Abstract
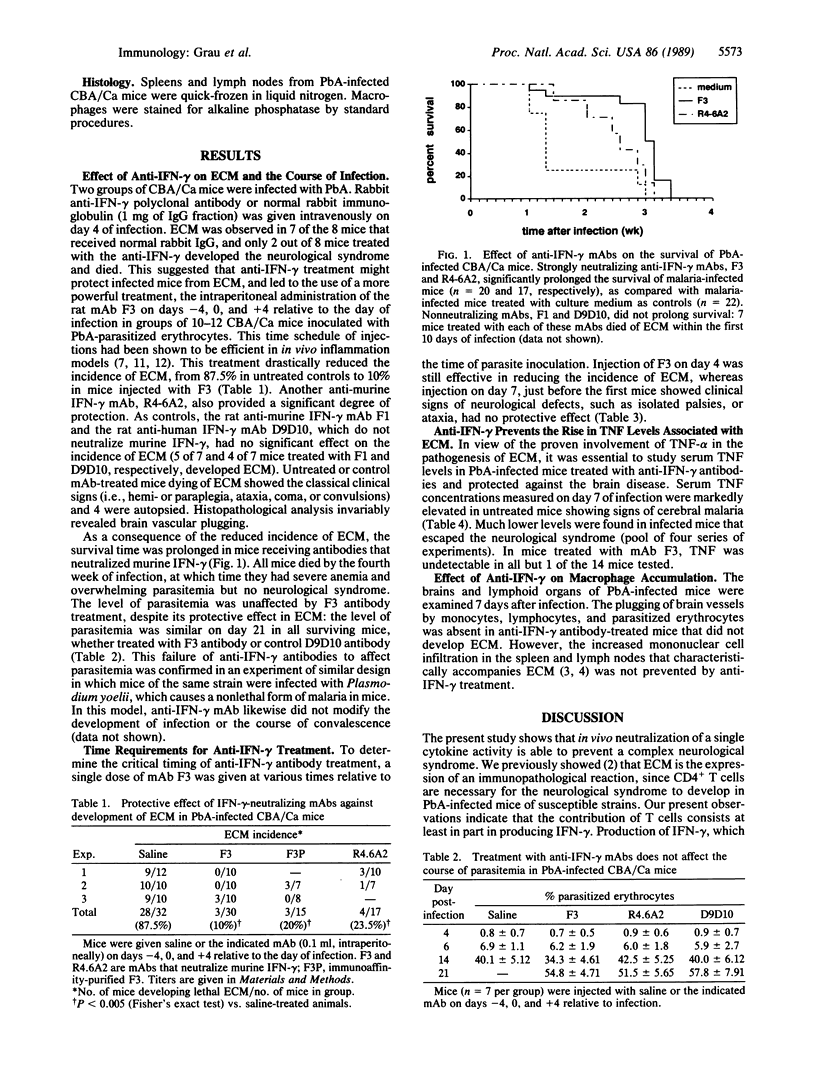
Experimental cerebral malaria (ECM), a lethal hyperacute neurological syndrome associated with high blood levels of tumor necrosis factor, develops in genetically susceptible (CBA/Ca) mice 7 days after infection with Plasmodium berghei ANKA strain. Injections of neutralizing monoclonal antibody against recombinant murine interferon gamma, not later than 4 days after infection, markedly reduced the incidence of ECM and the elevation in serum levels of tumor necrosis factor. This treatment prevented the cerebral lesions (plugging of brain vessels by monocytes, lymphocytes, and parasitized erythrocytes). In contrast, the extent of macrophage infiltration in lymphoid organs (which is a characteristic feature of mice developing ECM), as well as the course of infection, remained unaffected by the antibody treatment. Protected mice died at a later time of severe anemia and overwhelming parasitemia, the usual outcome of P. berghei infection in mice that are not susceptible to ECM. The present data indicate that interferon gamma constitutes an important link in the cytokine network that leads to brain vessel inflammation in experimental malaria. It is proposed that interferon gamma released by activated CD4+ T cells acts by augmenting both production and action of tumor necrosis factor.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal B. B., Eessalu T. E., Hass P. E. Characterization of receptors for human tumour necrosis factor and their regulation by gamma-interferon. Nature. 1985 Dec 19;318(6047):665–667. doi: 10.1038/318665a0. [DOI] [PubMed] [Google Scholar]
- Billiau A., Heremans H., Vandekerckhove F., Dijkmans R., Sobis H., Meulepas E., Carton H. Enhancement of experimental allergic encephalomyelitis in mice by antibodies against IFN-gamma. J Immunol. 1988 Mar 1;140(5):1506–1510. [PubMed] [Google Scholar]
- Billiau A., Heremans H., Vandekerckhove F., Dillen C. Anti-interferon-gamma antibody protects mice against the generalized Shwartzman reaction. Eur J Immunol. 1987 Dec;17(12):1851–1854. doi: 10.1002/eji.1830171228. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Hunt N. H., Butcher G. A., Cowden W. B. Inhibition of murine malaria (Plasmodium chabaudi) in vivo by recombinant interferon-gamma or tumor necrosis factor, and its enhancement by butylated hydroxyanisole. J Immunol. 1987 Nov 15;139(10):3493–3496. [PubMed] [Google Scholar]
- Collart M. A., Belin D., Vassalli J. D., de Kossodo S., Vassalli P. Gamma interferon enhances macrophage transcription of the tumor necrosis factor/cachectin, interleukin 1, and urokinase genes, which are controlled by short-lived repressors. J Exp Med. 1986 Dec 1;164(6):2113–2118. doi: 10.1084/jem.164.6.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druilhe P., Rhodes-Feuillette A., Canivet M., Gentilini M., Periês J. Circulating interferon in patients with Plasmodium falciparum, P. ovale and P. vivax malaria. Trans R Soc Trop Med Hyg. 1982;76(3):422–423. doi: 10.1016/0035-9203(82)90207-3. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Fajardo L. F., Piguet P. F., Allet B., Lambert P. H., Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987 Sep 4;237(4819):1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Kindler V., Piguet P. F., Lambert P. H., Vassalli P. Prevention of experimental cerebral malaria by anticytokine antibodies. Interleukin 3 and granulocyte macrophage colony-stimulating factor are intermediates in increased tumor necrosis factor production and macrophage accumulation. J Exp Med. 1988 Oct 1;168(4):1499–1504. doi: 10.1084/jem.168.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Piguet P. F., Engers H. D., Louis J. A., Vassalli P., Lambert P. H. L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. J Immunol. 1986 Oct 1;137(7):2348–2354. [PubMed] [Google Scholar]
