 10 | IM | 0 | Saline | 70% |
| 2 | 10 | IM | 1 mg/kg | Saline | 70% |
| 3 | 4a | IM | 0 | Saline | 0 (no operation) |
| 4 | 5a | IM | 1 mg/kg | Saline | 0 (no operation) |
Serum and spleen were obtained at killing for in vitro experiments.
IM = intramuscular.
The 70% hepatectomies in groups 1 and 2 were performed with the rats under methopfane anesthesia between 7:30 and 9:00 am by the method of Higgins and Anderson (8). Intra-muscular drugs or saline vehicle was given daily during the three days before the surgery and just after completion of the operation. A further dose was given 24 hr after operation, 2 hr before killing. At the same time as the final dose, 50 mCi [3H — ] thymidine/200 gm body wt was administered intraperitoneally. Liver DNA synthesis was judged by the incorporation of [3H — ] thymidine and by the percent of labeled hepatocyte nuclei as previously described (4). The mortality rate was zero in treated and untreated groups.
In Vitro Experiments
NK Cell Activity
In nude rats of groups 3 and 4 that had not been subjected to operation, the spleens were removed under sterile conditions at the time of death and NK cell preparations were made (9). Single cell suspensions prepared in RPMI1640 in 5% FCS by this technique have viability exceeding 97% by trypan blue exclusion. These cells were tested for their ability to lyse the NK-sensitive Moloney virus–induced mouse T-cell lymphoma YAC-1. The target tumor cell lines were grown in RPMI 1640 medium with 5% FCS and penicillin/streptomycin and subcultured two to three times per week until used.
The cytotoxicity of the NK cells was measured with a standard [51Cr]-release assay after labeling the target cells with 100 mCi of Na2 51Cr04/2 × 106 cells. NK cells were added to triplicate well round-bottom microplates (Costar, Cambridge, MA) that contained constant numbers of target cells to give an effector/target cell ratio of 1:100 or 1:200. After incubation at 37° C for 4 hr, the activity of the supernatant was determined in a gamma counter. Spontaneous isotope release (control) was obtained from wells receiving target cells and medium only, and total isotope release was obtained from wells receiving 1% Triton X-100 (Sigma Chemical Co., St. Louis, MO). The percent of cytotoxicity was calculated by: Percent cytotoxicity = 100 × [(Experimental release − Spontaneous release)/(Total release − Spontaneous release)].
Serum Stimulatory Activity
Sera at death were obtained from the FK 506–treated and untreated nonoperated nude rats in groups 3 and 4 and assayed for their hepatocyte stimulatory qualities. Target hepatocytes were isolated from 7-wk-old Fischer (F344) rats that weighed 180 to 200 gm. A modification of the two-step perfusion technique of Seglen (10) was used as described by Jirtle et al. (11). Viability was determined by trypan blue exclusion, and hepatocytes counted by hemocytometer were plated at a cell density of 1.5 × 105/well using 1.5 ml MEM + 5% FCS and 10 −7 molar insulin at 37° C in a 5% CO2 atmosphere. Hepatocyte DNA synthesis was determined with [3H — ] thymidine incorporation (4, 5). Serum or cellular proteins were determined by the method of Lowry et al. (12).
Statistical Analysis
Data were presented as mean ± S.E.M. Unpaired Student’s t test was used for statistical analysis of the data.
RESULTS
In Vivo Experiments
The nude rats not treated with FK 506 had a 16-fold increase in DNA synthesis and a commensurate increase of autographically labeled hepatocyte nuclei (Fig. 1). The regeneration response by both parameters was significantly greater when the animals received the 5-day course of preoperative treatment with FK 506 (Fig. 1).
Fig. 1.
