Abstract
Work in rodents has demonstrated that progenitor transplantation can achieve limited photoreceptor replacement in the mammalian retina; however, replication of these findings on a clinically relevant scale requires a large animal model. To evaluate the ability of porcine retinal progenitor cells to survival as allografts and integrate into the host retinal architecture, we isolated donor cells from fetal green fluorescent protein (GFP)-transgenic pigs. Cultures were propagated from the brain, retina, and corneo-scleral limbus. GFP expression rapidly increased with time in culture, although lower in conjunction with photoreceptor markers and glial fibrillary acid protein (GFAP), thus suggesting downregulation of GFP during differentiation. Following transplantation, GFP expression allowed histological visualization of integrated cells and extension of fine processes to adjacent plexiform layers. GFP expression in subretinal grafts was high in cells expressing vimentin and lower in cells expressing photoreceptor markers, again consistent with possible downregulation during differentiation. Cells survived transplantation to the injured retina of allorecipients at all time points examined (up to 10 weeks) in the absence of exogenous immune suppression without indications of rejection. These findings demonstrate the feasibility of allogeneic progenitor transplantation in a large mammal and the utility of the pig in ocular regeneration studies.
Introduction
There has been a large body of recent work showing that stem and progenitor cells can be isolated from the mammalian nervous system and expanded in culture (Martinez-Serrano et al., 1995; Palmer et al., 1997; Reynolds and Weiss, 1992; Ryder et al., 1990). Interest in these cells has been heightened by evidence that such cells can integrate back into host tissues and differentiate into local cell types following transplantation to animal models of disease. Results of this kind have been of particular interest in the setting of the central nervous system (CNS), including the retina, brain, and spinal cord where endogenous repair frequently falls short of providing adequate functional recovery following injury. Furthermore, there are currently no restorative treatments available for neural or retinal degenerative diseases, such as Parkinson's disease, multiple sclerosis, retinitis pigmentosa, and macular degeneration. Here again, various cell transplantation strategies have shown promise (Brustle et al., 1999; Klassen et al., 2004; Kuehn et al., 2006; MacLaren et al., 2006; Ostenfeld et al., 1999; Rubio et al., 1999; Young et al., 2000)
A fundamental challenge common to all cellular transplantation studies of this type is the need for unambiguous identification of donor cells within the host milieu. One strategy is to use xenogeneic cells that either have identifying cytological features (Couly et al., 1992), or that can be subsequently labeled by species-specific antibodies (Klassen and Lund, 1990). These methods have been successful in certain applications, but are of no use in models where xenografts are poorly tolerated. An important example in this respect is the pig (Warfvinge et al., 2005, 2006). Another method, and one that has been previously used in the pig, is prelabeling of cells with vital dyes such as DAPI, PKH26, or PHK67 (Klassen et al., 2007). This method can be used to identify grafted cells, but is limited by diffusion of the dye out of the donor cells as well as uptake and incorporation by cells of the host. In addition, these dyes will tend to be rapidly lost in situations where donor cells are dividing and, in any case, the fine details of cellular morphology are not well visualized using this method. The preferred method for labeling donor cells is the use of a reporter gene such as an enzyme or fluorescent protein. While these markers are not entirely without potential problems, including irregular expression (Swenson et al., 2007), possible cellular fusion (Terada et al., 2002), and physiological side effects (Devgan, et al., 2004; Guo et al., 2007; Huang et al., 2000; Liu et al., 1999), they allow detailed visualization of donor cell cytoarchitecture, and are remarkably stable and well-tolerated in many different cell types.
One method of labeling cells with a reporter gene is to first harvest the cells, then introduce the transgene, for example, via the use of viral vectors or electroporation. While these methods can be used on many different cell types, when possible, it is preferable to derive the cells directly from a viable, healthy animal already transgenic for the particular gene, as has been done with GFP mice (Klassen et al., 2004). As opposed to rodents, large mammalian models better approximate the efficacy of stem cells in the therapeutic setting. One such animal model is the pig; however, GFP-transgenic animals have been unavailable in this species until recently (Park et al., 2001). Here, we derive progenitor cells from the CNS and corneo-scleral limbus of GFP-transgenic pigs, and show for the first time porcine progenitor cells are capable of morphological integration into the host retina. In addition, we demonstrate that allogeneic porcine progenitor cells can survive for at least 10 weeks in immunocompetent hosts.
Materials and Methods
Donor and recipient animals
The donor animals for tissue and progenitor cell isolation were pigs of the NT5 line, transgenic for GFP (Fig. 1A). These transgenic animals express the green fluorescent protein in all nucleated cells and were generated using a CMV promoter in a replication-deficient retrovirus vector, as previously described (Park et al., 2001). Briefly, transgenic porcine zygotes and, ultimately, fertile pigs were obtained following nuclear transfer from porcine fibroblasts modified to express the enhanced version of GFP. The fetal eye shown in Figure 1B is that of a pig of the NT92 line, transgenic for enhanced GFP on a β-actin promoter, and shown at 45 days gestational age.
