Abstract
Purpose
We prospectively determined whether the change in tumor volume (TV) during 2 months of neoadjuvant androgen suppression therapy (nAST) measured using conventional 1.5 Tesla endorectal magnetic resonance imaging (eMRI) was associated with the risk of recurrence after radiation (RT) and 6 months of AST.
Patients and Methods
Between 1997 and 2001, 180 men with clinical stage T1c-T3cN0M0 adenocarcinoma of the prostate were registered. Fifteen were found to be ineligible and the institutional MR radiologist could not assess the TV in 32, leaving 133 for analysis. Multivariable Cox regression analysis was used to assess whether a significant association existed between eMRI-defined TV progression during nAST and time to recurrence adjusting for prostate-specific antigen (PSA) level, Gleason score (8 to 10 or 7 vs. 6 or less) and stage (T3 vs. T1-2).
Results
After a median follow up of 6.7 years and adjusting for known prognostic factors, there was a significant increase in the risk of PSA failure (HR, 2.3 [95% CI, 1.1–4.5; p = 0.025) in men with eMRI-defined TV progression during nAST. Specifically, adjusted estimates of PSA failure were significantly higher (p = 0.032) in men with, compared with men without, eMRI-defined TV progression reaching 38% vs. 19%, respectively, by 5 years.
Conclusion
Eradicating intraprostatic hormone refractory prostate cancer (HRPC) by maximizing local control and randomized trials assessing whether survival is improved when agents active against HRPC are combined with maximal local therapy are needed in men who progress based on eMRI during nAST.
Keywords: Prostate cancer, Magnetic resonance imaging, Radiation therapy, Androgen suppression therapy
INTRODUCTION
Imaging localized prostate cancer remains a diagnostic challenge. The results of the Radiology Diagnostic Oncology Group–sponsored prospective randomized trial (1) found no benefit for pelvic magnetic resonance imaging (MRI) performed with a body coil over transrectal ultrasound (TRUS) for staging men with clinically localized prostate cancer. Single-institution studies (2–4) performing MRI with an endorectal (e) coil have shown promise in men with palpable and intermediate- or high-risk disease where the overall accuracy of these modalities approximate 80% for the identification of occult extraprostatic disease. However, for the majority of men who present based on serial prostate-specific antigen (PSA) screening with nonpalpable disease, which imaging modality, if any, provides reliable information about the local extent of the disease remains an open question.
While defining the anatomic extent of prostate cancer at presentation remains under investigation (5) and important in guiding management, the ability to define tumor response to neoadjuvant therapy based on imaging remains a high priority because it provides the early identification of men with disease that is resistant to current standards of care. After these men are identified, they would be eligible for entry to randomized clinical trials designed to evaluate the impact on survival of adding therapies aimed at overcoming treatment resistance to the current standards of practice. For this reason the Cancer and Leukemia Group B (CALGB) performed a prospective assessment of the ability of eMRI to provide this information in men undergoing external beam radiation therapy (RT) and 6 months of androgen suppression therapy (AST) for prostate cancer. Here we report the results of that study whose purpose was to assess whether an association existed between tumor volume (TV) progression defined using eMRI during 2 months of neoadjuvant androgen suppression therapy (nAST) and time to PSA failure after registration adjusting for PSA level, Gleason score, and clinical stage in men undergoing RT and 6 months of AST.
PATIENTS AND METHODS
Patient population and registration
Between May 31, 1997, and April 30, 2001, 180 men (median age, 70 [range, 52–81] years) with 1992 American Joint Commission on Cancer clinical stage (6) T1c-T3cN0M0 adenocarcinoma of the prostate cancer as per the digital rectal examination and who had an Eastern Cooperative Oncology Group performance status of 1 or less were enrolled. Each patient was registered through a main member institution prior to the initiation of therapy. Patients were excluded if they had prior radiation therapy to the pelvis or AST or chemotherapy for prostate cancer. All prostate needle biopsy specimens underwent central review by a pathologist with expertise in genitourinary pathology. All men also had a bone scan and a computed tomographic or MRI scan of the pelvis. Patients were excluded if they were found to have radiographic evidence of pelvic lymph node or distant metastatic disease.
