Abstract
Sylvatic plague, caused by the bacterium Yersinia pestis, is a flea-borne disease that primarily affects rodents but has been detected in over 200 mammal species worldwide. Mammalian carnivores are routinely surveyed as sentinels of local plague activity, since they can present antibodies to Y. pestis infection but show few clinical signs. In Boulder County, Colorado, USA, plague epizootic events are episodic and occur in black-tailed prairie dogs. Enzootic hosts are unidentified as are plague foci. For three years, we systematically sampled carnivores in two distinct habitat types to determine whether carnivores may play a role in maintenance or transmission of Y. pestis and to identify habitats associated with increased plague prevalence. We sampled 83 individuals representing six carnivore species and found only two that had been exposed to Y. pestis. The low overall rate of plague exposure in carnivores suggests that plague may be ephemeral in this study system, and thus we cannot draw any conclusions regarding habitat-associated plague foci or temporal changes in plague activity. Plague epizootics involving prairie dogs were confirmed in this study system during two of the three years of this study, and we therefore suggest that the targeting carnivores to survey for plague may not be appropriate in all ecological systems.
Key Words: Boulder County, Colorado, Cynomys ludovicianus, disease ecology, pathogen dispersal, Yersinia pestis
Introduction
Yersinia pestis, the bacterium that causes plague, is highly pathogenic to a variety of mammal species, including the black-tailed prairie dog (Cynomys ludovicianus), which has been deemed a species of conservation concern (Reading et al. 2002). It is widely believed that plague is maintained by one or more enzootic, or maintenance, host species that are partially resistant to infection (Gage and Kosoy 2005). Enzootic periods are punctuated by epizootic events whereby an amplifying host becomes infected and plague spreads rapidly (Webb et al. 2006), potentially over long distances (Girard et al. 2004, Snäll et al. 2008). Although reservoir species for plague in North America have not been identified, likely candidates include species such as rodents and carnivores that show variation in susceptibility to plague (Barnes et al. 1982) and are found in close association with epizootic hosts such as prairie dogs (Gage and Kosoy 2005).
Carnivores are attractive candidates for plague reservoir species because they are able to acquire Y. pestis by multiple routes of infection. Similar to other mammal species, carnivores may become infected by bites from infected fleas. Dozens of flea species in western North America have been found to be naturally infected with plague (Hubbard 1968; Gage and Kosoy 2005) and carnivores routinely harbor fleas that are typical of other mammal species (Hubbard 1968; Lewis 2002; Brinkerhoff 2008), including prairie dogs (Brinkerhoff et al. unpublished data). Carnivores often acquire the fleas of their prey, as fleas will abandon a newly-dead host and immediately begin questing for a new host (Gage et al. 1994). Carnivorous mammals may also become infected by ingesting infected prey (Barnes 1982; Thomas et al. 1989), and then serving as an infectious source for fleas. During prairie dog plague epizootics, carnivores may be attracted to prairie dog colonies as infected prey may be more easily captured (Salkeld and Stapp 2006), thus increasing the likelihood of both flea-borne and ingestive routes of plague infection.
Carnivore species are often surveyed to determine levels of local plague activity (see Salkeld and Stapp 2006 for a review). Most studies of carnivores as potential reservoirs or sentinels of plague are done opportunistically (Salkeld and Stapp 2006) rather than being carried out systematically with the goal of testing specific hypotheses. Furthermore, the duration of Y. pestis antibody persistence in wild carnivores has not been established. Persistence of Y. pestis antibodies has been studied in feral and captive domestic dogs (Rust et al. 1971a, b; Taylor et al. 1981; Barnes 1982) as well as in captive wildlife species (Barnes 1982), but differences in stress levels among captive and free-ranging or wild and domestic species might cause differences in the duration of antibody response (Aviles and Monroy 2001). Given the uncertainty surrounding plague antibody persistence in wildlife species, it cannot be concluded that a high proportion of plague antibody-positive individuals necessarily indicates recent plague activity, since time of exposure cannot be accurately determined.
