Abstract
Although herons and egrets in the family Ardeidae frequently have been associated with viruses in the Japanese encephalitis virus serocomplex, communal nesting colonies do not appear to be a focus of early season and rapid amplification of West Nile virus (WNV) in California. Evidence for repeated WNV infection was found by testing living and dead nestlings collected under trees with mixed species ardeid colonies nesting above in an oak grove near the University of California arboretum in Davis and in a Eucalyptus grove at a rural farmstead. However, mosquito infection rates at both nesting sites were low and positive pools did not occur earlier than at comparison sites within the City of Davis or at the Yolo Bypass wetlands managed for rice production and waterfowl habitat. Black-crowned night herons (Nycticorax nycticorax) were the most abundant and frequently infected ardeid species, indicating that WNV may be an important cause of mortality among nestlings of this species.
Key Words: West Nile virus, ardeidae, Black-crowned night heron, snowy egret, cattle egret, great egret, California
Introduction
Vector-borne pathogen amplification requires frequent contact among vertebrate and vector hosts which may be enabled when aggregates of either species intersect in time and space. This is especially important for West Nile virus (Flaviviridae, Flavivirus, WNV) at temperate latitudes in North America, where transmission is limited to the warm summer months of the year and amplification is dependent upon repeated contact among highly susceptible avian hosts and moderately susceptible Culex vectors (Kramer et al. 2008; Reisen and Brault 2007). A hallmark of the ongoing WNV epidemic in North America has been the repeated association between communally roosting American crows (Corvus brachyrhynchos, AMCR) and tangential transmission of WNV to humans (Eidson 2005; Nielsen and Reisen 2007; Reisen et al. 2006; Ruiz et al. 2004). However, these and other Corvidae disperse into family groups at nesting sites during the critical vernal amplification period and therefore may play less of a role during vernal amplification than communally nesting susceptible species in the families Icteridae and Ardeidae.
Herons and egrets (Ciconiiformes, Ardeidae) have long been associated with the epidemiology of Flaviviruses within the Japanese encephalitis virus (JEV) serocomplex, including JEV in Japan (Buescher et al. 1959) and India (Jamgaonkar et al. 2003; Rodrigues et al. 1981), Murray Valley encephalitis virus in Australia (Boyle et al. 1983) and St Louis encephalitis virus (SLEV) in Panama (Adames et al. 1993). In California, several species of ardeids frequently form large communal nesting groups during spring and summer that may include thousands of nests concentrated within favorable habitat. Previously, we investigated the role of one communal nesting site situated within the flooded remnants of a Tamarix stand at the Finney-Ramer Wildlife Refuge in Imperial County, California (Reisen et al. 2005). Although considerable WNV activity was documented at the Refuge by seroconversions of sentinel chickens and infection in Culex tarsalis mosquitoes, there was little evidence of transmission to nestling ardeids, even though several species were found experimentally to be highly susceptible to infection and competent hosts for the virus. In agreement, vector infection and sentinel seroconversion rates at this Refuge were not statistically different from those documented at a nearby comparison site within the Imperial Wildlife Unit that lacked a communal ardeid nesting colony. We concluded that nestling ardeids at Finney-Ramer may have been protected by the failure of Cx. tarsalis to traverse open water during host-seeking flights, a phenomenon described previously for other avian groups roosting or nesting over water (Lothrop and Reisen 2001). However, ardeids also form nesting colonies over land and these birds may be vulnerable to Cx. tarsalis blood feeding and WNV infection. The location of two moderate-sized communal ardeid nesting colonies of similar species composition near Davis in Yolo County, California, afforded the opportunity to revisit the role of communally nesting ardeids in WNV amplification.
Our current research addresses the notion that WNV competent ardeid species that communally nest during spring may be important in rapid and focal viral amplification. We addressed this hypothesis by documenting WNV infection of birds at communal nesting sites and by comparatively measuring the incidence of mosquito infection at these nesting sites and at comparison areas in nearby wetland and urban habitats lacking communal ardeid nesting colonies.
