Abstract
PURPOSE
To evaluate peripapillary retinalnerve fiber layer (RNFL) thickness using spectral-domain optical coherence tomography (SD-OCT) in patients with autosomal recessive cone-rod dystrophy (CRD).
DESIGN
Cross-sectional study.
METHODS
Eleven patients (22 eyes) with CRD were studied, including 4 patients with identified ABCA4 gene mutations. Peripapillary RNFL thickness was measured in 16 segments from 4 quadrants. The analyses were based on age- and disc size-adjusted normative data. An abnormal thinning was considered when RNFL thickness measurements were under the 5th percentile in at least 2 out of 4 segments in a quadrant. Mean RNFL thickness was quantitatively compared to normative data obtained from 134 subjects.
RESULTS
Eight patients (73%) had peripapillary RNFL thinning in at least one quadrant of at least one eye, including 3 out of 4 patients with known ABCA4 gene mutations. Peripapillary RNFL thinning in the temporal quadrant was most commonly seen in 11 (79%) of 14 eyes with thinning in at least one quadrant. Significant thinning of the overall peripapillary RNFL was observed in CRD patients compared to that of controls (p=0.0002). Subgroup analysis showed that 8 (89%) of 9 patients who were older than 40 years had thinning in at least one quadrant of at least one eye.
CONCLUSIONS
Peripapillary RNFL thinning was commonly observed in our patients with autosomal recessive CRD. The results confirm that the inner retinal structures can be affected in outer retinal disease. Careful evaluation of the inner retina may be important in determining the success rate of potential treatments for predominantly outer retinal diseases.
INTRODUCTION
Cone-rod dystrophy (CRD) is a progressive inherited photoreceptor degenerative disease in which cone photoreceptor function is most often more impaired than rod photoreceptor function. Rod involvement occurs in concurrence with or subsequent to cone involvement.1 Patients characteristically present with impairment of color vision and visual acuity. Central scotomas are usually detected at an early stage, followed by various degrees of peripheral or midperipheral visual field defects. Electroretinogram (ERG) recordings typically show predominant loss in cone function, but reduction of a- and b-wave amplitudes for rods are also seen.2, 3 Fundus examination often shows various degrees of macular involvement, ranging from minimal and nonspecific pigmentary changes, a characteristic “bull’s eye maculopathy”, to a geographic atrophicappearing macular scar at a later stage.4 Diffuse peripheral or midperipheral pigmentary degenerative changes with waxy pallor of the optic disc and vascular attenuation similar to those seen in patients with retinitis pigmentosa (RP) are not infrequently observed.5 An initial history of photoaversion with an impairment of central vision and color vision is usually helpful for differentiating CRD from RP, in which nyctalopia and peripheral visual field loss are more prominent at an early stage.
CRDs are predominantly inherited in an autosomal recessive fashion, however, autosomal dominant6, 7 and X-linked recessive transmissions have also been reported.8, 9 Variations in multiple genes have been shown to cause this phenotype.1 Mutations in the ABCA4 gene account for 30-65% of autosomal recessive CRD.5, 10-12 The ABCA4 protein is a member of the ATP-binding cassette (ABC) superfamily whose products are transmembrane proteins involved in energy-dependent transport of a wide spectrum of substrates across cell membranes.13 The ABCA4 gene is transcribed exclusively in photoreceptors, and the protein transports vitamin A derivatives in the outer segment disc membranes.14 Mutations in this gene have also been reported in patients with age-related macular degeneration,15, 16 autosomal recessive Stargardt disease17 and autosomal recessive RP.18
In 1987, Newman et al reported that clinically evident RNFL thinning could be detected on fundus photography in various diseases of the outer retina, including Best macular dystrophy, Leber congenital amaurosis, Stargardt disease, choroideremia, rodcone dystrophy and CRD.19 However, an accurate observation of wedge-shaped RNFL defects on fundus examination is often technically difficult especially when detection is attempted against a background of generalized retinal pigment epithelial atrophy. More recent studies have shown that spectral-domain optical coherence tomography (SD-OCT) can be a sensitive tool to detect peripapillary RNFL thinning in patients with RP 20 and juvenile X-linked retinoschisis (XLRS) (accepted for publication in Eye). The purpose of our study was to evaluate peripapillary RNFL thickness measurements using SD-OCT in patients with autosomal recessive CRD, including those with known ABCA4 gene mutations. The presence of RNFL defects in this group of patients would have potential impact on patient selection in future therapeutic trials.