The timing of plague outbreaks in prairie dogs is irregular, suggesting that plague sporadically occurs in the plains habitats in which prairie dogs are found (Cully and Williams 2001). However, the sequence of recent plague outbreaks in prairie dogs in Boulder County, Colorado is suggestive of west-to-east transmission out of the Rocky Mountains (S. Collinge et al., unpublished data). Mechanisms to account for this pattern have not been described and may depend on mammal movement and seasonality of plague occurrence in different habitat types. Since prairie dogs do not occur in mountainous sites, the movement of plague from the foothills to the plains must depend on other species if plague is indeed resident in the front-range of the Rocky Mountains. Compared to rodents and other small-bodied mammals, carnivores have large home ranges and may travel long distances in relatively short periods of time (Rosatte 2002). During epizootic periods, plague can spread quite quickly over distances of tens of kilometers, a pattern that is more congruous with carnivore movement than with small rodent or prairie dog movement (Girard et al. 2004; Snäll et al. 2008). Furthermore, carnivore movement patterns may change during disease epizootics and can result in long-distance disease transmission by carnivores (Greenwood et al. 1997).
With the goal of identifying spatial patterns of plague prevalence and to determine seasonal patterns in plague emergence, we systematically tested wild-caught carnivores for plague exposure during spring and late summer months for three years. Specifically, our objective was to test the following hypotheses:
If the foothills of the Rocky Mountain Front Range serve as an enzootic focus of plague activity, carnivores sampled in this habitat type should show higher rates of plague exposure than carnivores sampled in grassland habitats.
Levels of plague exposure and plague prevalence in carnivores should be highest during spring and early summer months when plague epizootics are typically observed to begin to spread among prairie dog colonies
Plague exposure in carnivores should vary by species, with those that routinely prey on prairie dogs showing highest rates of exposure.
Materials and Methods
We sampled carnivores during spring (April-June) and late summer (August and September) trapping sessions at a total of six trapping locations in Boulder County, Colorado (40°00′54″ N, 105°16′12″ W) from 2004 to 2006. All trapping sites were located within two kilometers of active prairie dog colonies. In 2004, two sites (A and B) were trapped for three-week sessions consisting of one week of pre-baiting and two weeks of active trapping (Fig. 1). In 2005 and 2006, four sites (A, B, C, and D) were trapped seasonally for two-week trapping sessions with no pre-baiting. Sampling sites were categorized as either foothills sites (A and C) or grassland sites (B and D) based on topography and elevation with plains sites located at least 10 km from the foothills by straight-line distance. Foothills sites were characterized by relatively high elevation (roughly 1900 m), rocky outcrops, steep topography, and ponderosa pine forest. Grassland sites were characterized by relatively low elevation (roughly 1550 m), mixed agriculture consisting of cattle grazing and crop plantations, and riparian woodland. Supplemental trapping in response to local prairie dog die-offs was also conducted at one additional site in 2005 (site E) and 2006 (Site F); these sites were located less than 10 km from the foothills and were therefore not included in site-type comparisons of seroprevalence. Sites E and F were not sampled seasonally and are also excluded from seasonal comparisons.
FIG. 1.
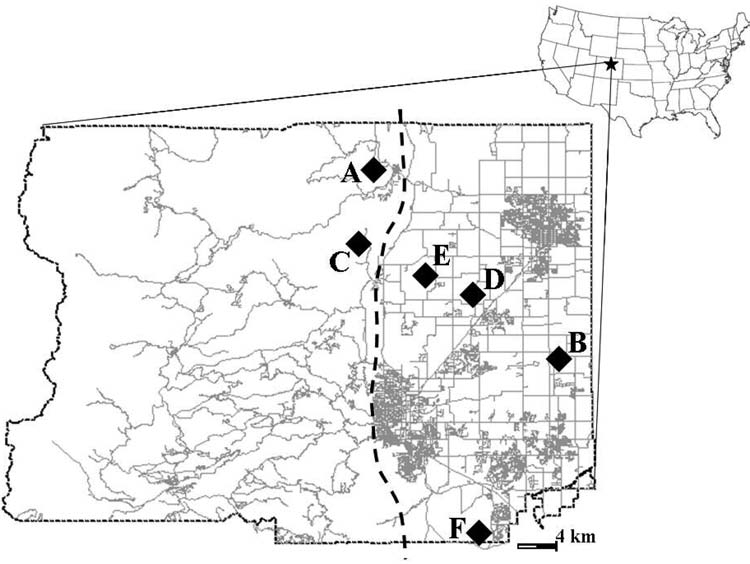
Map representing the location of Boulder County in Colorado as well as the locations of the six sites where carnivores were sampled. The dashed vertical line approximates the eastward extent of the Colorado Front Range foothills.
