Abstract
This essay is a reflection on the ways the X-ray structures of the ribosome are helping in the interpretation of cryogenic electron microscopy (cryo-EM) density maps showing the translating ribosome in motion. Through advances in classification methods, cryo-EM and single-particle reconstruction methods have recently evolved to the point where they can yield an array of structures from a single sample (“story in a sample”), providing snapshots of an entire subprocess of translation, such as translocation or decoding.
Keywords: protein synthesis, cryo-electron microscopy, molecular machines, dynamics
1. X-ray Crystallography and Cryo-EM of the Ribosome
The publication of the pioneering X-ray structures of the bacterial 30S and 50S ribosomal subunits by last year’s Nobel Laureates in Chemistry[1,2,3] and, as Harry Noller has put it, “the whole enchilada” of the 70S ribosome,[4] has increased our ribosomal structural knowledge base immensely, with ripple effects still being felt throughout biology. But those who might have expected that the atomic-resolution structure of this massive RNA–protein complex would itself offer an immediate insight into the mechanism of translation were thoroughly disappointed. I like to compare this situation to that of a visit to Earth by a Martian who wants to understand how an automobile works. She looks under the hood of a parked car, perhaps even takes the engine apart, but still has no clue. It is clear that she will have much better luck if she is able to see the engine in motion.
Similarly, in order to understand the complex processes of translation we need information about the translational machinery in motion. Dynamic information of this kind is provided through snapshots of ribosomes performing their work, under conditions that most closely mimic their native conditions. The technique of cryogenic electron microscopy (cryo-EM), combined with single-particle reconstruction,[5,6] has made the collection of snapshots possible, and it has given us many electron density maps depicting the ribosome in different states along the translation reaction path (for a recent review, see ref. [7]). Incidentally, the EMDB, depository of EM density maps of macromolecules, now has 73 entries all relating to the ribosome, the single most populated component of this database. However, as long as we lacked the ability to interpret them at atomic resolution, prior to the year 2000 —which has been called the “annus mirabili” of the ribosome — these density maps were of limited value in analyzing the mechanism of translation. An immediate effect of the emergence of atomic-resolution X-ray structures was that they enabled us to build models from the observed density maps, and thereby determine their meaning in terms of the conformation the ribosome assumes and the binding configurations of its functional ligands (transfer RNAs, translation factors, etc.).
Flexible fitting methods designed to “mold” an atomic-resolution X-ray structure into a low-resolution density map are thus of crucial importance for deriving atomic interpretations of such density maps. Depending on the resolution of the density map, the meaning of the term “atomic interpretation” varies. At the resolution of the current maps (examples: refs. [8, 9]), which is in the range of 6–7Å, the density does not determine the conformation of the underlying structure unambiguously. Rather, it is appropriate to regard the fitted structure as representative of an entire ensemble of structures that agree with the observed density.[10] The members of this ensemble differ in the exact positions of RNA nucleosides and amino acid side chains, while generally agreeing on the trajectory of the phosphate backbone of the ribosomal and transfer RNAs and the Cα backbone of the ribosomal proteins and translation factors.
However, compared with these finer details of the model derived by fitting, changes in ribosome conformation observed by cryo-EM and single-molecule fluorescent resonance transfer (smFRET) are substantial, with the most dramatic one occurring during messenger RNA–transfer RNA translocation, as predicted by Alexander Spirin[11] and first observed in my laboratory.[12] In this process, distances between peripheral components of the two subunits change by up to 15Å.[13] The A-site finger, a long curved helix of the 23S ribosomal RNA within the 50S subunit that is linked to the 30S subunit, performs a sideways and upside-down motion as the subunits rotate with respect to each other, a directional motion that is in line with the molecular dynamics properties of an RNA kink–turn hinge element located at the base of the helix.[14] The ribosomal protein L1, sitting atop a flexible RNA stalk formed by helices 76, 77, and 78 of the 23S ribosomal RNA, swings along an arc of 20Å[13] as it assists in the shuttling of the tRNA.[13,15,16]
Through cryo-EM experiments, smFRET studies (see ref. [17] for an overview), and by examinaing the connectivity of the architectural framework in ribosome structures obtained by X-ray crystallography (see refs. [17,18]), we have come to appreciate the fact that the ribosome structure is intrinsically flexible and likely capable of sampling a large, multidimensional range of conformations. This range is multidimensional in the sense that several domains and peripheral components may perform independent motions, although allosteric coupling of motions in components separated by distances of more than 100 Å has also been observed, as well.[16,20,21]
X-ray crystallography and cryo-EM — at least in the way they have been practiced thus far — share the feature that they sample conformational space in a sporadic, unsystematic way. The advantage of X-ray crystallography is that it gives us structures on the atomic length scale; however, the conformations that are captured are not authentic in the sense that they do not necessarily reflect functional states of the molecule, but are instead primarily determined by the energetics of crystal formation in which intermolecular forces play an important role. Conversely, the advantage of cryo-EM is that it gives us structures, or snapshots, that capture the molecule as it performs its work, but — for reasons that have to do with the residual conformational variability even in defined states — it is quite difficult to attain atomic resolution. Traditionally the timing of the snapshots provided by cryo-EM is defined by chemical interventions: either by the use of an antibiotic (“throwing a spanner in the works,” see ref. [22]), a nonhydrolyzable GTP analog (e.g., ref. [13]), or a suitable mutation that stabilizes an intermediate or transition state (e.g., ref. [21]). It is obviously not possible, relying on those interventions, to choose the time points at will.