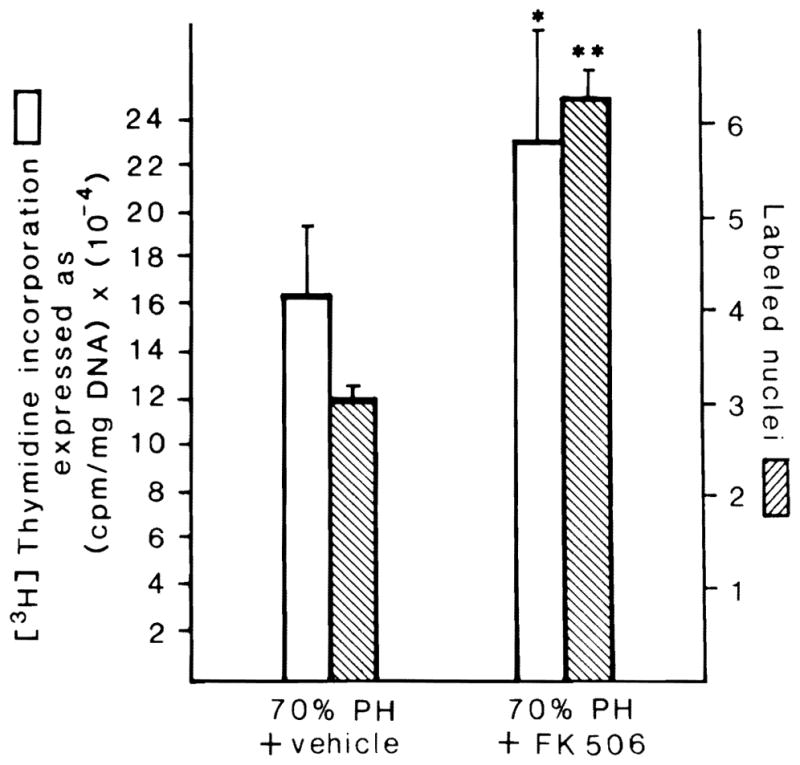
[3H] thymidine incorporation and percentage of labeled hepatocyte nuclei 24 hr after 70% hepatectomy in nude (NIH-ENU) rats treated (right) or not treated (left) with FK 506. Mean ± S.E.M. (p < 0.05* or <0.01**). Ten animals in each group.
In Vitro Experiments
NK Cell Activity
The splenic NK cell cytolytic activity was vigorous when the cells were added at an effective target ratio of 100:1 or 200:1. This cytolytic activity was neither increased nor decreased by a 5-day course of FK 506 (Fig. 2), findings similar to those reported by Markus et al. (13) in normal Fischer rats.
Fig. 2.
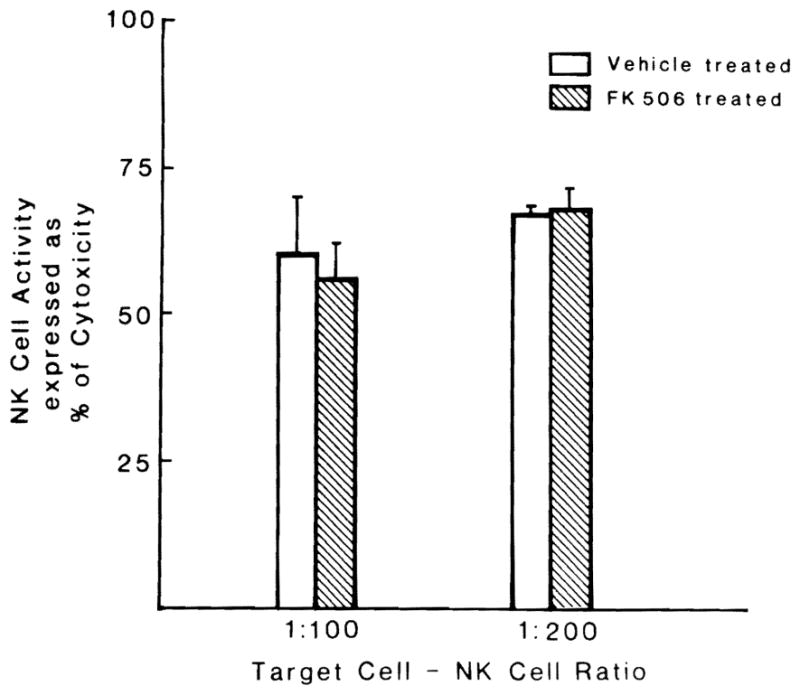
Lytic activity of NK cells isolated from nude rats treated and untreated with FK 506. NK cells were added at a ratio of either 1:100 or 1:200 to target lymphoma cells. Each bar = four experiments. Mean ± S.E.M.
Hepatocyte Stimulatory Factors in Serum
The serum of vehicle-treated unoperated nude rats (group 3) caused slight stimulation of F344 hepatocytes in culture (Fig. 3), an effect of normal rat serum that is well known (5, 14).
Fig. 3.