METHODS
Subjects
This study included 4 patients with a diagnosis of autosomal recessive CRD and disease-causing variants in the ABCA4 gene. An additional 7 patients who had the same clinical diagnosis, including 3 patients where no ABCA4 mutations were detected by screening with single-strand conformation polymorphism analysis (SSCP), as well as 4 patients with unavailable genetic test results, were enrolled in the study. Genetic testing techniques were previously described.5,21 Seven CRD patients with either positive or negative results for ABCA4 gene mutations whose names were listed in our genetic database participated after receiving a telephone invitation. Other patients were prospectively recruited when seen in the Electrophysiology and Inherited Retinal Disease unit at the Illinois Eye and Ear Infirmary. The diagnosis of CRD was established based on clinical presentation and ERG findings. All patients were examined by two authors (SP and GAF).
Exclusion criteria included known optic nerve diseases or anomalies (glaucoma or glaucoma suspects, optic disc drusen, optic neuropathy, optic pit or coloboma), known other retinal diseases (diabetic retinopathy, hypertensive retinopathy), uveitis, intraocular pressure (IOP) higher than 20 mmHg or a previous history of ocular hypertension, refractive error of more than ± 6 D sphere or ± 3 D cylinder, previous intraocular or refractive surgery, a diagnosis of diabetes mellitus, and inability to hold reasonable fixation, or media opacity that precluded a high-quality OCT examination.
Data Collection, Ocular Examination and Psychophysical Tests
Patient characteristics were collected, including date of birth, gender, race, medical and ophthalmic history, onset of visual impairment, genetic testing results, as well as pedigree information. All patients underwent a comprehensive ocular examination, including best-corrected visual acuity (BCVA) measurement using either a Snellen projection chart or a Feinbloom Distance Test Chart for the Partially Sighted, slit-lamp examination, intraocular pressure measurement with Goldmann applanation tonometry, and dilated fundus examination with direct and indirect ophthalmoscopy. Color fundus photographs were obtained in all patients. Each patient underwent ERG testing obtained by either of two procedures previously described.22, 23 The recording techniques adhered to an international standard for clinical electrophysiologic measurements.24 ERG measurements were compared with either 90% tolerance limits or to an appropriate range obtained from a normally sighted control population.
Optical Coherence Tomography
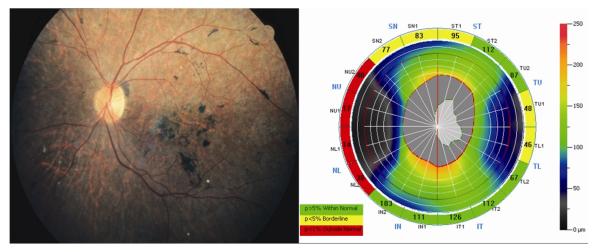
SD-OCT scanning was performed on all subjects using Optovue technology(RTVue Model-RT100 version 3.5; Optovue Inc., Fremont, CA). The NHM4 protocol was used for peripapillary RNFL analysis. Peripapillary RNFL thickness was measured at a diameter of 3.45 mm around the center of the optic disc with a total of 2,225 A-scans. The results were displayed in a color map using customized software with normative data adjusted for age and optic disc size. A peripapillary RNFL thickness map was shown as a numerical value and the color code in each of 16 segments for the 4 quadrants: superior (46 ° -135 °), nasal (316°-45 ° for the right and 136 ° -225 ° for the left), inferior ((226 ° -315 °), and temporal (136 ° -225 ° for the right and 316°-45 ° for the left). An abnormally thin RNFL was encoded yellow and red for values less than the 5th and 1st percentiles, respectively. RNFL measurements not considered as thin (the 5th percentile or more) were demonstrated in green. Peripapillary RNFL thinning in an individual quadrant was considered when red or yellow coding presented in at least 2 out of 4 segments of the quadrant. An example is shown in the Figure (Right).