Evidently there is some sort of uncertainty principle at work: if we want to see a molecule at atomic resolution, we need to immobilize it in a crystal. On the other hand, if we want to see how the molecule moves in “real life,” in the pursuit of its function, we have to liberate it from crystal confinement, but this comes at the price of lower resolution. This is not to say that atomic resolution of a large asymmetric assembly such as the ribosome will never be achieved by single-particle reconstruction, but it is an uphill struggle, and even when this is accomplished in some not-too-distant future, it will still be far from routine.
2. Advances Toward a New Approach of Cryo-EM: Finding a Story in the Sample
The single-particle reconstruction method was initially conceived with the premise that the molecules comprising the sample would exist in a single conformation; if some small percentage were in different conformations or binding states, this would be treated as a “contamination,” with the three-dimensional density map essentially representing the prevailing majority. Thus in the case of the ribosome, sample preparation methods have traditionally focused on some kind of chemical intervention that attempted to bring all, or close to all, ribosomes into lockstep. However, as classification methods were refined, more and more cases of heterogeneity were discovered, and in response to this challenge, the aims of the studies have now widened to characterize every subpopulation encountered by a separate reconstruction. Thus, for instance, a sample in which the pre-translocational ribosome was incubated with elongation factor G in complex with a non-hydrolyzable GTP analog (EF-G•GDPNP) was found to consist of two subpopulations, one in which EF-G•GDPNP and a hybrid P/E transfer RNA were bound to the ribosome, and another in which classical A/A, P/P, and E/E transfer RNAs were bound to the ribosome.[13,23]
A further step in this evolution of the method has now been taken: what if we left out the chemical intervention — the attempt to bring all of the ribosomes in the sample into lockstep — altogether? Sufficiently populated states, if characterized by a distinct conformation of the ribosome, would be represented by sizeable fractions of the population, which could be separated by classification and hence independently reconstructed. All conformations and binding states that the ribosome assumes in the equilibrated sample will then be on display at once, often with a logical sequence or “story line” related to the physical movement of ligands.
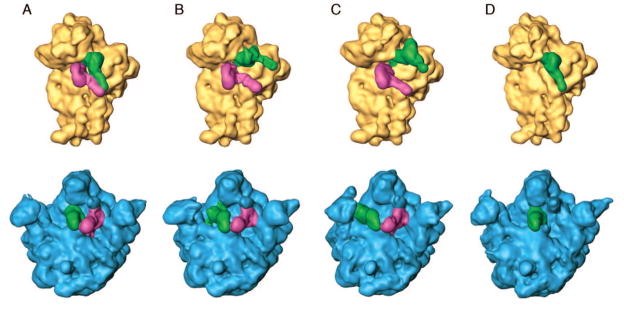
This “story-in-a-sample” approach is now being taken increasingly by several groups practicing cryo-EM (see ref. [24]). Recent work by Fischer and coworkers,[25] in which close to 2 million particles were analyzed, gives us an idea of the rich number of intermediate states in elongation that require characterization. Back-translocation alone, which is a relatively slow process that essentially reverts part of the process of messenger RNA–transfer RNA translocation, [26] was resolved into dozens of different configurations of the ribosome-transfer RNA complex. Similarly, we have recently discovered the presence of at least three coexisting states in a pre-translocational ribosome sample that was prepared at physiological Mg2+ concentrations in the absence of EF-G: in addition to the nonratcheted and ratcheted states of the pre-translocational ribosome already described,[27] a well-populated intermediate state of inter-subunit rotation has now been discovered (Figure 1).[28]
Figure 1.