Methods and Materials
Study area
Two nesting sites were studied in Yolo County, California near the City of Davis (38°N, 121°W). Site 1 consisted of mixed Cattle egret (Bubulcus ibis; CAEG), Snowy egret (Egretta thula; SNEG) and Black-crowned night heron (Nyc-ticorax nycticorax; BCNH) nests within a stand of oak (Quercus spp.) trees at the University of California arboretum, just to south of the campus. The nesting site was surrounded by the campus, parkland and agricultural fields. Site 2 consisted SNEG, BCNH, Great egret (Ardea alba; GREG) and to a less extent CAEG nests within a stand of Eucalyptus trees at a farmstead ca. 3 km north of Davis which raised goats and cattle and was flanked on all sides by row crops and pasture. A wetland constructed to process sewage effluent and a rice growing area and bird sanctuary within the Yolo Bypass were located 3–5 km to the east and produced large numbers of Cx. tarsalis mosquitoes during summer.
Sampling and diagnostics
Older ardeid chicks frequently push younger, weaker or sick nest mates out of the nest. In addition, older nestlings frequently wander about in the canopy near their nest and are attacked by parents or chicks residing in neighboring nests causing them to fall to the ground. This resulted in a large number of dead, injured, and displaced chicks stranded on the floor beneath both communal nesting sites. Weekly during the nesting season we collected recently dead birds and submitted these for necropsy to the California Animal Health and Food Safety Laboratory as part of the Dead Bird Surveillance Program managed by the California Department of Public Health (McCaughey et al. 2003). Kidney snips and other tissues taken at necropsy were sent to the Center for Vectorborne Diseases (CVEC) for testing for WNV RNA by singleplex RT-PCR using primers described previously (Lanciotti et al. 2000). Living birds were collected by hand, identified to species and approximate age, and a 0.2 ml blood sample collected by jugular puncture; 0.1 mL was expelled into 0.4 ml of virus diluent (phosphate buffered saline, fetal bovine sera and antibiotics), frozen in the field on dry ice, and then stored at −80°C until tested for virus, while the second 0.1 mL was expelled into 0.9 mL of PBS, clarified by centrifugation and stored at −20°C until tested for antibody at the BSL3 laboratory at CVEC. Healthy birds were banded and released, whereas small, dehydrated or injured birds were taken to avian rehabilitation centers. Sera were tested for infectious virus by standard plaque assay on Vero cell culture, and if negative, screened for antibodies by enzyme immunoassay (EIA) (Chiles and Reisen 1998). An EIA was considered positive if the ratio of the mean optical density of two antigen positive wells over the antigen negative well for each bird was >2. EIA positives then were confirmed by plaque reduction neutralization tests (PRNTs) against WNV and St. Louis encephalitis viruses. A 90% reduction of >50–75 plaques at a dilution ≥1:20 confirmed EIA results, whereas titers >4× the competing virus were considered to specifically identify either WNV or SLEV. Collection and bleeding of wild birds was done under University of California Internal Animal Care and Use Committee protocols, State of California Fish and Game scientific collecting permits and a USGS Master Station Bird Banding permit.
Mosquitoes were collected weekly from April through October during 2005–2007 at the two ardeid nesting sites described above and at comparison sites at the Yolo Bypass wetland area and within the City of Davis by 4–21 dry ice-baited CDC traps (Newhouse et al. 1966). Additional mosquitoes (mostly gravid Cx. pipiens) were collected using gravid female traps (Cummings 1992). Mosquitoes were returned alive to our laboratory at Davis where they were anesthetized with triethylamine, enumerated by species, sorted into pools of ≤50 females each, and frozen until tested for WN, SLE and western equine encephalomyelitis viral RNA by multiplex real time RT-PCR. Maximum likelihood estimates of infection incidence were calculated using Excel add-in software (Biggerstaff 2003).
We used two other measures to detect WNV transmission at Site 2. During 2007 sentinel chicken flocks each consisting of 5 hens were positioned at Site 2 and at a control farm site ca. 2 km to the west. Hens were bled weekly and sera tested by EIA as described above. Year end samples of House sparrows (Passer domestica, HOSP) were collected at Site 2 and the control flock site. HOSPs were collected by mist-net, identified to species, sex and age, banded using USGS bands, a 0.1 ml blood sample collected by jugular venipuncture, and released. Blood samples were expelled into 0.9 ml of saline, clarified by centrifugation and then tested for antibody by EIA as described above.