This trial has been registered on the National Institutes of Health website (http://www.clinicaltrials.gov, with NCT# 00002889). Before study entry, all men signed an institution review board–approved, protocol-specific informed consent form in accordance with federal and institutional guidelines. Patient registration and data collection were managed by the CALGB Statistical Center. Data quality was ensured by careful review of data by CALGB Statistical Center staff and by the study chairperson.
eMRI protocol
Before beginning the study, all radiologists met and agreed on the criteria for interpretation and designed and approved the imaging data forms. The meeting also involved review of multiple cases to ensure reader agreement on the definition of focal tumor, focal low signal area in the peripheral zone, without corresponding T1W abnormality. In addition, the radiology principal investigator was available for questions throughout the study by telephone, e-mail, and regularly scheduled conference calls.
A conventional 1.5-Tesla magnet (General Electric Health Care, Milwaukee, WI) was used for all MRI studies. The imaging technique involved: external multicoil arrays combined with an endorectal coil (Medrad Inc, Indianola, PA) that were used to allow imaging of the entire prostate and surrounding structures (7). Baseline prostate images were not obtained until at least 3 weeks (median, 1.0 [range, 0.8–3.0] months) after prostate biopsy to reduce the artifact that can be introduced from biopsy related intraprostatic hemorrhage (2). All prostate images were obtained using the same parameters, with both T2W and T1W sequences: a 10–12 cm field of view and a 256× 256 matrix interpolated to 512 pixels (256 pixel pairs) yielded a resolution of 0.4 mm per pixel pair. This can be compared with a 30-cm field of view when the body coil is used, which yields a resolution of 1.2 mm per pixel pair. The T2W long repetition time/echo time pulse sequences used in this study were as follows: fast spin echo, 4000/100eff, section thickness 3 mm, interleaved acquisition (3-mm skip), field of view 10 cm, 256 × 256 matrix, right-to-left phase encoding, no phase wrap, flow compensation, 90° flip angle, and axial, sagittal, and angled coronal planes.
For all MRI examinations, the radiologists were blinded to the clinical data. Prostate cancer was detected on either axial, sagittal, or coronal T2-weighted MRIs as focal areas of hypointense signal, located in the peripheral zone by the institutional body MRI radiologist; all other causes of low T2-weighted signal, such as postbiopsy hemorrhage, were ruled out by comparison with T1-weighted images (8). All tumor volumes (TV) were measured along three perpendicular axes and summed. The volumes were calculated using an ellipsoid approximation per the formula: volume = π/6 × length (anterior to posterior) × width (right to left) × height (superior to inferior).
Definition of radiologic progression
Disease progression on the second eMRI was defined as a >10% increase in the eMRI-defined TV measured after 2 months of nAST when compared with baseline. This definition was selected before the study initiation and was derived from a pilot study (9) showing that TV changes <5% could arise from measurement differences when different radiologists read the same pair of scans. By selecting a >10% change, we ensured that the cut point was outside the coefficient of variation when the same difference was measured by multiple readers. The percent change in TV was calculated using the formula: 100% × [TV at 2 months – TV at baseline]/[TV at baseline].
Treatment technique and quality assurance
Androgen suppression therapy consisted of 6 months of both a luteinizing hormone–releasing hormone agonist (leuprolide acetate) or goserelin and an antiandrogen (flutamide). The AST was administered for 2 months after the baseline eMRI, 2 months throughout RT, and then for 2 months after the RT. The choice of the luteinizing hormone–releasing hormone agonist was left to physicians’ discretion, but flutamide use was required. The recommended dosing for leuprolide acetate was 7.5 mg intramuscularly each month or 22.5 mg intramuscularly every 12 weeks and flutamide 250 mg by mouth three times daily. The alternative treatment that was used was goserelin at 3.6 mg subcutaneously each month or 10.8 mg subcutaneously every 12 weeks and flutamide 250 mg by mouth three times daily. The flutamide was to be administered at least 24 h before the start of leuprolide acetate or goserelin.