FIGURE.
Fundus photograph (Left) of the left eye from a patient with autosomal recessive cone-rod dystrophy (patient number 2) which shows generalized retinal pigment epithelial atrophic changes, pigment clumping at the posterior pole, peripapillary region and midperiphery, vascular attenuation, without clinically-apparent optic disc pallor. Peripapillary retinal nerve fiber layer thickness measurements (Right) from the same eye were considered abnormally thin in three quadrants including, the nasal (4 out of 4 segments with red encoding), superior (3 out of 4 segments with yellow encoding) and temporal quadrant (2 out of 4 segments with yellow encoding).
The OCT machine provided internal fixation. However, most of the patients with CRD had poor visual acuity and were unable to see the fixation target. They were asked to direct their gaze in a direction to facilitate visualization of their optic disc. Each peripapillary RNFL scan was completed within 0.39 seconds, while the patients maintained their eye position. Only scans with good centration were included for analysis. The scans with low or irregular signal strength index (SSI) and visibly misaligned segmentation lines were excluded. Three scans were performed in each eye to ensure reproducibility. When an initial set of 3 scans was not reproducible, additional scans were performed to provide at least 3 high-quality scans with acceptable reproducibility of the color coding in at least 2 out of 3 scans in each eye. A total of 17 patients with autosomal recessive CRD underwent OCT examination; however, high-quality images could not be obtained from 4 patients with ABCA4 mutations and 2 patients with pending genetic testing results. Included for analysis were data from the remaining 11 patients with high-quality OCT images.
Additional macular scans were also performed using the MM5 protocol, which is presented as 5×5 mm macular thickness maps with results compared to color codes based on age-similar customized normative data. Scan acquisition time required for each of the MM5 scans was 0.78 seconds.
Data Analysis
Peripapillary RNFL thickness was analyzed in each quadrant based on the color codes which, as mentioned, were referenced to customized normative data adjusted for age and optic disc size. Mean circumferential peripapillary RNFL thickness from all 4 quadrants in each eye was quantitatively compared to normative data which were obtained from 268 eyes of 134 control subjects (mean age of 44.1 ± 15.5 years). These normative data were provided from the analyzer. The thickness measurements from our patients with CRD were analyzed compared to those of the normal subjects using an unpaired Student t-test. Probability values under 0.05 were considered statistically significant.
RESULTS
Twenty-two eyes of 11 patients were included in this study. Patient characteristics are shown in Table 1. Mean (SD) age of the patients was 47.7 (18.9) years, with a median of 54.4 years (range 10.9-68.8 years). Female patients predominated (64%). Caucasians were the majority (64%), followed by African American (18%) and Palestinian (18%). Visual acuity ranged from 20/50 to counting fingers, with mean logMAR acuity of 1.43 (equivalent to 20/540). Mean (SD) of IOP was 14.8 (1.7) mmHg (range, 11-17 mmHg). Four patients from different families (numbers 1-4) were found to harbor plausible disease-causing mutations in the ABCA4 gene (Table 1). No ABCA4 variants were found in three patients after screening with SSCP, three patients refused to have blood tests for genetic evaluation, and a genetic result was pending in one patient.
TABLE 1.