“Story in a sample:” Reconstructions from four classes of the same ribosome sample (pre-translocational ribosome complex in the absence of EF-G), obtained by Maximum-likelihood classification. Small and large subunits have been computationally separated so that the tRNA positions become visible. Apparently, three different states co-exist in equilibrium, differing in ribosome conformation and positions of the two tRNAs. A — Unratcheted ribosome, tRNAs in classical (A/A, P/P) positions; B — intermediate rotation of the small subunit, intermediate positions of tRNAs; C — ratcheted ribosome, tRNAs in hybrid (A/P, P/E) positions. D — In addition, a class of ribosomes is found in which the A-site is empty, probably because it was never occupied. Adapted from ref. [28].
Thus, as classification methods in cryo-EM are refined, we see more and more evidence for the fact that the structures in real life form a quasi-continuum, and that the fluctuations of molecules among different states are essential to their function. Energy barriers separating different states in the ribosome are often small, and are easily overcome in the thermal environment. [17,29,30]
Many processes, however, are elusive to the “story-in-the-sample” approach since they are fast relative to the time required for specimen preparation in cryo-EM experiments, which is on the order of a few seconds. Thus, transient states are not observable with the usual blotting and freeze-plunging technique. The recent emergence of nanotechnologically machined mixing and spraying devices with millisecond time resolution[31] which revive Berriman and Unwin’s pioneering spray-freeze technique[32] is an exciting development which should make even fast-moving processes such as aminoacyl-transfer RNA selection during decoding amenable to time-resolved studies.
3. Conclusions
The dynamics of the ribosome as it performs its work are being increasingly revealed through acquisition of series of cryo-EM density maps, each reconstructed from ribosome complexes captured in the same state. The X-ray structures of bacterial ribosomal subunits and whole ribosomes obtained from the pioneering achievements by the groups of Ada Yonath, Tom Steitz, Venki Ramakrishnan and Harry Noller over the last decade have enabled us to obtain atomic models that sketch out the conformation of the ribosome and the binding configuration of its functional ligands in each state. Recent progress in the development of classification techniques has made it possible to visualize all significantly populated states coexisting in the sample at once. This development, augmented by time-resolved cryo-EM imaging techniques in the millisecond time regime and supplemented by rapidly evolving developments in smFRET studies of ribosome dynamics, should greatly expand our mechanistic understanding of translation in the years to come.
Acknowledgments
I would like to thank Ruben Gonzalez for a critical reading and helpful comments. This work was supported by HHMI and NIH R01 GM29169.
Biography

Joachim Frank is a structural biologist who developed the single-particle re- construction technique in cryo-electron microscopy. For the past 25 years, his research has focused on the structure and dynamics of the translational apparatus. One of his discoveries is the ratchet movement of the ribosome during translocation. A cryo-EM density map of the ribosome from Haloarcula marismortui has helped in the initial phasing of Thomas Steitz’ X-ray structure of the large subunit. Frank did much of this work while at the Wadsworth Center in Albany, New York, and has recently moved to Columbia University where he has a joint appointment with the Department of Biochemistry and Molecular Biophysics and the Department of Biological Sciences. He is a Howard Hughes Investigator and a member of the National Academy of Sciences.
References
- 1.Schluenzen F, Tocilj A, Zarivach R, Harms J, Gluehmann M, Janell D, Bashan A, Bartels H, Agmon I, Franceschi F, Yonath A. Cell. 2000;102:615–623. doi: 10.1016/s0092-8674(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 2.Ban N, Nissen P, Hansen J, Moore PB, Steitz TA. Science. 2000;289:905–920. doi: 10.1126/science.289.5481.905. [DOI] [PubMed] [Google Scholar]
- 3.Wimberly BT, Brodersen DE, Clemons WM, Jr, Morgan-Warren RJ, Carter AP, Vonrhein C, Hartsch T, Ramakrishnan V. Nature. 2000;407:327–339. doi: 10.1038/35030006. [DOI] [PubMed] [Google Scholar]