External beam RT was delivered using photons of at least 10 million volts using a three-dimensional conformal technique. A four-field technique or any other technique that optimized the dose distribution was permitted. Field shaping was performed with Cerrobend blocks or multileaf collimators that were at least five half-value layers thick. The target volumes were defined at the time of simulation. The original target volume included the prostate and seminal vesicles and any other gross disease as defined based on the baseline eMRI with a 1.0–1.5 cm margin. This prescription point was defined as the isocenter of the initial target volume and that point received 4,500 centigray (cGy) in 25 180-cGy fractions. The boost volume included the prostate and any other gross disease as defined based on the baseline eMRI with a minimum of 1.0-cm margin; this volume received 2,160 cGy delivered in 12 180-cGy fractions. RT was delivered daily, Monday through Friday, and 95% normalization was used, meaning that the dose to the prescription point was 70 Gy (66.6 × 1.05). All radiation fields including margins, technique, and dose were reviewed by the principal investigator to ensure that the treatment technique complied with protocol guidelines before the start of RT.
As part of the quality assurance program of the CALGB, members of the Data Audit Committee visit all participating institutions at least once every 3 years to review source documents. The auditors verify compliance with federal regulations and protocol requirements, including those pertaining to eligibility, treatment, adverse events, tumor response, and outcome in a sample of protocols at each institution. Such onsite review of medical records was performed for a subgroup of 14 patients (8%) of the 180 men treated under this study.
METHODS
Sample size calculation
The target sample size was 180 men and sample size was calculated based on the following assumptions: an accrual rate of 60 patients per year over a 35-month accrual period with a 24-month follow-up period, an equal proportion of patients were present in both groups (TV progression versus no progression). Furthermore, time to PSA failure was assumed to follow an exponential distribution. The power to detect an increased recurrence risk in men who progress on eMRI during nAST or a hazard ratio of 1.80 (equivalent to an increase in median time to PSA failure from 24 months in nonresponders to 43 months in responders, respectively) was 80% assuming a two-sided significance level of 0.05. Under the alternative hypothesis, 92 events (biochemical progressions) were expected at the end of the follow-up period.
Primary end point
The primary end point of this study was time to PSA failure. PSA failure was defined as a PSA >1.0 ng/mL and increasing by more than 0.2 ng/mL at two consecutive visits after treatment as well as per the former American Society of Therapeutic Radiology and Oncology consensus definition (10) (i.e., three consecutive rises with the date of PSA failure defined as the mid point between the PSA nadir and first rise). However, all men in this study who experienced a PSA nadir had the opportunity to achieve at least a 2 ng/mL rise in the PSA level above the nadir and before the initiation of AST for PSA failure because of the protocol requirement that salvage AST would be recommended as the PSA level approached 10 ng/mL. Therefore we were able to use the 2006 American Society of Therapeutic Radiology and Oncology consensus (Phoenix) definition (11) of a PSA value exceeding the PSA nadir + 2 ng/mL to define PSA recurrence in the current study. A single rise in PSA was also considered PSA failure if accompanied by a positive bone scan, pelvic MRI or computed tomography, or reinitiation of hormonal therapy or any other objective measure of disease.
Follow-up
Follow-up started on the day of registration and concluded on January 26, 2007, or the date of death, whichever came first; no patient was lost to follow-up. Patients were seen every 6 months for 3 years and annually thereafter. At each follow-up, a history and physical examination including a digital rectal examination, blood pressure, height, weight, an assessment of the Eastern Cooperative Oncology Group performance status, and a serum PSA level before the digital rectal examination was performed. At the time of PSA failure, in addition to the routine follow-up assessment, a pelvic computed tomography or MRI and a bone scan were also obtained.