Animals were trapped with three sizes (17.8 × 17.8 × 50.8cm, 25.4 × 30.5 × 81.3cm, and 40.1 × 50.8 × 106.7cm) of wire box traps (Tomahawk Live Trap Inc, Tomahawk, WI) (2004-2006) and #1.5 and #3 Victor Coil Soft Catch™ leg-hold traps (Woodstream Corporation, Lititz, PA) (2005 and 2006). Box traps were placed in sets containing three traps, one of each size. Trapping sets were placed in locations predicted to experience carnivore visitation, such as among rock outcrops, along creek beds, or along stream banks. Box trap sets were separated by at least 25 meters. Leg-hold traps were placed in sets of two traps and were located in areas predicted to be frequented by foxes and coyotes. Box traps were baited with raw chicken and commercial trapping lures were used for leg-hold traps. Traps were open and set for six consecutive days per week for two weeks at a time (12 total nights per trap per site). In all cases, traps were checked daily between 0600 and 0930. Equivalent numbers of box and leg-hold traps were used at all sites throughout the study. In addition to carnivore trapping, we received blood samples from domestic cats (Felis catus) collected by a local veterinarian.
An intramuscular injection of Telazol (Fort Dodge Animal Health, Fort Dodge, IA), delivered by a 1 m pole syringe at a rate of 10 mg/kg, was used to chemically anesthetize trapped animals. Each individual was fitted with a uniquely numbered aluminum ear tag (National Band and Tag Co., Newport, KY) and 2–5 ml of blood was collected for pathogen screening. A small volume of blood (∼100ul) was first applied to a nobuto filter paper strip and dried. Remaining blood was divided between 2 ml cryovials (Nalgene Co, Rochester, NY), stored at −20°C, and 10 ml EDTA vacutainer tubes (Becton, Dickinson and Co., Franklin Lakes, NJ). We identified each host to species and collected sex, measurement, and body weight data before the sedation wore off (30-90 min). All trapping was done in accordance with the guidelines set by and with the approval of the Institutional Animal Care and Use Committee (IACUC) at the University of Colorado.
Nobuto strips with bloods applied were tested for antifraction I (F1) antibodies to Y. pestis. Passive hemagglutination testing was first performed for screening. Yersinia pestis F1 antigen was produced, purified, and evaluated at the Centers for Disease Control and Prevention, Fort Collins, Colorado. Any positive samples then were tested by inhibition tests for confirmation (Chu 2000). For samples where F1 antibodies were detected, DNA was extracted from the corresponding whole blood sample using Qiagen DNEasy blood and tissue kits (Qiagen, Inc., Valencia, CA), and the DNA was tested for presence of Y. pestis by multiplex polymerase chain reaction (PCR) using Y. pestis-specific primers (Stevenson et al. 2003). We used a binomial probability function to obtain a maximum likelihood estimate (MLE) of plague prevalence based on the observed rate of plague exposure in carnivores. We calculated a 95% confidence interval for the MLE by determining the range of log-likelihood (LL) values such that the maximum log-likelihood (MLL) minus LL was less than or equal to 1.92.
Results
We collected and tested blood samples from a total of 83 individual carnivores. Of these samples, 76 came from five species of wild carnivores and the remaining seven came from domestic cats (F. catus) (Table 1). Precise location data for domestic cat samples were not available; however, all domestic cat samples were collected in close spatial (<3 km) and temporal (<2 months) proximity to a confirmed sylvatic plague event. Two individual carnivores were seropositive for exposure to plague and all others were negative. The two positive individuals include one striped skunk, Mephitis mephitis sampled at site B in 2004 and one red fox, Vulpes vulpes, sampled at site C in 2005. Both individuals showed relatively high antibody titers: 1:128 for the striped skunk and 1:512 for the red fox, respectively. Y. pestis DNA was not detected in blood samples from either individual by PCR. The plague-positive M. mephitis was recaptured and re-tested on 15 May 2005, at which time no Y. pestis antibodies were detected. Our MLE of plague prevalence in carnivores in this study system is 2.6% with a 95% confidence interval of 0.4%-7.9% (Fig. 2).
Table 1.