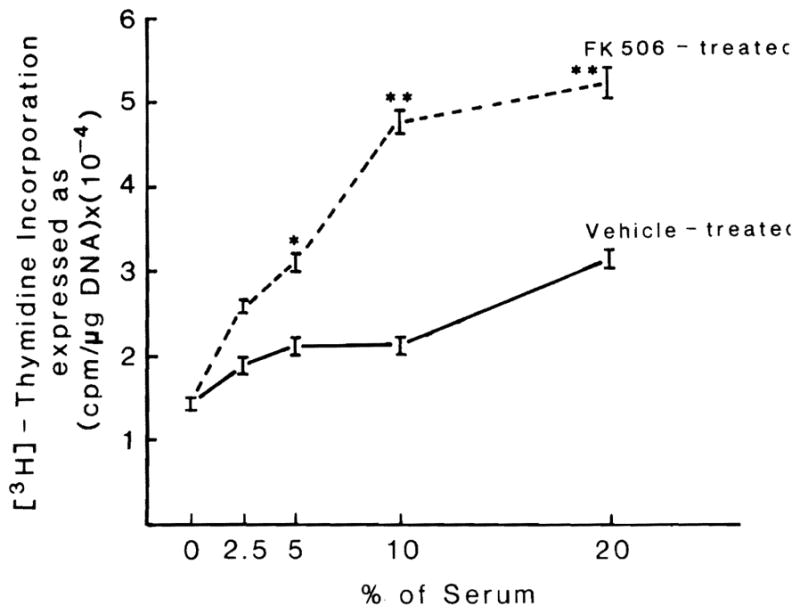
Effect on DNA synthesis of cultured hepatocytes of different concentrations of rat serum obtained from nude rats treated or not treated with FK 506. n = 5 in both experiments, (p < 0.05* or <0.01**).
In contrast, the serum of unoperated nude rats submitted to a 5-day course of FK 506 (group 14) significantly increased the rate of replication when added to the hepatocyte culture medium. This was significant at all of the higher volume serum enrichments (Fig. 3). This stimulatory effect of the rat sera from FK 506–treated nude rats was similar to that previously seen in operated immunologically competent Fischer rats treated with FK 506 (5).
DISCUSSION
These experiments suggest but do not prove that T helper or NK lymphocytes are unimportant in controlling liver regeneration, contrary to the speculation in the literature summarized elsewhere (4, 5, 7). In the nude rats in which the T cells were not present, the regeneration response was normal. Nevertheless, the FK 506, which targets T cells for its immunological action (15), augmented regeneration in the same way as in immunologically intact animals. The additional possibility (7) that NK cells were defunctionalized by the immunosuppressive therapy was essentially eliminated by our in vitro experiments and by in vivo experiments reported elsewhere (13).
Instead, the evidence, although circumstantial, is congruent with our previously enunciated hepatotrophic hypothesis that FK 506 and cyclosporine (16) affect basic growth control mechanisms that are independent of immunological pathways. Both FK 506 and cyclosporine have cytosolic binding sites that, although distinct, contain peptidyl-prolyl isomerase (17, 18), an enzyme that facilitates the breakdown of oligopeptide bonds and facilitates protein folding (19). Cellular growth regulation as epitomized by its hepatotrophic qualities (6,16; Francavilla A, Starzl TE, Porter K, et al. Manuscript submitted for publication, 1991), including regeneration (4, 5), is only one of the nonimmunological pleiotropic effects (20) of this ubiquitous immunophilin network after it binds FK 506 or cyclosporine (21).
The intriguing observation in our current experiments was the apparent development of hepatocyte stimulatory activity in the serum of FK 506–treated nude rats. The finding was similar to that recently reported in immunologically competent Fischer rats treated with this drug (5). At the moment, no clue exists as to the explanation for this induced stimulatory activity. The possibility has not been ruled out that what actually occurred instead of stimulation was removal or suppression of an inhibitory factor.
Acknowledgments
Aided by research grants from the Veterans Administration and Project Grant No. DK 29961 from the National Institutes of Health, Bethesda, Maryland.