Time to PSA failure analyses
Multivariable Cox regression analysis (12) was used to assess whether an association existed between eMRI-defined TV progression and time to PSA failure after registration, adjusting for baseline PSA level (continuous), Gleason score (8–10 or 7 vs. 6 or less), and clinical stage (T3 versus T1, T2). Treatment was initiated within 4 weeks of registration. The PSA values at registration were log transformed so that these values followed a normal distribution. The assumptions of the proportional hazards model were tested and no evidence was found against these assumptions. Hazard ratios (12) for PSA failure with associated 95% CI and p values were calculated for each covariate. PSA values were assessed for confounding from urinary retention, colonoscopy, cystoscopy, or Foley insertion within 1 week of the PSA assessment or prostatitis; if any of these were present at the time the PSA was measured (n = 2), these PSA values were excluded from the analyses. For the purpose of illustration, the method of Kaplan-Meier (13) was used to estimate freedom from PSA failure and the log–rank test was used to compare these estimates across men with or without evidence of eMRI-defined TV progression. These estimates were also displayed after adjusting for the covariates of PSA, Gleason score, and clinical stage. Men who did not experience an event were censored on the date of last follow-up. A chi-square metric (14) was used to compare the distributions of baseline patient and tumor characteristics at registration among men who experiences progression or not on eMRI during nAST. For the case of a small sample sizes in some groups (i.e., Race, Eastern Cooperative Oncology Group performance status), a Fisher exact test was used. All statistical tests were two-sided and a p value of 0.05 or less was considered statistically significant. CALGB statisticians performed all statistical analyses.
RESULTS
Description of the study cohort
Seven institutions contributed men to this study, of which 90% were from community-based centers. Of the 180 men registered, as shown in Fig. 1, 15 were ineligible because of having an inappropriate dose, technique and type of radiation, consent withdrawn, or no PSA data. TV could not be completely assessed by the institutional radiologist in 32 men who were excluded from the analysis, leaving 133 men who composed the study cohort. Of the 32 men in whom TV could not be completely assessed, the reasons included obscuring of the cancer from slow resolution of hemorrhage artifact from the biopsy in 22, large amounts of benign prostatic hyperplasia causing significant compression of the peripheral zone in 9, and cancer suspected but under the limit of detection of 0.5 mL in 1. Men who experienced TV progression on eMRI during nAST were significantly more likely to have Gleason score 8–10 disease (p = 0.03) or a PSA level of 4 ng/mL or less (p < 0.0001). A summary of the pretreatment baseline clinical and tumor characteristics of the 133 men composing the study cohort are listed in Table 1, stratified by whether they had eMRI TV progression during nAST or not and a diagram of the patient flow through the study is shown in Fig. 1.
Fig. 1.
A CONSORT patient flow diagram of the prospective Phase 2 assessment of the prognostic significance of changes in endorectal magnetic resonance imaging (eMRI) during neoadjuvant androgen suppression therapy (nAST) for the 180 registered men.
Table 1.
Comparison of the distribution of patient and tumor characteristics of the 133 men at registration stratified by whether they had eMRI-defined tumor volume progression or not after 2 months of neoadjuvant androgen suppression therapy
| Clinical characteristics | Number (%) | |
|---|---|---|
| Percent change in the eMRI-defined tumor volume during neoadjuvant AST | ||
| >10% increase | 22 (17%) | |
| <10% increase or decrease | 8 (6%) | |
| >10% decrease | 103 (77%) | |
| Prostate-specific antigen (PSA) level (ng/mL) | ||
| Median (IQR): 10.7 (6.3–19.1) | ||
| eMRI TV progression (n = 22) | No eMRI TV progression (n = 111) | |
| <4 | 8 (36%) | 5 (5%) |
| >4–10 | 6 (27%) | 44 (40%) |
| >10–20 | 5 (23%) | 34 (31%) |
| >20 | 3 (14%) | 28 (25%) |
| p value < 0.001 | ||
| Biopsy Gleason score | ||
| <6 | 7 (32%) | 30 (27%) |
| 7 | 6 (27%) | 61 (55%) |
| 8–10 | 9 (41%) | 20 (18%) |
| p value = 0.03 | ||
| Clinical tumor (T) category (6) | ||
| T1c | 6 (27%) | 29 (26%) |
| T2a | 5 (23%) | 22 (20%) |
| T2b | 4 (18%) | 22 (20%) |
| T2c | 3 (14%) | 14 (13%) |
| T3a | 3 (14%) | 12 (11%) |
| T3b | 0 (0%) | 5 (5%) |
| T3c | 1 (5%) | 7 (6%) |
| p value = 0.97 | ||
| Patient age at registration (y) | ||
| Median (IQR) 70 (65–74) | ||
| <60 | 5 (23%) | 14 (13%) |
| 61–64 | 0 (0%) | 14 (13%) |
| 65–69 | 3 (14%) | 31 (28%) |
| 70–74 | 10 (45%) | 29 (26%) |
| >75 | 4 (18%) | 23 (21%) |
| p value = 0.09 | ||
| Race | ||
| Caucasian | 18 (81%) | 98 (88%) |
| African-American | 3 (14%) | 11 (10%) |
| Other | 1 (5%) | 2 (2%) |
| p value = 0.47 | ||
| ECOG performance status | ||
| 0 | 21 (95%) | 109 (98%) |
| 1 | 1 (5%) | 2 (2%) |
| p value = 0.42 | ||
Abbreviations: TV = tumor volume; AST = androgen suppression therapy; eMRI = endorectal magnetic resonance image; IQR = interquartile range; ECOG = Eastern Cooperative Oncology Group.