Proportion of Each Carnivore Species from Each Habitat Type that Tested Positive for Y. Pestis Exposure with Sample Sizes in Parentheses
| Species | Foothills habitat | Grassland habitat | Other habitat type | TOTAL |
|---|---|---|---|---|
| Canis latrans | 0 (0/2) | 0 (0/5) | 0 (0/4) | 0 (0/11) |
| Vulpes vulpes | 33.3 (1/3) | 0 (0/0) | 0 (0/0) | 33.3 (1/3) |
| Mephitis mephitis | 0 (0/19) | 11.1 (1/9) | 0 (0/1) | 3.4 (1/29) |
| Spilogale gracilis | 0 (0/5) | 0 (0/0) | 0 (0/0) | 0 (0/5) |
| Procyon lotor | 0 (0/2) | 0 (0/26) | 0 (0/0) | 0 (0/28) |
| Felis catus | 0 (0/0) | 0 (0/0) | 0 (0/7) | 0 (0/7) |
| TOTAL | 2.4 (2/83) |
FIG. 2.
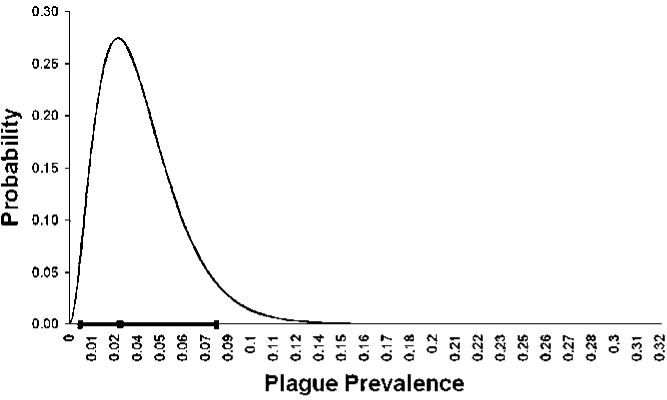
Probability density function across possible values of plague prevalence given our observed rate of plague exposure in Boulder County carnivores. The black dot on the x-axis represents the most likely prevalence value (2.6%) and the dark line represents the 95% confidence interval.
In 2004 there was no known plague activity in prairie dogs in Boulder County. In 2005, epizootic activity was observed in several prairie dog colonies and was also confirmed in samples collected from squirrels and fleas in the northern portion of the study area (Fig. 3). In 2006, several more plague events were observed, most of which were located in relatively close spatial proximity to the plague events of 2005 (Fig. 3). Twenty-one individual carnivores, representing five species, were sampled within two months and two kilometers of the plague events observed in 2005 and 2006. Only one of these 21 carnivores tested positive for plague exposure (Table 2).
FIG. 3.
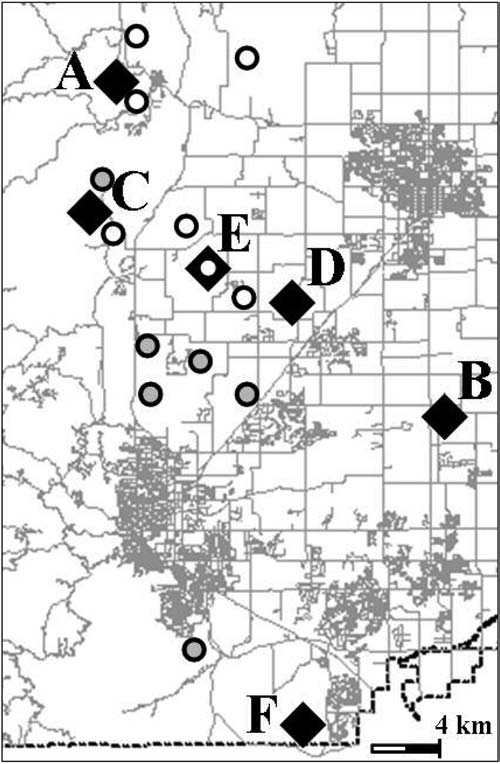
Locations of sampling sites relative to sites at which plague was observed in 2005 (open circles) and 2006 (gray-filled circles).
Table 2.