Demographic Characteristics and Ocular Examination in Patients with Autosomal Recessive Cone-Rod Dystrophy
| Subject No. |
Age (years) |
Gender | Race | Genetic Testing Results for the ABCA4 Gene |
Visual Acuity |
Fundus Findings | ||
|---|---|---|---|---|---|---|---|---|
| OD | OS | Macula | Pigmentation | |||||
| 1 | 60 | Female | Caucasian | IVS40+5 G>A & cys54tyr exon 3, heterozygous |
20/400 | 20/400 | Pigment clumping | Posterior pole, midperiphery |
| 2 | 59 | Female | Caucasian | glu328stop exon 8 & val767asp, heterozygous |
3/200 | 3/400 | Pigment clumping | Posterior pole, midperiphery |
| 3 | 44 | Male | Caucasian | ala1038val exon 21 & leu541pro exon 12, heterozygous a |
5/400 | 5/200 | Pigment clumping | Periphery |
| 4 | 54 | Female | African American |
val989ala exon 20, heterozygous | 20/400 | CF | Pigment clumping | Posterior pole, peripapillary |
| 5 | 42 | Male | Palestinian | None detected b | 20/50 | 20/400 | Drusen-like lesions, hypopigmentation |
- |
| 6 | 45 | Female | Palestinian | None detected b | 20/70 | 20/200 | Drusen-like lesions, hypopigmentation |
- |
| 7 | 63 | Female | African American |
None detected b | 20/200 | 20/200 | Geographic atrophic lesions | - |
| 8 | 69 | Female | Caucasian | No genetic testing | 2/400 | 2/700 | Pigment granularity | - |
| 9 | 62 | Female | Caucasian | Pending | 20/200 | 20/400 | Bull’s eye-like lesions | - |
| 10 | 16 | Male | Caucasian | No genetic testing | 10/300 | 10/200 | Geographic atrophic lesions | - |
| 11 | 11 | Male | Caucasian | No genetic testing | 10/120 | 10/160 | Bull’s eye-like lesions and pigment granularity |
Few at midperiphery |
CF = counting fingers; OD = right eye; OS = left eye; “-” = absent
These two variants constitute a complex allele
Screening with single-strand conformation polymorphism analysis (SSCP)
Color Code Referenced Peripapillary RNFL Analysis
Eight (73%) of 11 patients showed peripapillary RNFL thinning using our criteria of at least one quadrant in at least one eye, including 3 (numbers 1-3) out of 4 patients (numbers 1-4) who harbored plausible disease-causing variations in the ABCA4 gene (Table 2). Of the 8 patients with thinning, 6 had thinning in both eyes, and 2 had thinning in one eye. There were 4 patients who had thinning in 2 or more quadrants in at least one eye. Interestingly, 3 patients with a known ABCA4 gene mutation had thinning in 2 or more quadrants in both eyes. There were 14 (64%) of 22 eyes which presented with peripapillary RNFL thinning in at least one quadrant. Thinning in the temporal quadrant was most commonly seen in 11 (79%) of 14 eyes, followed by the nasal (7 eyes, 50%), inferior (5 eyes, 36%), and superior quadrants (4 eyes, 29%). The presence of RNFL thinning was not necessarily symmetrical. All patients were older than 40 years old, except for 2 patients (numbers 10-11). Peripapillary RNFL thinning, in at least one quadrant of at least one eye, was seen in 8 (89%) of 9 patients whose ages were greater than 40 years.
TABLE 2.
Quadratic Distribution of Peripapillary Retinal Nerve Fiber Layer Thinning in Patients with Autosomal Recessive Cone-Rod Dystrophy Based on Color-Coded Referenced Normative Data
| Subject No. |
Side | Superior | Nasal | Inferior | Temporal |
|---|---|---|---|---|---|
| 1 | OD | + | - | - | + |
| OS | - | + | - | + | |
| 2 | OD | - | + | + | + |
| OS | + | + | - | + | |
| 3 | OD | + | + | + | + |
| OS | + | + | + | + | |
| 4 | OD | - | - | - | - |
| OS | - | - | - | - | |
| 5 | OD | - | - | - | + |
| OS | - | - | - | + | |
| 6 | OD | - | - | + | - |
| OS | - | - | - | - | |
| 7 | OD | - | - | - | + |
| OS | - | - | - | - | |
| 8 | OD | - | + | - | - |
| OS | - | + | + | - | |
| 9 | OD | - | - | - | + |
| OS | - | - | - | + | |
| 10 | OD | - | - | - | - |
| OS | - | - | - | - | |
| 11 | OD | - | - | - | - |
| OS | - | - | - | - |
“+” = present; “-” = absent
Non-Color Code Referenced Peripapillary RNFL Analysis
Table 3 shows mean thickness of the peripapillary RNFL in each quadrant and in overall circumferential thickness. The overall peripapillary RNFL thickness measurements of our patients were compared with those from the 268 eyes of 134 control subjects. There was no statistically significant difference in ages between our cohort of patients and the controls (mean age of 47.7 + 18.9 versus 44.1 + 15.5, p = 0.468). By comparing our patients to controls, there was a statistically significant reduction of the thickness in the overall peripapillary circumferential RNFL measurements in the patients (p = 0.0002). When evaluating each quadrant separately, a statistically significant reduction in peripapillary RNFL thickness was observed in the temporal (p <0.0001), nasal (p = 0.003) and inferior quadrants (p = 0.005).