Percentages may not sum to 100% because of rounding.
Tumor volume changes on eMRI and time to PSA failure
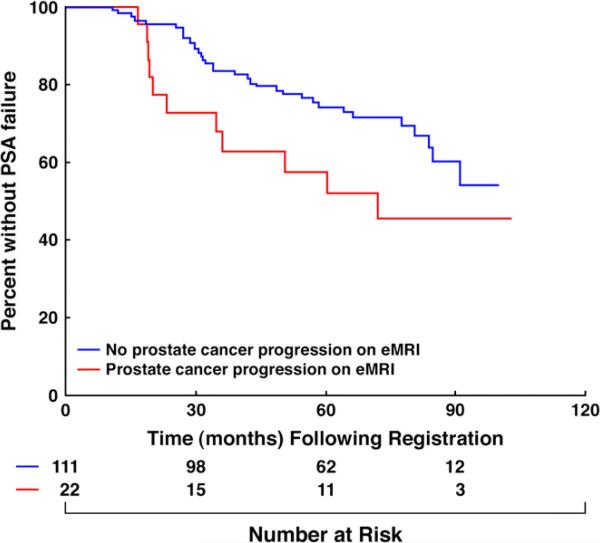
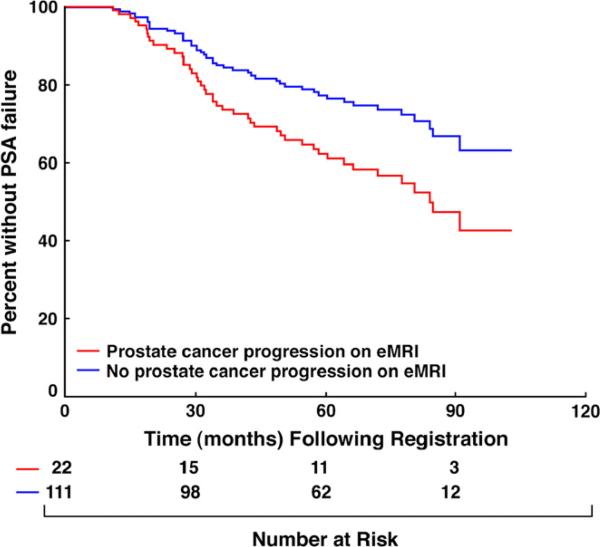
After a median follow-up of 6.7 years in living men, 45 men experienced PSA failure; 42 based on the Phoenix definition and 3 because of the initiation of salvage AST for an initial posttreatment median PSA level of 13.3 ng/mL (range, 6.3–22.6 ng/mL). The time in months when 50% of men who progressed based on eMRI vs. all others had experienced PSA failure was 72 months and not estimable because of censorship, respectively. After adjusting for known prognostic factors, there was a significant increase in the risk of PSA failure (HR, 2.3 [95% CI, 1.1–4.5]; p = 0.025) in men with eMRI-defined TV progression during nAST compared with those without TV progression. This increase in risk approached significance (HR, 1.9 [1.0– 3.8]; p = 0.06) in the unadjusted analysis as shown in Table 2 and illustrated in Fig. 2. Estimates of PSA failure adjusted for PSA level, Gleason score, and clinical T-category were significantly higher (adjusted log–rank p value = 0.032) in men with, compared with men without, eMRI-defined TV progression during nAST reaching 38% (95% CI, 16–54) vs. 19% (95% CI, 11–27) respectively by 5 years as illustrated in Fig. 3.