Individual Carnivores Sampled in Close Spatial and Temporal Proximity to Prairie Dog Die-Offs or Other Confirmed Plague Occurrence. All Individuals Tested Negative for Plague Exposure Except for One V. Vulpes, Marked with an Asterisk
| Carnivore species | Sampling site | Approximate distance to plague activity | Time between carnivore sampling and plague event |
|---|---|---|---|
| Canis latrans | E | 200m | 1 month |
| Canis latrans | D | 1.3 km | 1 month |
| Canis latrans | C | 1 km | 2 months |
| Mephitis mephitis | A | 500m | 2 weeks |
| Mephitis mephitis | A | 1 km | 2 months |
| Mephitis mephitis | C | 200 m | 2 months |
| Mephitis mephitis | C | 1 km | 2 months |
| Mephitis mephitis | C | 1.2 km | 1 month |
| Procyon lotor | C | 1 km | 1 month |
| Procyon lotor | C | 1 km | 2 months |
| Procyon lotor | D | 1.3 | 2 months |
| Procyon lotor | D | 1.3 | 2 months |
| Procyon lotor | D | 1.3 | 2 months |
| Procyon lotor | D | 1.3 | 2 months |
| Procyon lotor | D | 1.3 | 2 months |
| Procyon lotor | D | 1.3 | 2 months |
| Procyon lotor | D | 1.3 | 2 months |
| Procyon lotor | D | 1.3 | 1 months |
| Spilogale gracilis | A | 1 km | 2 months |
| Spilogale gracilis | C | 1km | 2 months |
| Vulpes vulpes* | C | 500m | 1 month |
Discussion
We detected very low rates of plague exposure among the six species of carnivores we tested in this study (Table 2). This result contrasts the general patterns of plague exposure in carnivores sampled in other western North American ecosystems (Salkeld and Stapp 2006). We found no evidence to support any of the hypotheses we tested. We failed to detect any relationship between plague exposure and habitat type, suggesting that habitat does not affect Y. pestis exposure in carnivores. We also failed to detect a relationship between plague exposure in carnivores and season; plague exposure in carnivores was not measurably higher during the early trapping session and did not coincide with epizootic plague events in prairie dogs. Although one plague-positive carnivore was detected in close spatial and temporal proximity to a prairie dog die-off, the other was detected in a year and a location in which no plague activity was observed. On the contrary, many individual carnivores of several species were sampled in close spatial and temporal proximity to plague epizootics in prairie dogs and tested negative for exposure to Y. pestis (Table 2). We were not able to draw any conclusions about interspecific variation in plague exposure and we found no evidence to suggest that common predators of prairie dogs, such as coyotes, are more likely to become exposed to plague than other carnivore species.
Our comprehensive estimate of plague prevalence in carnivores (2.6%; Fig. 2) is substantially lower than the mean value for western North America (15.9%, N = 21,826) reported by Salkeld and Stapp (2006). Differences between the results of this study and published records of plague exposure in carnivore species are intriguing and are not easily explained. For example, plague exposure in coyotes sampled in the western United States, as summarized by Salkeld and Stapp (2006), indicated a mean seroprevalence rate of 14% (N = 17,403). Coyote seroprevalence in this study was 0% (N = 11), though with our relatively small sample size, we cannot refute a non-zero prevalence rate; there is a 19% chance of finding 0/11 positive individuals assuming 14% exposure. However, we detected very low exposure to plague in species whose sample sizes were 2–3 times higher than coyotes: striped skunks showed seroprevalence of only 3.4% and we failed to detect any exposure to plague in raccoons. Previous reports of plague exposure for these species average 10% for striped skunks (N = 92) and 13% (N = 438) for raccoons (Salkeld and Stapp 2006). The probabilities associated with our observations (Table 1) assuming 10% and 13% prevalence rates for striped skunks and raccoons are 0.2 and 0.02, respectively. Moreover, the probability of encountering 2 positive carnivores out of a sample of 76 individuals assuming a seroprevalence rate of 15.9% (Salkeld and Stapp 2006) is roughly 0.0002.
The low rates of seroprevalence that we detected in carnivores could be explained by at least two causes. First, it is possible that Y. pestis is absent between epizootics or is present at nearly undetectable levels in this study system. Support for this hypothesis comes from the lack of detection of plague in other mammal species in this study area during inter-epizootic periods; repeated sampling of the small rodent community assumed to contain enzootic host species resulted in very low (<1/1000) rates of plague exposure both during and between epizootic events (S. Collinge et al., unpublished data). If a small rodent or carnivore reservoir species were to exist in this system, a higher measure of plague exposure would be expected. In lieu of a persistent reservoir population, it is possible that Y. pestis is periodically reintroduced into this study system from other locations. The observed patterns of plague activity in our system may be reflective of repeated introduction rather than low-level enzootic activity since very little evidence of plague exists between epizootic events and very few non-prairie dog mammals are found to have been exposed to Y. pestis during epizootic events.