TABLE 3.
Mean Circumferential Retinal Nerve Fiber Layer Thickness in Autosomal Recessive Cone-Rod Dystrophy (CRD) Patients Compared to That of a Control Population
| Quadrant | CRD Patients (μm, mean ± SD) |
Controls (μm, mean ± SD) |
P-Value |
|---|---|---|---|
| Superior | 125.04 ± 31.09 | 129.03 ± 17.83 | 0.347 |
| Nasal | 68.69 ± 25.48 | 78.43 ± 13.54 | 0.003a |
| Inferior | 128.31 ± 30.11 | 139.78 ± 17.08 | 0.005a |
| Temporal | 62.27 ± 16.93 | 79.63 ± 12.03 | <0.0001a |
| Overall | 96.08 ± 23.44 | 106.72 ± 11.34 | 0.0002a |
Statistically significant
Macular Scan Analysis
The mean central 1-mm macular thickness was calculated using data from 18 eyes of 9 patients. The macular thickness measurements from two patients (numbers 3 and 10) were excluded due to misaligned segmentation lines automatically drawn by the OCT program. The central macular thickness ranged from 108 to 262 μm, with a median of 168 μm. There was a statistically significant difference in mean central macular thickness between CRD patients and controls (175.2 ± 42.1 μm versus 265.8 ± 23.9 μm, p < 0.0001).
DISCUSSION
Using SD-OCT, in previous studies from our group we found that peripapillary RNFL thinning may be observed in patients with certain inherited retinal diseases, including RP20 and XLRS (accepted for publication in Eye). The presence of peripapillary RNFL thinning of at least one quadrant in at least one eye was seen in 44% and 42% of patients with RP and XLRS, respectively, compared to 73% of patients with CRD in this study.
Scan centration at the optic disc is a key factor for an accuracy of OCT peripapillary RNFL measurement. Although the OCT machine provides internal fixation which facilitates scan acquisition, most of our patients were unable to see the fixation target. In our study, because of this issue with patient fixation, high-quality scans were obtained in 11 (65%) out of 17 patients who were examined. We cannot rule out the possibility that the 6 patients excluded from analysis might have had similar or even more advanced RNFL thinning than those presented. Therefore, our results may have possibly underestimated the prevalence of peripapillary RNFL thinning in autosomal recessive CRD patients.
In autosomal recessive CRD patients, the peripapillary RNFL thinning was most prominent in the temporal quadrant. This might, in part, be explained by the cone distribution in the posterior pole and the fact that most patients with CRD primarily present with macular involvement and its related symptoms prior to more apparent changes in the peripheral retina. Nonetheless, impairment of visual acuity did not always parallel the presence of such thinning in the temporal quadrant. For instance, patient number 5 retained visual acuity of 20/50 in one eye, despite an observation of peripapillary RNFL thinning in the temporal quadrant.
The results from our studies confirm that inner retinal structures may be affected in outer retinal degenerative diseases. However, the mechanism currently remains unclear. We speculate that this might result from either transsynaptic degeneration or, yet to be identified, a mechanism by which there is a more direct involvement of inner retinal layers as a consequence of outer retinal photoreceptor cell degeneration.
Our study suggests that peripapillary RNFL thinning is commonly observed in patients with autosomal recessive CRD, including those with identified ABCA4 gene mutations. The data also show that patients in an older age group are more likely to have peripapillary RNFL thinning than are younger patients, which is consistent with a similar observation in patients with XLRS. The degrees of thinning were usually similar between both eyes of the same patient, although asymmetry was also observed. Evaluation of the inner retinal structures using SD-OCT may have relevance in determining the success rate of potential treatments, such as the use of retinal neurotrophic factors or gene-directed therapy, for primarily outer retinal diseases.