Table 2.
Hazard ratios (12) for prostate-specific antigen failure (11) and associated p values for the time to prostate-specific antigen failure analyses
| Covariate* | Number of men | Number of events | Analysis type | HR (95% CI) | p value |
|---|---|---|---|---|---|
| eMRI-defined TV progression | 22 | 11 | Adjusted | 2.3 (1.1–4.9) | 0.025 |
| Unadjusted | 1.9 (1.0–3.8) | 0.06 | |||
| Baseline PSA level in ng/mL (continuous)† | 133 | 45 | Adjusted | 1.02 (1.007–1.03) | 0.001 |
| Baseline eMRI defined TV in mL (continuous) | 133 | 45 | Adjusted | 1.01 (0.99–1.03) | 0.43 |
| Gleason score 8–10 | 29 | 16 | Adjusted | 1.8 (0.84–3.7) | 0.13 |
| Gleason score 7 | 67 | 16 | Adjusted | 0.68 (0.32–1.4) | 0.31 |
| Clinical stage T3N0M0 | 28 | 15 | Adjusted | 1.9 (0.95–4.0) | 0.07 |
Abbreviations: ASTRO = American Society for Therapeutic Radiology and Oncology; HR = hazard ratio; CI = confidence interval; eMRI: = endorectal magnetic resonance imaging; TV = tumor volume; PSA = prostate-specific antigen.
Baseline groups for categorical covariates are no eMRI-defined TV progression (n = 111 and 34 events), Gleason score <6 (n = 37 and 13 events) and clinical stage T1,2N0M0 (n = 105 and 30 events) (6).
PSA levels were log transformed.
Fig. 2.
Kaplan Meier estimates (13) of prostate-specific antigen failure-free survival (11) stratified by the presence or absence of endorectal magnetic resonance imaging–defined tumor progression during neoadjuvant androgen suppression therapy. Log–rank p value = 0.06.
Fig. 3.
Prostate-specific antigen (PSA) failure-free survival (11) estimates stratified by the presence or absence of endorectal magnetic resonance imaging–defined tumor progression during neoadjuvant androgen suppression therapy and adjusted for PSA level, Gleason score, and clinical stage. Adjusted log–rank p value = 0.032. eMRI = endorectal magnetic resonance imaging.
DISCUSSION
In the current multi-institutional CALGB study, 133 men treated with RT and 6 months of AST for T1c-T3c adenocarcinoma of the prostate underwent assessment of the eMRI-defined TV at baseline and after 2 months of nAST. The results revealed a statistically significant increase in the risk of PSA failure after registration in men with TV progression on eMRI during 2 months of nAST after adjusting for known prognostic factors.
To our knowledge, this is the first study to provide physicians with the ability to identify what appears to be intraprostatic hormone-refractory prostate cancer (HRPC) and a resulting increased risk of recurrence after RT and AST after only 2 months of nAST using 1.5 Tesla eMRI scan, a routinely available imaging device. The clinical significance of this finding is that it permits the early identification of men for randomized clinical trials designed to evaluate whether survival is improved when therapies (15, 16) active against HRPC are combined with RT and AST. In addition, it argues for complete ablation of the prostate using radical prostatectomy or high-dose radiation techniques to eradicate intraprostatic HRPC in men who progress on eMRI despite the use of nAST. Both of these efforts should lead to a decrease in recurrence, thereby reducing the expense of long-term AST at relapse and subsequent therapy that should favorably impact the cost-effectiveness of the two eMRI studies.