Results and Discussion
Bird collections
A total of 81 dead juvenile ardeid birds were tested from Sites 1 and 2, of which 12 (15%) kidney samples taken at necropsy tested positive for WNV RNA by singleplex RT-PCR (Table 1). Although the proportion of dead birds positive for WNV RNA did not differ significantly among species (ξ2 = 1.91, df = 3, P = 0.56), most of the dead birds found on the ground under nests were BCNH (52% of total); by comparison relatively few CAEG (7%) were found dead even though they were abundant at Site 1. Although the numbers of birds or nests were not censused, these mortality data generally agreed with the visual abundance of the different species and our previously published experimental infections where 2 of 3 BCNH and 0 of 4 CAEG nestlings succumbed during days 1–7 post infection (Reisen et al. 2005).
Table 1.
Summary of Dead and Live Bird Collections at Communal Ardeid Nesting Colonies at Davis, California, During 2005–2007, that Were Tested for West Nile Virus (WNV) Infection or Antibodies
| |
|
|
Dead birds |
Live birds |
|||
|---|---|---|---|---|---|---|---|
| Year | Site | Species | Tested | WNV RNA* | Tested | WNV aby** | WNV viremia*** |
| 2005 | Site 1 | BCNH | 5 | 1 | 11 | 3 | nd |
| CAEG | 2 | 1 | 5 | 1 | nd | ||
| SNEG | 1 | 0 | 7 | 0 | nd | ||
| 2006 | Site 1 | BCNH | 7 | 0 | 14 | 0 | 0 |
| CAEG | 2 | 0 | 11 | 0 | 0 | ||
| SNEG | 1 | 0 | 2 | 0 | 0 | ||
| 2006 | Site 2 | BCNH | 10 | 3 | 75 | 11 | 8 |
| CAEG | 0 | 0 | 3 | 0 | 0 | ||
| GREG | 4 | 1 | 6 | 6 | 0 | ||
| SNEG | 9 | 2 | 40 | 0 | 0 | ||
| 2007 | Site 2 | BCNH | 20 | 2 | 170 | 3 | 0 |
| CAEG | 2 | 1 | 7 | 0 | 0 | ||
| GREG | 4 | 0 | 5 | 1 | 0 | ||
| SNEG | 14 | 1 | 112 | 3 | 0 | ||
| Totals | 81 | 12 | 468 | 28 | 8 | ||
Kidney samples tested by RT-PCR.
Sera screened for antibody (aby) by EIA and confirmed by PRNT.
Sera tested for virus by Vero cell plaque assay.
At total of 468 juvenile ardeids of different ages were collected alive on the ground under colonial nesting Sites 1 and 2, of which 28 (6%) were positive by EIA for antibodies against WNV (Table 1). Seroprevalence varied significantly among species (ξ2 = 9.8, df = 3, P = 0.02), being highest for GREG (15.0%) and lowest for SNEG (2.4%). Of the 28 positives, 22 were confirmed by PRNT, with titers against WNV ≥1:80 and 4× the titers against SLEV that were ≤1:40. Five SNEG with EIA P/N ratios ranging from 2.1–2.8 had PRNT titers ≤1:20 and were not confirmed. A single BCNH tested in 2007 had equivocal 1:80 titers against both WNV and SLEV, indicating possible maternal antibody. Dual infection was unlikely, because SLEV has not been found in California since the arrival of WNV (Reisen et al. 2008). These serological results were markedly different than observed previously in Imperial County (Reisen et al. 2005), where antibody prevalence and titers were low and PRNT results non-specific. In addition, PRNT titers in Finney-Ramer ardeid chicks decreased with age and were attributed to maternal antibody due to limited evidence of virus transmission within the colony.
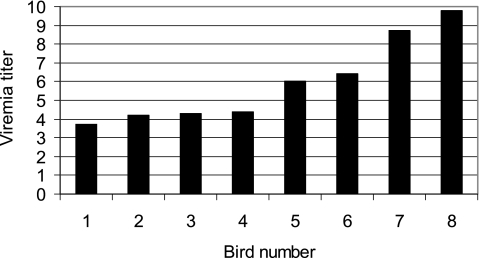
Of 435 live birds bled during 2006 and 2007 and tested for viremia, 8 of 245 (3.3%) BCNH were positive for WNV and exhibited titers with a mean ± 95% confidence interval = 5.94 ± 1.57 log10 plaque forming units (PFU) per mL of blood (range = 3.7–9.8 log10 PFU/mL). Two birds had elevated titers >8 log10 PFU/mL, whereas 4 had titers <5 log10 PFU/mL (Fig. 1), the threshold for most Culex mosquito infection (Komar et al. 2003). These data verified that some naturally infected immature BCNH were extremely competent hosts.