Acknowledgement
A.Funding/support: Foundation Fighting Blindness, Owings Mills, Maryland (GAF, RA, EMS); Grant Healthcare Foundation, Chicago, Illinois (GAF); NIH core grant EY01792 (GAF); NIH grant EY13435 (RA); NIH grant EY016822 (EMS); departmental grant from Research to Prevent Blindness (GAF, RA, EMS); the Howard Hughes Medical Institute (EMS) and the Foundation Fighting Blindness,Toronto, Ontario, Canada (RA)
E. Other acknowledgements We are thankful to Ms.Jana Zernant-Rajang for genetic analysis.
Biographic sketch
Sirichai Pasadhika, MD, completed his medical degree and ophthalmology residency training at Chulalongkorn University, Bangkok, Thailand. His extensive post-residency trainings include a vitreo-retinal fellowship in Bangkok, a Graham-Lovett clinical fellowship in vitreo-retinal surgery at the Sydney Eye Hospital in Australia, a clinical uveitis fellowship at Oregon Health & Science University in Portland, and a clinical-research fellowship in inherited retinal disease and electrophysiology at the University of Illinois at Chicago.

Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
B. Financial disclosures: The authors have no financial or proprietary interest in any of the products or techniques mentioned in this article.
D. Conformity of author information The protocol was approved by an institutional review board of the University of Illinois at Chicago, and conducted in accordance with the principles of the Declaration of Helsinki and the HIPAA compliance. Informed consent was obtained from all patients.
A Table of Contents Statement: Using spectral-domain optical coherence tomography, 73% of autosomal recessive cone-rod dystrophy patients were observed to have peripapillary retinal nerve fiber layer thinning in at least one quadrant of at least one eye. The results confirm that the inner retinal structures can be affected in outer retinal disease. Careful evaluation of the inner retina may be important in determining the success rate of potential treatments, such as gene-directed therapy, for predominantly outer retinal diseases.
REFERENCES
- 1.Hamel CP. Cone rod dystrophies. Orphanet J Rare Dis. 2007;2:7. doi: 10.1186/1750-1172-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yagasaki K, Jacobson SG. Cone-rod dystrophy. Phenotypic diversity by retinal function testing. Arch Ophthalmol. 1989;107:701–708. doi: 10.1001/archopht.1989.01070010719034. [DOI] [PubMed] [Google Scholar]
- 3.Szlyk JP, Fishman GA, Alexander KR, Peachey NS, Derlacki DJ. Clinical subtypes of cone-rod dystrophy. Arch Ophthalmol. 1993;111:781–788. doi: 10.1001/archopht.1993.01090060069025. [DOI] [PubMed] [Google Scholar]
- 4.Krill AE, Deutman AF, Fishman M. The cone degenerations. Doc Ophthalmol. 1973;35:1–80. doi: 10.1007/BF00234530. [DOI] [PubMed] [Google Scholar]
- 5.Fishman GA, Stone EM, Eliason DA, Taylor CM, Lindeman M, Derlacki DJ. ABCA4 gene sequence variations in patients with autosomal recessive cone-rod dystrophy. Arch Ophthalmol. 2003;121:851–855. doi: 10.1001/archopht.121.6.851. [DOI] [PubMed] [Google Scholar]
- 6.Miki T, Kiyonaka S, Uriu Y, et al. Mutation associated with an autosomal dominant cone-rod dystrophy CORD7 modifies RIM1-mediated modulation of voltagedependent Ca2+ channels. Channels (Austin) 2007;1:144–147. doi: 10.4161/chan.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitiratschky VB, Nagy D, Zabel T, et al. Cone and cone-rod dystrophy segregating in the same pedigree due to the same novel CRX gene mutation. Br J Ophthalmol. 2008;92:1086–1091. doi: 10.1136/bjo.2007.133231. [DOI] [PubMed] [Google Scholar]