- Heremans H., Dijkmans R., Sobis H., Vandekerckhove F., Billiau A. Regulation by interferons of the local inflammatory response to bacterial lipopolysaccharide. J Immunol. 1987 Jun 15;138(12):4175–4179. [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Le J., Vilcek J. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab Invest. 1987 Mar;56(3):234–248. [PubMed] [Google Scholar]
- Mellouk S., Maheshwari R. K., Rhodes-Feuillette A., Beaudoin R. L., Berbiguier N., Matile H., Miltgen F., Landau I., Pied S., Chigot J. P. Inhibitory activity of interferons and interleukin 1 on the development of Plasmodium falciparum in human hepatocyte cultures. J Immunol. 1987 Dec 15;139(12):4192–4195. [PubMed] [Google Scholar]
- Murray H. W., Spitalny G. L., Nathan C. F. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-gamma. J Immunol. 1985 Mar;134(3):1619–1622. [PubMed] [Google Scholar]
- Philip R., Epstein L. B. Tumour necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induced by itself, gamma-interferon and interleukin-1. Nature. 1986 Sep 4;323(6083):86–89. doi: 10.1038/323086a0. [DOI] [PubMed] [Google Scholar]
- Rhodes-Feuillette A., Bellosguardo M., Druilhe P., Ballet J. J., Chousterman S., Canivet M., Périès J. The interferon compartment of the immune response in human malaria: II. Presence of serum-interferon gamma following the acute attack. J Interferon Res. 1985 Winter;5(1):169–178. doi: 10.1089/jir.1985.5.169. [DOI] [PubMed] [Google Scholar]
- Ruff M. R., Gifford G. E. Purification and physico-chemical characterization of rabbit tumor necrosis factor. J Immunol. 1980 Oct;125(4):1671–1677. [PubMed] [Google Scholar]
- Sandvig S., Laskay T., Andersson J., De Ley M., Andersson U. Gamma-interferon is produced by CD3+ and CD3- lymphocytes. Immunol Rev. 1987 Jun;97:51–65. doi: 10.1111/j.1600-065x.1987.tb00516.x. [DOI] [PubMed] [Google Scholar]
- Schofield L., Ferreira A., Altszuler R., Nussenzweig V., Nussenzweig R. S. Interferon-gamma inhibits the intrahepatocytic development of malaria parasites in vitro. J Immunol. 1987 Sep 15;139(6):2020–2025. [PubMed] [Google Scholar]
- Schofield L., Villaquiran J., Ferreira A., Schellekens H., Nussenzweig R., Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987 Dec 17;330(6149):664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- Spitalny G. L., Havell E. A. Monoclonal antibody to murine gamma interferon inhibits lymphokine-induced antiviral and macrophage tumoricidal activities. J Exp Med. 1984 May 1;159(5):1560–1565. doi: 10.1084/jem.159.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge J. E., Bowersox O., Tribble H., Lee S. H., Shepard H. M., Liggitt D. Toxicity of tumor necrosis factor is synergistic with gamma-interferon and can be reduced with cyclooxygenase inhibitors. Am J Pathol. 1987 Sep;128(3):410–425. [PMC free article] [PubMed] [Google Scholar]
- Wyler D. J. Steroids are out in the treatment of cerebral malaria: what's next? J Infect Dis. 1988 Aug;158(2):320–324. doi: 10.1093/infdis/158.2.320. [DOI] [PubMed] [Google Scholar]