FIG. 1.
Viremia titers in log10 PFU per mL for eight infected Black-crowned night herons collected at Site 2 during 2006.
Mosquito abundance and infection
Overall, 39,619 Culex females collected at dry ice-baited and gravid traps were tested for WNV, SLEV and WEEV RNA in 2,210 pools, of which 43 were positive for WNV (Table 2). None were positive for SLEV or WEEV. Of the WNV positive pools, 31 were collected within the City of Davis during the 2006 outbreak (Nielsen et al. 2008). Although considerably more Cx. tarsalis (35,498 females in 1335 pools) than Cx. pipiens (4121 in 875 pools) were tested, the infection rate for Cx. pipiens (3.15 per 1000) was higher than for Cx. tarsalis (0.85 per 1000). This may be because Cx. tarsalis abundance was highest near larval habitats associated with rice fields and wetlands at the Yolo Bypass resulting in collections that possibly consisted mostly of newly-emerged nulliparous females, and because about half of the Cx. pipiens tested were collected by gravid traps and had digested at least one blood meal, thereby increasing their chances of being infected. Cx. tarsalis abundance during 2006 and 2007 at Site 2, the Yolo Bypass and the City of Davis generally were highest during periods when nestlings were observed at Site 2 (Fig. 2). Adult birds were present several weeks before this time, but juveniles were not observed on the ground within the Eucalyptus grove until the period indicated.
Table 2.
Mean Abundance (Females Collected per Dry Ice-Baited Trap Night) and Infection Rate (Maximum Likelihood Estimate of Positive Females per 1000 Estimated by the Method of Biggerstaff) of Culex Tarsalis and Cx. Pipiens Mosquitoes Collceted at each Site per Year. IR is the Infection Rate and LL and UL are the Lower and Upper Limits of the 95% Confidence Interval About This Mean
| Site | Culex species | Year | Traps | F/TN* | Tested | Pools | WNV+ | IR | LL | UL | 1st Pos |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Site 1 | tarsalis | 2005 | 3 | 1.4 | 24 | 8 | 0 | ||||
| 2006 | 3 | 4.4 | 230 | 21 | 0 | ||||||
| pipiens | 2005 | 3 | <0.1 | 72 | 5 | 0 | |||||
| 2006 | 3 | 1.6 | 89 | 18 | 1 | 12.1 | 0.7 | 62.4 | 11-Jul | ||
| Site 2 | tarsalis | 2006 | 3 | 113.7 | 2,490 | 56 | 2 | 0.8 | 0.1 | 2.7 | 18-Jul |
| 2007 | 3 | 73.0 | 2,039 | 54 | 0 | ||||||
| pipiens | 2006 | 3 | 0.7 | 137 | 15 | 2 | 16.7 | 3.0 | 59.4 | 18-Jul | |
| 2007 | 3 | <0.1 | 37 | 9 | 0 | ||||||
| Yolo | tarsalis | 2006 | 4 | 213.6 | 8,423 | 178 | 6 | 0.7 | 0.2 | 1.5 | 18-Jul |
| 2007 | 4 | 202.1 | 3,815 | 85 | 1 | 0.3 | 0.0 | 1.3 | 17-Aug | ||
| Davis | tarsalis | 2006** | 21 | 19.2 | 16,411 | 778 | 20 | 1.2 | 0.8 | 1.9 | 11-Jul |
| 2007 | 5 | 11.2 | 2,066 | 155 | 1 | 0.5 | 0.0 | 2.4 | 21-Jul | ||
| pipiens | 2006** | 21 | 2.1 | 3,177 | 673 | 9 | 2.9 | 1.4 | 5.2 | 4-Jul | |
| 2007 | 5 | 1.9 | 609 | 155 | 1 | 1.6 | 0.1 | 7.9 | 11-Sep | ||
| Total | 39,619 | 2,210 | 43 | 1.1 |
Dry ice baited traps only
Data from Nielsen et al. (2007)
FIG. 2.