- 8.Jalkanen R, Mantyjarvi M, Tobias R, et al. X linked cone-rod dystrophy, CORDX3, is caused by a mutation in the CACNA1F gene. J Med Genet. 2006;43:699–704. doi: 10.1136/jmg.2006.040741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebenezer ND, Michaelides M, Jenkins SA, et al. Identification of novel RPGR ORF15 mutations in X-linked progressive cone-rod dystrophy (XLCORD) families. Invest Ophthalmol Vis Sci. 2005;46:1891–1898. doi: 10.1167/iovs.04-1482. [DOI] [PubMed] [Google Scholar]
- 10.Ducroq D, Rozet JM, Gerber S, et al. The ABCA4 gene in autosomal recessive cone-rod dystrophies. Am J Hum Genet. 2002;71:1480–1482. doi: 10.1086/344829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klevering BJ, Yzer S, Rohrschneider K, et al. Microarray-based mutation analysis of the ABCA4 (ABCR) gene in autosomal recessive cone-rod dystrophy and retinitis pigmentosa. Eur J Hum Genet. 2004;12:1024–1032. doi: 10.1038/sj.ejhg.5201258. [DOI] [PubMed] [Google Scholar]
- 12.Maugeri A, Klevering BJ, Rohrschneider K, et al. Mutations in the ABCA4 (ABCR) gene are the major cause of autosomal recessive cone-rod dystrophy. Am J Hum Genet. 2000;67:960–966. doi: 10.1086/303079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11:1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 14.Sun H, Nathans J. Mechanistic studies of ABCR, the ABC transporter in photoreceptor outer segments responsible for autosomal recessive Stargardt disease. J Bioenerg Biomembr. 2001;33:523–530. doi: 10.1023/a:1012883306823. [DOI] [PubMed] [Google Scholar]
- 15.Allikmets R, Shroyer NF, Singh N, et al. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science. 1997;277:1805–1807. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- 16.Allikmets R. Further evidence for an association of ABCR alleles with age-related macular degeneration. The International ABCR Screening Consortium. Am J Hum Genet. 2000;67:487–491. doi: 10.1086/303018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allikmets R, Singh N, Sun H, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15:236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Mir A, Paloma E, Allikmets R, et al. Retinitis pigmentosa caused by a homozygous mutation in the Stargardt disease gene ABCR. Nat Genet. 1998;18:11–12. doi: 10.1038/ng0198-11. [DOI] [PubMed] [Google Scholar]
- 19.Newman NM, Stevens RA, Heckenlively JR. Nerve fibre layer loss in diseases of the outer retinal layer. Br J Ophthalmol. 1987;71:21–26. doi: 10.1136/bjo.71.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walia S, Fishman GA. Retinal nerve fiber layer analysis in RP patients using Fourier-domain OCT. Invest Ophthalmol Vis Sci. 2008;49:3525–3528. doi: 10.1167/iovs.08-1842. [DOI] [PubMed] [Google Scholar]
- 21.Jaakson K, Zernant J, Kulm M, et al. Genotyping microarray (gene chip) for the ABCR (ABCA4) gene. Hum Mutat. 2003;22:395–403. doi: 10.1002/humu.10263. [DOI] [PubMed] [Google Scholar]
- 22.Peachey NS, Fishman GA, Derlacki DJ, Alexander KR. Rod and cone dysfunction in carriers of X-linked retinitis pigmentosa. Ophthalmology. 1988;95:677–685. doi: 10.1016/s0161-6420(88)33128-3. [DOI] [PubMed] [Google Scholar]
- 23.Fishman GA, Farber MD, Derlacki DJ. X-linked retinitis pigmentosa. Profile of clinical findings. Arch Ophthalmol. 1988;106:369–375. doi: 10.1001/archopht.1988.01060130395029. [DOI] [PubMed] [Google Scholar]
- 24.Marmor MF, Holder GE, Seeliger MW, Yamamoto S. Standard for clinical electroretinography (2004 update) Doc Ophthalmol. 2004;108:107–114. doi: 10.1023/b:doop.0000036793.44912.45. [DOI] [PubMed] [Google Scholar]