Second, it is possible that exposed animals clear Y. pestis infection quickly and the detection window of a positive antibody response is short. Laboratory studies of domestic and wild carnivore species, as well as studies of free-ranging domestic dogs, indicate that positive titer responses are measurable for 4–8 months following an initial inoculation with Y. pestis (Rust et al. 1971b; Taylor et al. 1981; Barnes 1982). Our data suggest that antibody deterioration from a relatively high level titer count (1:128) to undetectable levels may be roughly equivalent between natural and laboratory conditions, though without intermittent samples between the initial positive sample and the negative sample collected several months later, we cannot precisely assess the duration Y. pestis antibodies persistence.
Y. pestis antibody persistence in wild carnivore species is rarely discussed as a factor that might influence the results of plague surveillance studies and precise estimates of antibody persistence are unavailable for wildlife species. However, the window of positive antibody detection is clearly long enough to result in non-zero levels of detection of plague exposure (Hopkins and Gresbrink 1982, Thomas and Hughes 1992, McGee et al. 2006, Salkeld and Stapp 2006), so it is unlikely that a short window of antibody presence would explain the low levels of plague detection in this study. Furthermore, plague-exposed carnivores may be found when no plague activity is observed in other species (e.g. Hopkins and Gresbrink 1982; Salkeld and Stapp 2006) suggesting that antibody persistence may be enough to remain detectable well beyond epizootic events.
Laboratory studies indicate that circulating Y. pestis antibody levels tend to peak three to four weeks following exposure (Rust et al. 1971b; Barnes 1982). In the field, this could result in a multi-month lag between plague activity in prairie dogs and antibody detection in carnivores. However, we found no evidence of this phenomenon; of the two individuals that tested positive for plague exposure, neither was sampled in the months following an observed epizootic event. To explain our observations, it is most reasonable to assume that very few individuals became exposed to Y. pestis during the epizootic events of 2005 and 2006.
The overall low seroprevalence of plague in carnivores observed in our study suggests that carnivore surveillance may not be a reliable measure of plague activity in this study system. Our data were collected from a highly localized area and it should be noted that plague ecology is likely to depend on the suite of potential reservoir and susceptible mammalian species in a given region. For example, the northern grasshopper mouse (Onychomys leucogaster) is a potentially important species to Y. pestis maintenance and transmission (Stapp 2007) that is absent from Boulder County, Colorado (S. Collinge et al., unpublished data). The dynamics of Y. pestis infection in Boulder County mammals may therefore make carnivore surveillance an unreliable method for determining local plague activity if mammalian species key to Y. pestis maintenance are lacking. Given the rates of Y. pestis exposure in carnivores reported from other systems where plague regularly occurs (e.g. Cavanaugh et al. 1965; Thomas and Hughes 1992), the most parsimonious explanation for our results is that Y. pestis is uncommon in Boulder County, between and during epizootic events.
The utility of carnivore serosurveys for plague is highest when conducted over large spatial scales and when tracking changes in plague activity in one or multiple locations over time. Such methods have been used to identify plague foci (Cavanaugh et al. 1965), although in some systems, plague prevalence in carnivores does not reconcile with disease rates in human or other mammal species (Hopkins and Gresbrink 1982). Carnivore serosurveys are often convenient and can be relatively cost-effective when they are done opportunistically or when they are “piggybacked” onto other wildlife projects. In many cases, such surveys can yield important information about variation in pathogen exposure across space and time. However, local pathogen transmission dynamics may preclude carnivore serosurveys from being a universally applicable surveillance technique.