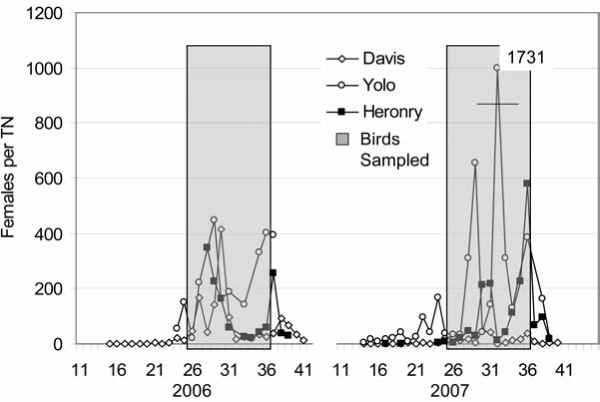
Arithmetic mean abundance of Cx. tarsalis females per trap-night (TN) at the City of Davis (Davis), the Yolo Bypass (Yolo), and communal ardeid nesting Site 2 (Heronry) plotted as a function of weeks during 2006 and 2007. Shown in grey shading are the periods when dead and living juvenile birds were collected under trees at Site 2.
Mosquito infection was measured at two ardeid nesting sites, wetlands at the Yolo Bypass and at multiple sites within the City of Davis to test our hypothesis that communal ardeid nesting sites form foci of early and rapid virus amplification (Table 2). Although infected birds were collected during 2005 and a positive mosquito pool was collected in 2006 at Site 1, mosquito abundance was low and inadequate for assessment, and this site was abandoned after 2006. An outbreak of WNV occurred within Davis during 2006 (Nielsen et al. 2008), but WNV positive birds were not detected at Site 1 during this period.
Mosquito abundance was much greater at Site 2 located relatively near the Yolo Bypass and the City of Davis water treatment plant with an adjacent constructed wetland (Fig. 2) than at Site 1; however, relatively few pools tested positive for WNV RNA during the 2006 outbreak year or the following summer (Table 2). In addition, the timing of infection at Sites 1 and 2 was not earlier than observed within the City of Davis or at the Yolo Bypass. These results were unexpected because dead and live ardeids collected at Site 2 frequently showed signs of infection and juvenile BCNH were competent hosts for WNV (Reisen et al. 2005). We considered that some of the avian infection observed at Site 2 could be attributed to bird-bird transmission due to parental feeding, fecal-oral contamination, frequent fighting on the nests, or scavenging of injured or dead nestlings.
There was additional evidence for virus transmission at Site 2. During 2007, 3 of 5 sentinel chickens deployed at Site 2 and 1 of 5 chickens deployed at the comparison site seroconverted to WNV. HOSP mist-netted at Site 2 at the end of 2006 also showed evidence for local WNV transmission (3 EIA positive hatching year (HY) birds of 44 tested). These data were similar to 2007 year end seroprevalence for HOSP at the sentinel chicken comparison site (2 EIA positives of 29 tested, including 1 HY and 1 after hatching year bird (AHY)).
In summary, ardeids nesting at two colonies in trees over land near Davis showed evidence of repeated infection with WNV. These findings strongly contrasted results with the same ardeid species communally nesting over water in Imperial County, where there was little evidence of WNV infection using the same viremia and antibody measures, despite the fact that WNV was repeatedly detected in Cx. tarsalis collected in vegetation surrounding the pond (Reisen et al. 2005). Overall, WNV infection incidence at Finney-Ramer was 3.1 per 1000 Cx. tarsalis tested during the transmission season, considerably higher than documented for Cx. tarsalis at the Yolo County ardeid sites (Table 2). In Imperial County, any birds falling or being pushed out of their nest and into the pond would either drown or be able to climb back into the Tamarisk, so there were no dead birds available for necropsy. Collectively these data supported our previous conclusions that bird species nesting (Reisen et al. 2005) or roosting (Lothrop et al. 2002) over water were protected from host-seeking Cx. tarsalis and therefore WNV infection.