Several points require further consideration. First, the primary end point of this study, PSA failure, has been shown to represent a clinical heterogeneous population characterized by the PSA doubling time (17, 18). However, given that PSA failure was calculated using the 2006 American Society of Therapeutic Radiology and Oncology consensus definition (11), there is a high likelihood that many of the PSA failure events observed in this study will translate into metastases and cancer death because this definition of PSA failure (11) has been shown to be significantly associated with these clinical end points. Second, prior studies (19–22) have suggested that the value of the PSA nadir after AST in similar and more advanced disease states then in the current study was significantly associated with time to PSA failure. However, some men in those studies (19–22) and others (23) with an undetectable serum PSA after AST subsequently become androgen independent and died of prostate cancer. Therefore some men with an undetectable serum PSA may have persistent intraprostatic HRPC that this study has shown serial eMRI can identify. Therefore, further study is needed to assess whether the information provided on time to PSA recurrence from the value of the PSA nadir in the neoadjuvant setting or from the changes seen in the eMRI-defined TV after 2 months of AST are independent or associated. Third, 17% of men in this study were observed to have TV progression by eMRI criteria during nAST. Whether intraprostatic eMRI-defined TV progression and a subsequent higher risk of PSA failure was due to elevated intraprostatic androgen levels (24) despite castrate serum levels of testosterone requires further study. Finally, community radiologists rendered 90% of the eMRI readings in this study. We believe that this is a significant strength of the study because it shows that the results are generalizable to the community setting where most of medicine is practiced.
In conclusion, these data provide evidence supporting that image-based biomarkers of hormonal resistance in men treated using RT and AST add significantly to other conventional predictors of recurrence such as Gleason score, PSA level, and clinical stage. Specifically, after adjusting for known prognostic factors, men with eMRI-defined TV progression during nAST remained at high risk for recurrence. Therefore eradicating intraprostatic HRPC by maximizing local control and randomized trials assessing whether survival is improved when agents active against HRPC are combined with maximal local therapy are needed in men who progress based on eMRI during nAST.
Acknowledgments
University of Chicago, Chicago, IL: Gini Fleming, M.D., supported by CA41287; CALGB Statistical Center, Duke University Medical Center, Durham, NC: Stephen George, Ph.D., supported by CA33601; Dana-Farber Cancer Institute, Boston, MA: Eric P. Winer, M.D., supported by CA32291; Memorial Sloan-Kettering Cancer Center, New York, NY: Clifford Hudis, M.D., supported by CA77651; Medical University of South Carolina, Charleston, SC: Mark Green, M.D., supported by CA03927; Vermont Cancer Center, Burlington, VT: Hyman B. Muss, M.D., supported by CA77406; University of California at San Francisco, San Francisco, CA: Alan P. Venook, M.D., supported by CA60138. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Research for CALGB 9682 was supported, in part, by grants from the National Cancer Institute (CA31946) to the Cancer and Leukemia Group B (Richard L. Schilsky, MD, Chairman) and to the CALGB Statistical Center (Stephen George, PhD, CA33601).
Footnotes
Conflict of interest: none.
REFERENCES
- 1.Rifkin MD, Zerhouni EA, Gatsonis CA, et al. Comparison of magnetic resonance imaging and ultrasonography in staging early prostate cancer. Results of a multi-institutional cooperative trial. New Engl J Med. 1990;323:621–626. doi: 10.1056/NEJM199009063231001. [DOI] [PubMed] [Google Scholar]
- 2.Huzjan R, Sala E, Hricak H. Magnetic resonance imaging and magnetic resonance spectroscopic imaging of prostate cancer. Nat Clin Pract Urol. 2005;2:434–442. doi: 10.1038/ncpuro0296. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Hricak H, Kattan MW, et al. Prediction of organ-confined prostate cancer: Incremental value of MR imaging and MR spectroscopic imaging to staging nomograms. Radiology. 2006;238:597–603. doi: 10.1148/radiol.2382041905. [DOI] [PubMed] [Google Scholar]