Acknowledgments
This research was funded by grants to RJB from the Boulder County Parks and Open Space Department, the Boulder County Nature Association, the Museum of Natural History at the University of Colorado, the Colorado Chapter of the Wildlife Society, the Beverly Sears Fund, and the Department of Ecology and Evolutionary Biology at the University of Colorado. Additional support was provided though an Edna Bailey Sussman internship grant to RJB and research grants from the National Center for Environmental Research (NCER) STAR program of the US-EPA (R-82909101-0) and the NSF/NIH joint program in Ecology of Infectious Diseases (DEB-0224328) to SKC et al. Logistical support was provided by Mark Brennan, Sheldon Frost, Kevin Grady, Rob Alexander, Al Petkus, and Silvia Iorio. We would also like to thank Dave Armstrong, Jason Knouft, Andy Martin, Michelle Sauther, and two anonymous reviewers who provided comments on an earlier version of this manuscript.
Disclosure Statement
No competing interests exist.
References
- Aviles HO. Monroy FP. Toxoplasma gondii: cold stress-induced modulation of antibody responses. Exp Parasitol. 2001;99:89–96. doi: 10.1006/expr.2001.4658. [DOI] [PubMed] [Google Scholar]
- Bangert RK. Slobodchikoff CN. Conservation of prairie dog ecosystem engineering may support arthropod beta and gamma diversity. J Arid Environ. 2004;67:100–115. [Google Scholar]
- Barnes AM. Surveillance and control of bubonic plague in the United States. Symp Zool Soc of London. 1982;50:237–270. [Google Scholar]
- Brinkerhoff RJ. Habitat-associated differences in flea assemblages of striped skunks (Mephitis mephitis) Comp Parasitol. 2008;75:127–131. [Google Scholar]
- Cavanaugh DC. Thorpe BD. Bushman JB. Nicholes PS. Rust JH. Detection of an enzootic plague focus by serological methods. B World Health Organ. 1965;32:197–203. [PMC free article] [PubMed] [Google Scholar]
- Chu MC. Laboratory manual of plague diagnostic tests. Centers for Disease Control and Prevention, Division of Vector-Borne Diseases, Fort Collins, Colorado. 2000. p. 129.
- Collinge SK. Johnson WC. Ray C. Matchett R, et al. Testing the generality of a trophic cascade model for plague. Eco-Health. 2005a;2:102–112. [Google Scholar]
- Collinge SK. Johnson WC. Ray C. Matchett R, et al. Landscape structure and plague occurrence in black-tailed prairie dogs on grasslands of the western USA. Landscape Ecol. 2005b;20:941–955. [Google Scholar]
- Gage KL. Ostfeld RS. Olson JG. Nonviral vector-borne zoonoses associated with mammals in the United States. J Mammal. 1994;76:695–715. [Google Scholar]
- Gage KL. Kosoy MY. Natural history of plague: perspectives from more than a century of research. Annu Rev Entomol. 2005;50:505–528. doi: 10.1146/annurev.ento.50.071803.130337. [DOI] [PubMed] [Google Scholar]
- Gese EM. Schultz RD. Johnson MR. Williams ES, et al. Serological survey for diseases in free-ranging coyotes (Canis latrans) in Yellowstone National Park, Wyoming. J Wildl Dis. 1997;33:47–56. doi: 10.7589/0090-3558-33.1.47. [DOI] [PubMed] [Google Scholar]
- Girard JM. Wagner DM. Vogler AJ. Keys C. Allender CJ, et al. Differential plague-transmission dynamics determine Yersinia pestis population genetic structure on local, regional, and global scales. Proc Nat Acad Sci. 2004;101:8408–8413. doi: 10.1073/pnas.0401561101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood RJ. Newton WE. Pearson GL. Schamber GL. Population and movement characteristics of radio-collared striped skunks in North Dakota during an epizootic of rabies. J Wildl Dis. 1997;33:226–241. doi: 10.7589/0090-3558-33.2.226. [DOI] [PubMed] [Google Scholar]
- Hopkins DD. Gresbrink RA. Surveillance of sylvatic plague in Oregon by serotesting carnivores. Am J Public Health. 1982;72:1295–1297. doi: 10.2105/ajph.72.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard CA. Fleas of Western North America. Iowa State College Press; Ames, IA: 1968. 533 pp. [Google Scholar]