Based on relatively low infection rates among dead and living ardeid juveniles and low infection rates among host-seeking and oviposition site questing Culex mosquitoes, we concluded that the two communal ardeid nesting colonies sampled in Yolo County were not foci for early season or rapid WNV amplification. There was no indication that virus amplified initially at these sites and then spread to other areas. During the outbreak of 2006, initial virus activity was detected in dead corvids (Western scrub-jays, Aphelocoma californica, WESJ; AMCR; and Yellow-billed magpies, Pica nuttalli, YBMP) at residences on the west side of Davis (Nielsen and Reisen 2007) and away from both ardeid colonies and the Yolo Bypass. Results generally were similar during 2007 when overall WNV activity was less (i.e., fewer positive dead and living birds and fewer positive mosquitoes).
Our research continues to implicate corvids and Culex as important amplifying hosts for WNV in California (Nielsen and Reisen 2007; Reisen et al. 2006). AMCRs and YBMPs disperse into reproductive groups in spring and then coalesce into communal roosts during midsummer (Caccamise et al. 1997), when most human cases occur. In areas supporting large populations of these species, the risk of human infection seemed to be delineated by the range of foraging flights about these communal roosts (Reisen et al. 2006). In contrast, in Bakersfield where highly territorial and relatively evenly dispersed WESJs are the dominant corvid, WNV amplification and human cases has tended to be more evenly dispersed. To date, our attempts to implicate other communally nesting or roosting bird species in WNV amplification have remained unsuccessful.
Acknowledgements
We especially thank the Tauzer family for permission to sample birds and mosquitoes and house sentinel chickens on their property. This research was funded, in part, by Research Grant RO1 AI55607 from the National Institute of Allergy and Infectious Diseases, NIH, the Sacramento-Yolo Mosquito and Vector Control District, and special funds from the Mosquito Research Program allocated annually through the Division of Agriculture and Natural Resources, University of California.
Disclosure Statement
No competing interests exist.
References
- Adames AJ. Dutary B. Tejera H. Adames E. Galindo P. (The relationship between mosquito vectors and aquatic birds in the potential transmission of 2 arboviruses (published erratum appears in Rev Med Panama 1993 Sep;18(3):242)) Rev Med Panama. 1993;18:106–119. [PubMed] [Google Scholar]
- Biggerstaff BJ. Pooled infection rate. www.cdc.gov/ncidod/dvbid/westnile/software.htm. 2003. pp. 1–5.www.cdc.gov/ncidod/dvbid/westnile/software.htm
- Boyle DB. Dickerman RW. Marshall ID. Primary viraemia responses of herons to experimental infection with Murray Valley encephalitis, Kunjin and Japanese encephalitis viruses. Aust J Exp Biol Med Sci. 1983;61(Pt 6):655–664. doi: 10.1038/icb.1983.62. [DOI] [PubMed] [Google Scholar]
- Buescher EL. Scherer WF. McClure HE. Moyer JT. Rosenberg MZ. Yoshi M. Okada Y. Ecologic studies of Japanese encephalitis virus in Japan. IV. Avian Infection. Am J Trop Med Hyg. 1959;8:689–697. doi: 10.4269/ajtmh.1959.8.678. [DOI] [PubMed] [Google Scholar]
- Caccamise DF. Reed LM. Romanowski J. Stouffer PC. Roosting behavior and group territoriality in American crows. The Auk. 1997;114:628–637. [Google Scholar]
- Chiles RE. Reisen WK. A new enzyme immunoassay to detect antibodies to arboviruses in the blood of wild birds. J Vector Ecol. 1998;23:123–135. [PubMed] [Google Scholar]
- Cummings RF. Design and use of a modified Reiter gravid mosquito trap for mosquito-borne encephalitis surveillance in Los Angeles County, California. Proc Mosq Vector Control Assoc Calif. 1992;60:170–176. [Google Scholar]
- Eidson M. Dead crow density and West Nile virus monitoring, New York. Emerg Infect Dis. 2005;11:1370–1375. doi: 10.3201/eid1109.040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamgaonkar AV. Yergolkar PN. Geevarghese G. Joshi GD. Joshi MV. Mishra AC. Serological evidence for Japanese encephalitis virus and West Nile virus infections in water frequenting and terrestrial wild birds in Kolar District, Karnataka State, India. A retrospective study. Acta Virol. 2003;47:185–188. [PubMed] [Google Scholar]