References
- 1.Makowka L, Svanas G, Esquivel C, Venkataramanan R, Todo S, Iwatsuki S, Van Thiel DH, et al. The effect of cyclosporine on hepatic regeneration. Surg Forum. 1986;37:352–354. [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn D, Lai HS, Romovacek H, Makowka L, Van Thiel DH, Starzl TE. Cyclosporin A augments the regeneration response after partial hepatectomy in the rat. Transplant Proc. 1988;20(suppl 3):850–852. [PMC free article] [PubMed] [Google Scholar]
- 3.Kim YI, Calne RY, Nagasue N. Cyclosporin A stimulates proliferation of the liver cells after partial hepatectomy in rats. Surg Gynecol Obstet. 1988;166:317–311. [PubMed] [Google Scholar]
- 4.Francavilla A, Barone M, Todo S, Zeng Q, Porter KA, Starzl TE. Augmentation of rat liver regeneration by FK 506 compared with cyclosporine. Lancet (8674) 1989;2:1248–1249. doi: 10.1016/s0140-6736(89)91853-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francavilla A, Barone M, Starzl TE, Zeevi A, Scotti C, Carrieri G, Mazzaferro V, et al. FK 506 as a growth control factor. Transplant Proc. 1990;23:90–92. [PMC free article] [PubMed] [Google Scholar]
- 6.Starzl TE, Porter KA, Mazzaferro V, Todo S, Fung J, Francavilla A. Hepatotrophic effects of FK 506 in dogs. Transplantation. 1991;51:67–70. doi: 10.1097/00007890-199101000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Itoh H, Abo T, Sugawara S, Kanno A, Kumagai K. Age-related variation in the proportion and activity of murine liver natural killer cells and their cytotoxicity against regenerating hepatocytes. J Immunology. 1988;141:315–323. [PubMed] [Google Scholar]
- 8.Higgins GM, Anderson RM. Experimental pathology of liver. I. Restoration of liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 9.Van der Brink MRM, Hunt LE, Hiserodt JC. In vitro treatment with monoclonal antibody 323 selectively reliminates natural killer cells in rats. J Exp Med. 1990;171:197–210. doi: 10.1084/jem.171.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seglen PO. Preparation of isolated rat liver cell. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 11.Jirtle RL, Michalopoulos G, McLaine JR, Crowley J. Transplantation system for determining the clongenic survival of parenchymal hepatocytes exposed to ionizing radiation. Cancer Res. 1981;41:3512–3518. [PubMed] [Google Scholar]
- 12.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 13.Markus PM, Van der Brink MRM, Luchs BA, Fung JJ, Starzl TE, Hiserodt JC. Effects of in vivo treatment with FK 506 on NK and T-cell function in rats. Transplantation. doi: 10.1097/00007890-199104000-00037. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michalopoulos G, Cianciulli HD, Novotny R, Kligerman AD, Strom SC, Jirtle RL. Liver regeneration studies with rat hepatocytes in primary culture. Cancer Res. 1982;42:4673–4680. [PubMed] [Google Scholar]
- 15.Kino T, Hatanaka H, Miyata S, Inamura N, Nishiyama M, Yajima T, Goto T, et al. FK-506: a novel immunosuppressant isolated from a streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J Antibiotics. 1987;40:1256–1265. doi: 10.7164/antibiotics.40.1256. [DOI] [PubMed] [Google Scholar]
- 16.Mazzaferro V, Porter KA, Scotti-Foglieni CL, Venkataramanan R, Makowka L, Rossaro L, Francavilla A, et al. The hepatotrophic influence of cyclosporine. Surgery. 1990;107:533–539. [PMC free article] [PubMed] [Google Scholar]
- 17.Siekierka JJ, Hung SHY, Poe M, Lin CS, Sigal NH. A cytosolic binding protein for the immunosuppressant FK 506 has peptidylprolyl isomerase activity but is distinct from cyclophilin. Nature. 1989;341:755–757. doi: 10.1038/341755a0. [DOI] [PubMed] [Google Scholar]
- 18.Harding MW, Galat A, Uehling DE, Schreiber SL. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-proly isomerase. Nature. 1989;341:758–760. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]
- 19.Fischer G, Bang J. The refolding of urea-denatured ribonuclease A is catalyzed by peptidyl-prolyl cis-trans isomerase. Biocheir Biophys Acta. 1984;828:39–42. doi: 10.1016/0167-4838(85)90006-8. [DOI] [PubMed] [Google Scholar]
- 20.Starzl TE, Fung JJ. Contempo 89: transplantation. JAMA. 1989;261:2894–2895. [PMC free article] [PubMed] [Google Scholar]
- 21.Schreiber SL. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991;251:283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]