- 4.Nakashima J, Tanimoto A, Imai Y, et al. Endorectal MRI for prediction of tumor site, tumor size, and local extension of prostate cancer. Urology. 2004;64:101–105. doi: 10.1016/j.urology.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 5.Villers A, Puech P, Mouton D, et al. Dynamic contrast enhanced, pelvic phased array magnetic resonance imaging of localized prostate cancer for predicting tumor volume: correlation with radical prostatectomy findings. J Urol. 2006;176:2432–2437. doi: 10.1016/j.juro.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Beahrs OH, Henson DE, Hutter RVP, et al. American Joint Committee on Cancer Staging Manual. 4th ed. Lippincott; Philadelphia: 1992. pp. 181–186. [Google Scholar]
- 7.Tempany CMC, Zhou X, Zerhouni E, et al. Staging of prostate cancer MRI: Results of Radiology Diagnostic Oncology Group project comparison of three MR imaging techniques. Radiology. 1994;192:47–54. doi: 10.1148/radiology.192.1.8208963. [DOI] [PubMed] [Google Scholar]
- 8.Cheng D, Tempany CM. MR imaging of the prostate and bladder. Semin Ultrasound CT MR. 1998;19:67–89. doi: 10.1016/s0887-2171(98)90025-7. [DOI] [PubMed] [Google Scholar]
- 9.D'Amico AV, Chang HE, Garnick M, et al. Tumor volume changes on endorectal coil MRI during neoadjuvant androgen suppression: A new predictor of tumor response. Urology. 1998;51:287–292. doi: 10.1016/s0090-4295(97)00610-9. [DOI] [PubMed] [Google Scholar]
- 10.Cox JD, Gallagher MJ, Hammond EH, et al. Consensus statements on radiation therapy of prostate cancer: Guidelines for prostate re-biopsy after radiation and for radiation therapy with rising prostate-specific antigen levels after radical prostatectomy. American Society for Therapeutic Radiology and Oncology Consensus Panel. J Clin Oncol. 1999;17:1155–1165. doi: 10.1200/JCO.1999.17.4.1155. [DOI] [PubMed] [Google Scholar]
- 11.Roach M, 3rd, Hanks G, Thames H, Jr., et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 12.Cox DR. Regression models and life tables. J Royal Stat Soc B. 1972;34:187–220. [Google Scholar]
- 13.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Amer Stat Assoc. 1958;53:457–500. [Google Scholar]
- 14.Agresti A, editor. An introduction to categorical data analysis. 2nd ed. John Wiley & Sons; New York: 2002. pp. 16–52. [Google Scholar]
- 15.Tannock IF, de Wit R, Berry WR, et al. Tax 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 16.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 17.D'Amico AV, Moul J, Carroll P, et al. Surrogate marker for prostate cancer specific mortality following radical prostatectomy or radiation therapy. J Natl Cancer Inst. 2003;95:1376–1383. doi: 10.1093/jnci/djg043. [DOI] [PubMed] [Google Scholar]
- 18.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 19.Kwak C, Jeong SJ, Park MS, et al. Prognostic significance of the nadir prostate specific antigen level after hormone therapy for prostate cancer. J Urol. 2002;168:995–1000. doi: 10.1016/S0022-5347(05)64559-4. [DOI] [PubMed] [Google Scholar]
- 20.Morote J, Trilla E, Esquena S, et al. Nadir prostate-specific antigen best predicts the progression to androgen-independent prostate cancer. Int J Cancer. 2005;108:877–891. doi: 10.1002/ijc.11639. [DOI] [PubMed] [Google Scholar]
- 21.Hussain M, Tangen CM, Higano C, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: Data from Southwest Oncology Group Trial 9346 (INT-0162). J Clin Oncol. 2006;24:3984–3990. doi: 10.1200/JCO.2006.06.4246. [DOI] [PubMed] [Google Scholar]
- 22.Zelefsky MJ, Lyass O, Fuks Z, et al. Predictors of improved outcome for patients with localized prostate cancer treated with neoadjuvant androgen ablation therapy and three-dimensional conformal radiotherapy. J Clin Oncol. 1998;16:3380–3385. doi: 10.1200/JCO.1998.16.10.3380. [DOI] [PubMed] [Google Scholar]
- 23.D'Amico AV, McLeod DG, Carroll PR, et al. Time to an undetectable prostate-specific antigen (PSA) after androgen suppression therapy for postoperative or postradiation PSA recurrence and prostate cancer-specific mortality. Cancer. 2007;109:1290–1295. doi: 10.1002/cncr.22550. [DOI] [PubMed] [Google Scholar]
- 24.Mostaghel EA, Page ST, Lin Dw, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: Therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67:5033–5041. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]