- Komar N. Langevin S. Hinten S. Nemeth N. Edwards E. Hettler D. Davis B. Bowen R. Bunning M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer LD. Styer LM. Ebel GD. A Global Perspective on the Epidemiology of West Nile Virus. Annu Rev Entomol. 2008;53:61–81. doi: 10.1146/annurev.ento.53.103106.093258. [DOI] [PubMed] [Google Scholar]
- Lanciotti RS. Kerst AJ. Nasci RS. Godsey MS. Mitchell CJ. Savage HM. Komar N. Panella NA. Allen BC. Volpe KE. Davis BS. Roehrig JT. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lothrop HD. Lothrop B. Reisen WK. Nocturnal microhabitat distribution of adult Culex tarsalis (Diptera: Culicidae) impacts control effectiveness. J Med Entomol. 2002;39:574–582. doi: 10.1603/0022-2585-39.4.574. [DOI] [PubMed] [Google Scholar]
- Lothrop HD Reisen WK. Landscape affects the host-seeking patterns of Culex tarsalis (Diptera: Culicidae) in the Coachella Valley of California. J Med Entomol. 2001;38:325–332. doi: 10.1603/0022-2585-38.2.325. [DOI] [PubMed] [Google Scholar]
- McCaughey K. Miles SQ. Woods L. Chiles RE. Hom A. Kramer VL. Jay-Russel M. Sun B. Reisen WK. Scott TW. Hui LT. Steinlein DB. Castro M. Houchin A. Husted S. The California West Nile virus dead bird surveillance program. Proc Mosq Vector Control Assoc Calif. 2003;71:38–43. [Google Scholar]
- Newhouse VF. Chamberlain RW. Johnston JG., Jr Sudia WD. Use of dry ice to increase mosquito catches of the CDC miniature light trap. Mosq News. 1966;26:30–35. [Google Scholar]
- Nielsen CF. Armijos V. Wheeler SS. Carpenter TE. Kelley K. Boyce WM. Brown DA. Reisen WK. Risk factors associated with the 2006 West Nile virus outbreak in Davis, a residential community in Northern California. Am J Trop Med Hyg. 2008;78:53–62. [PMC free article] [PubMed] [Google Scholar]
- Nielsen CF. Reisen WK. Dead birds increase the risk of West Nile Virus infection in Culex mosquitoes (Diptera: Culicidae) in Domestic Landscapes. J Med Entomol. 2007;44:1007–1013. doi: 10.1603/0022-2585(2007)44[1067:wnvdci]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Reisen WK. Barker CM. Carney R. Lothrop HD. Wheeler SS. Wilson JL. Madon MB. Takahashi R. Carroll B. Garcia S. Fang Y. Shafii M. Kahl N. Ashtari S. Kramer V. Glaser C. Jean C. Role of corvids in epidemiology of West Nile virus in southern California. J Med Entomol. 2006;43:356–367. doi: 10.1603/0022-2585(2006)043[0356:rocieo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Reisen WK. Brault AC. West Nile virus in North America: perspectives on epidemiology and intervention. Pest Management Sci. 2007;63:641–646. doi: 10.1002/ps.1325. [DOI] [PubMed] [Google Scholar]
- Reisen WK. Lothrop HD. Wheeler SS. Kensington M. Gutierrez A. Fang Y. Garcia S. Lothrop B. Persistent West Nile virus transmission and the displacement St Louis encephalitis virus in southeastern California, 2003–2006. J Med Entomol. 2008;45:494–508. doi: 10.1603/0022-2585(2008)45[494:pwnvta]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen WK. Wheeler SS. Yamamoto S. Fang Y. Garcia S. Nesting Ardeid colonies are not a focus of elevated West Nile virus activity in southern California. Vector Borne Zoonotic Dis. 2005;5:258–266. doi: 10.1089/vbz.2005.5.258. [DOI] [PubMed] [Google Scholar]
- Rodrigues FM. Guttikar SN. Pinto BD. Prevalence of antibodies to Japanese encephalitis and West Nile viruses among wild birds in the Krishna-Godavari Delta, Andhra Pradesh, India. Trans Roy Soc Trop Med Hyg. 1981;75:258–262. doi: 10.1016/0035-9203(81)90330-8. [DOI] [PubMed] [Google Scholar]
- Ruiz MO. Tedesco C. McTighe TJ. Austin C. Kitron U. Environmental and social determinants of human risk during a West Nile virus outbreak in the greater Chicago area, 2002. Int J Health Geogr. 2004;3:8. doi: 10.1186/1476-072X-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]