- 4.Yusupov MM, Yusupova GZ, Baucom A, Lieberman K, Earnest TN, Cate JHD, Noller HF. Science. 2001;292:883–896. doi: 10.1126/science.1060089. [DOI] [PubMed] [Google Scholar]
- 5.Frank J. Three-dimensional Electron Microscopy of Macromolecular Assemblies. Oxford University Press; New York: 2006. [Google Scholar]
- 6.Frank J. Q Rev Biophys. 2009;42:139–158. doi: 10.1017/S0033583509990059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agirrezabala X, Frank J. Q Rev Biophys. 2009;42:159–200. doi: 10.1017/S0033583509990060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LeBarron J, Grassucci RA, Shaikh TR, Baxter WT, Sengupta J, Frank J. J Struct Biol. 2008;164:24–32. doi: 10.1016/j.jsb.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seidelt B, Innis CA, Wilson DN, Gartmann M, Armache JP, Villa E, Trabuco LG, Becker T, Mielke T, Schulten K, Steitz TA, Beckmann R. Science. 2009;326:1412–1415. doi: 10.1126/science.1177662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trabuco LG, Villa E, Mitra K, Frank J, Schulten K. Structure. 2008;16:673–683. doi: 10.1016/j.str.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spirin AS. Cold Spring Harbor Symp Quant Biol. 1969;34:197–207. doi: 10.1101/sqb.1969.034.01.026. [DOI] [PubMed] [Google Scholar]
- 12.Frank J, Agrawal RK. Nature. 2000;406:318–322. doi: 10.1038/35018597. [DOI] [PubMed] [Google Scholar]
- 13.Valle M, Zavialov A, Sengupta J, Rawat U, Ehrenberg M, Frank J. Cell. 2003;114:123–134. doi: 10.1016/s0092-8674(03)00476-8. [DOI] [PubMed] [Google Scholar]
- 14.Réblová K, Rázga F, Li W, Gao H, Frank J, Šponer J. Nucl Acids Res. 2010;38:1325–1340. doi: 10.1093/nar/gkp1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harms J, Schluenzen F, Zarivach R, Bashan A, Gat S, Agmon I, Bartels H, Franceschi F, Yonath A. Cell. 2001;107:679–688. doi: 10.1016/s0092-8674(01)00546-3. [DOI] [PubMed] [Google Scholar]
- 16.Fei J, Kosuri P, MacDougall DD, Gonzalez RL. Mol Cell. 2008;30:348–359. doi: 10.1016/j.molcel.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Frank J, Gonzalez RL. Ann Rev Biochem. 2010 doi: 10.1146/annurev-biochem-060408-173330. ANRV413-BI79-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noller HF. Science. 2005;309:1508–1514. doi: 10.1126/science.1111771. [DOI] [PubMed] [Google Scholar]
- 19.Korostelev A, Ermolenko DN, Noller HF. Curr Opin Chem Biol. 2008;12:674–683. doi: 10.1016/j.cbpa.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornish PV, Ermolenko DN, Staple DW, Hoang L, Hickerson RP, Noller HF, Ha T. Proc Natl Acad Sci USA. 2009;106:2571–2576. doi: 10.1073/pnas.0813180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fei J, Bronson JE, Hofman JM, Srinivas RL, Wiggins CH, Gonzalez RL. Proc Natl Acad Sci USA. 2009;106:15702–15707. doi: 10.1073/pnas.0908077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spahn CMT, Prescott CD. J Mol Med. 1996;74:423–439. doi: 10.1007/BF00217518. [DOI] [PubMed] [Google Scholar]
- 23.Scheres SHW, Gao H, Valle M, Herman GT, Eggermont PPB, Frank J, Carazo JM. Nature Meth. 2007;4:27–29. doi: 10.1038/nmeth992. [DOI] [PubMed] [Google Scholar]
- 24.Spahn CMT, Penczek PA. Curr Opin Struct Biol. 2009;19:623–631. doi: 10.1016/j.sbi.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer N, Wintermeyer W, Stark H, Konevega AL, Rodnina M. Abstract, EMBO Conference on Protein Synthesis and Translational Control; September 9–13, 2009.Heidelberg: EMBL; [Google Scholar]
- 26.Konevega AL, Fischer N, Semenkov YP, Stark H, Wintermeyer W, Rodnina MV. Nat Struct Mol Biol. 2007;14:318–324. doi: 10.1038/nsmb1221. [DOI] [PubMed] [Google Scholar]
- 27.Agirrezabala X, Lei J, Brunelle JL, Ortiz-Meoz RF, Green R, Frank J. Mol Cell. 2008;32:190–197. doi: 10.1016/j.molcel.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agirrezabala X, Liao H, Fu J, Brunelle JL, Ortiz-Meoz RF, Schreiner E, Schulten K, Green R, Frank J. in preparation. [Google Scholar]
- 29.Frank J, Gao H, Sengupta J, Gao N, Taylor DJ. Proc Natl Acad Sci USA. 2007;104:19671–19678. doi: 10.1073/pnas.0708517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munro JB, Sanbonmatsu KY, Spahn CMT, Blanchard SC. Trends Biochem Sci. 2009;34:390–400. doi: 10.1016/j.tibs.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu Z, Shaikh TR, Barnard D, Meng X, Mohamed H, Yassin A, Mannella CA, Agrawal RK, Lu TM, Wagenknecht T. J Struct Biol. 2009;168:388–395. doi: 10.1016/j.jsb.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berriman J, Unwin PNT. Ultramicroscopy. 1994;56:241–252. doi: 10.1016/0304-3991(94)90012-4. [DOI] [PubMed] [Google Scholar]