- Kausrud KL. Viljugrein H. Frigessi A. Begon M, et al. Climatically-driven synchrony of gerbil populations allows large-scale plague outbreaks. Proc Roy Soc B-Biol Sci. 2007;274:1963–1969. doi: 10.1098/rspb.2007.0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RE. A review of the North American species of Oropsylla Wagner and Ioff, 1926 (Siphonaptera: Ceratophyllidae: Ceratophyllinae) J Vect Ecol. 2002;27:184–206. [PubMed] [Google Scholar]
- McGee BK. Butler MJ. Pence DB. Alexander JL, et al. Possible vector dissemination by swift foxes following a plague epizootic in black-tailed prairie dogs in northwestern Texas. J Wildl Dis. 2006;42:415–420. doi: 10.7589/0090-3558-42.2.415. [DOI] [PubMed] [Google Scholar]
- Reading RP. Clark TW. McCain L. Miller BJ. Black-tailed prairie dog conservation: a new approach for a 21st century challenge. End Sp Update. 2002;19:162–178. [Google Scholar]
- Rosatte RC. Long distance movement by a coyote, Canis latrans, and red fox, Vulpes vulpes, in Ontario: implications for disease spread. Can Field Nat. 2002;116:129–131. [Google Scholar]
- Rust JH. Cavanaugh DC. O'Shita R. Marshall JD. The role of domestic animals in the epidemiology of plague. I. Experimental infection of dogs and cats. J Infect Dis. 1971;124:522–526. doi: 10.1093/infdis/124.5.522. [DOI] [PubMed] [Google Scholar]
- Rust JH. Miller BE. Bahamnyar M. Marshall JD, et al. The role of domestic animals in the epidemiology of plague. II. Antibody to Yersinia pestis in sera of dogs and cats. J Infect Dis. 1971;124:527–531. doi: 10.1093/infdis/124.5.527. [DOI] [PubMed] [Google Scholar]
- Salkeld DJ. Stapp P. Seroprevalence rates and transmission of plague (Yersinia pestis) in mammalian carnivores. Vector-Borne Zoonot. 2006;6:231–239. doi: 10.1089/vbz.2006.6.231. [DOI] [PubMed] [Google Scholar]
- Salkeld DJ. Eisen R. Stapp P. Wilder AP, et al. The role of swift foxes (Vulpes velox) and their fleas in plague (Yersinia pestis) outbreaks. J Wildl Dis. 2007;42:425–431. doi: 10.7589/0090-3558-43.3.425. [DOI] [PubMed] [Google Scholar]
- Snäll T. O'Hara R. Ray C. Collinge SK. Climate-driven spatial dynamics of plague among prairie dog colonies. Am Nat. 2008;171:238–248. doi: 10.1086/525051. [DOI] [PubMed] [Google Scholar]
- Stapp P. Antolin MF. Ball M. Patterns of extinction in prairie-dog metapopulations: plague outbreaks follow El Nino events. Front Ecol Environ. 2004;2:235–240. [Google Scholar]
- Stapp P. Rodent communities in active and inactive colonies of black-tailed prairie dogs in short grass steppe. J Mammal. 2007;88:241–249. [Google Scholar]
- Stevenson HL. Bai Y. Kosoy MY. Montenieri JA, et al. Detection of novel Bartonella strains and Yersinia pestis in prairie dogs and their fleas (Siphonaptera: ceratophyllidae and pulicidae) using multiplex polymerase chain reaction. J Med Entomol. 2003;40:329–337. doi: 10.1603/0022-2585-40.3.329. [DOI] [PubMed] [Google Scholar]
- Taylor P. Gordon DH. Isaacson M. The status of plague in Zimbabwe. Ann Trop Med Parasit. 2003;75:165–173. [Google Scholar]
- Thomas RE. Beard ML. Quan TJ. Carter LG, et al. Experimentally indiced plague onfection in the northern grasshopper mouse (Onychomys leucopus) acquired by consumption of infected prey. J Wildl Dis. 1989;25:477–480. doi: 10.7589/0090-3558-25.4.477. [DOI] [PubMed] [Google Scholar]
- Thomas CU. Hughes PE. Plague surveillance by serological testing of coyotes (Canis latrans) in Los Angeles County, California. J Wildl Dis. 1992;28:610–613. doi: 10.7589/0090-3558-28.4.610. [DOI] [PubMed] [Google Scholar]
- Webb CT. Brooks CP. Gage KL. Antolin MF. Classic flea-borne transmission does not drive plague epizootics in prairie dogs. Proc Nat Acad Sci. 2006;103:6236–6241. doi: 10.1073/pnas.0510090103. [DOI] [PMC free article] [PubMed] [Google Scholar]