FIG. 1.
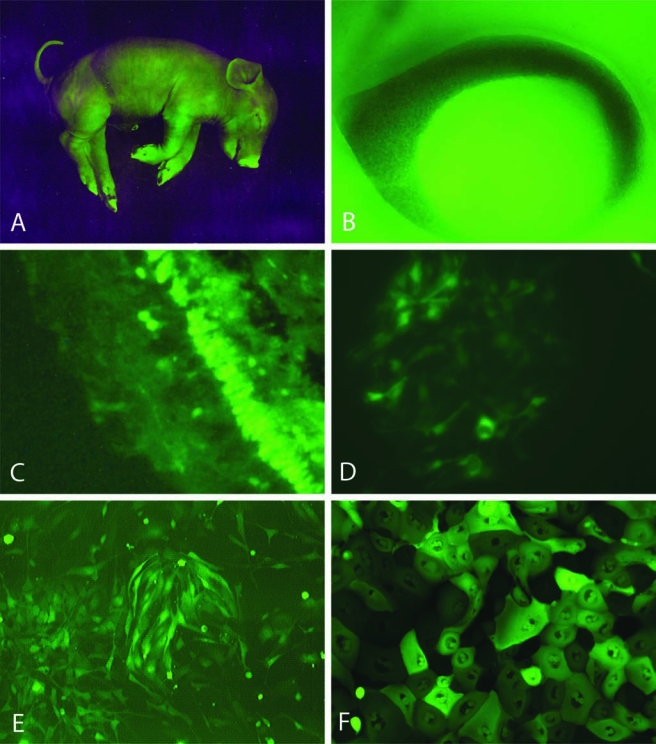
GFP-transgenic pigs are a source of GFP+ progenitor cells. Viewed under fluorescent illumination, a near-term piglet exhibits widespread green fluorescence, particularly in the hooves and snout (A). The eye of a 43-day gestational age GFP-transgenic fetus (NT92 line, this example only) shows an intense green reflex from the ocular interior, visible through the developing pupillary aperture (B). The retina from a transgenic adult shows a mosaic pattern of GFP expression in the mature structure (C). Cultures from tissue taken at 60 days gestational age show GFP+ cells with characteristic morphology derived from the retina (D), forebrain (E), and corneo-scleral limbus (F). Photograph in (A) courtesy of Max Aguilera-Hellweg, photograph in (B) courtesy Kristin Whitworth. (See paper online for Fig. 1 in color.)
The recipients used in transplantation experiments were female Danish Landrace pigs of 4 months age and weight approximately 30 kg. No immunosuppressive treatment was used, either locally or systemically, at any time in these experiments. All live animal work was performed according to IACUC-approved protocols and in compliance with NIH and institutional guidelines, including the Danish Animal Experiment Inspectorate.
Tissue harvest and cell isolation
For tissue collection, a pregnant sow at 60 days of gestation was placed under terminal anesthesia and the uterine horns and fetuses were removed through an abdominal incision. Tissue was stored on ice and transported the same day from Columbia, Missouri, to either Orange County, California, or Boston, Massachusetts.
Porcine forebrain progenitors were obtained using methods described previously (Schwartz et al., 2005). Briefly, after enzymatic digestion the cell-containing tissue homogenates were grown in fibronectin-coated flasks and incubated in growth medium containing FBS (10%, by vol.; HyClone, Logan, UT; hyclone.com) ). Growth medium consisted of high glucose DMEM/F12 (Irvine Scientific, Irvine, CA; irvinesci.com), 40 ng/mL bFGF, 20 ng/mL hEGF, 20 ng/mL hPDGF-AB, 2 mM L-glutamine, BIT 9500 (10% by vol.; StemCell Technologies, Vancouver, BC, Canada; stemcell.com), and antibiotics. Within 24 h, media was exchanged for growth medium without FBS. Cultures were subsequently re-fed by exchanging 50% of the media every 2–3 days.
Retinal progenitors were also obtained using methods similar to those previously described (Klassen et al., 2007). Briefly, pooled retinal tissue homogenates were plated on fibronectin and incubated with medium containing high-glucose DMEM-F12 (Irvine Scientific), 20 ng/mL hEGF, 2 mM L-glutamine, BIT 9500 (10% by vol.), antibiotics, and FBS (10% by vol.). Within 24 h, all medium was exchanged for growth medium without any FBS. Cultures were subsequently re-fed by 100% medium exchanges every 2–3 days.
Progenitor cells from the corneo-scleral limbus were grown using previously established methods. Briefly, isolated anterior segment tissue samples from 60-day-old (midgestational) transgenic GFP-expressing pig fetuses were delivered to the UC Irvine Medical Center on ice within 48 h of harvest. The cornea-scleral rims were gently cleaned of uveal tissue, rinsed using PBS supplemented with antibiotics, and placed on collagen type IV-coated cell culture dishes. Serum-free keratinocyte medium supplemented with recombinant EGF, L-glutamine, and bovine pituitary extract (Gibco, Invitrogen Corp., Carlsbad, CA) was used. The cultures were incubated under 5% CO2 at 37°, with media change performed every two days.
Determination of percentage of transgenic cells showing GFP expression
Cells from the fetal GFP-transgenic pig retina were grown in T75 flasks and photomicrographs taken at selected time points from 1 to 36 days. Images were obtained using green fluorescence and bright-field channels separately and these were then merged. Cells were counted through a transparent 36-square grid covering the entire image and the percentage of cells expressing GFP was calculated from the total.
Immunocytochemistry
Cells were subjected to immunocytochemical analysis using previously established protocols (Schwartz et al., 2003). Cells were fixed by 10-min immersion in 4% paraformaldehyde in 0.1 M PBS buffer and then washed in PBS buffer + 0.05% sodium azide. A blocking solution of TBS (Trisbuffered saline), 0.3% Triton X-100, and 3% donkey serum (Jackson Immunoresearch, West Grove, PA; jacksonimmuno.com) was then applied for 15 min. The cells were then rinsed twice in TBS buffer. Primary antibodies (Table 1) were diluted in TBS, 0.3% Trition X-100, and 1.5% donkey serum at previously determined concentrations, applied to samples, and left overnight at 5°C on a mechanical rocker. The next day the cells were rinsed with TBS. All secondary antibodies were donkey-derived (Jackson Immunoresearch) and diluted 1:100 in 250 μL TBS, 0.3% Triton X-100, and 1.5% donkey serum. Secondary antibodies were applied to samples and again left overnight at 5°C on a rocker. The following day, slides were rinsed with TBS three times. Coverslips were mounted with Prolong® Antifade Kit (Molecular Probes, Eugene, OR; probes.invitrogen.com) and photomicrographs recoded via an Olympus IX70 Microscope and Macrofire digital camera (Optronics, Goleta, CA; optronics.com) using Image Pro Plus 4.5 with AFA plugin 4.5 software.
Table 1.
Primary Antibodies for Immunocytochemistry
| Antigen | Species | Supplier | Product code | Dilution |
|---|---|---|---|---|
| AQP4 | rabbit | Chemicona | AB359450ul | 1:100 |
| CD15 | mouse | BDPharmb | 559045 | 1:100 |
| DCX | goat | Santa Cruzc | SC-8066 | 1:100 |
| GFAP | guinea pig | Chemicon | AB1540 | 1:200 |
| Ki-67 | mouse | BDPharm | 56003 | 1:200 |
| NCAM | rabbit | Chemicon | AB5032 | 1:100 |
| nestin | mouse | BDPharm | 611658 | 1:400 |
| PSA-NCAM | mouse | Chemicon | MAB5324 | 1:100 |
| recoverin | rabbit | Chemicon | AB5431P | 1:100–200 |
| rhodopsin | mouse | R. Moldayd | 4D2 | 1:500 |
| Sox-2 | goat | Santa Cruz | SC-17320 | 1:50 |
| synapsin 1 | rabbit | Sigmae | SX193 | 1:1200 |
| transducin | rabbit | CytoSignalf | TF15 | 1:1000 |
| Beta-3-tubulin | mouse | Chemicon | MAB1637 | 1:100 |
| vimentin | mouse | Sigma | V 6630 | 1:200 |
Temecula, CA; chemicon.com.
Franklin Lakes, NJ; bdpharma.com.
Santa Cruz, Ca; scbt.com.
Gift of R. Molday, Univ. British Columbia.
St. Louis, MO; sigmaaldrich.com.
Irvine, CA; cytosignal.com.
Transplantation
Progenitor cell tranplantation surgery was carried out in 4-month-old female Danish Landrace pigs in the manner previously described, with minor modifications (Klassen et al., 2007; Warfvinge et al., 2005, 2006). All animals were preanesthetized with intramuscular injections of 15 mg midazolam (DormicumA; Roche, Hvidovre, Denmark; www.roche.dk) and a composition consisting of [zolazepam 11.9 mg/mL and tiletamin 11.9 mg/mL (Zoletin 50 Vet, Virbac SA, Carros CEDEX, France; www.virbac.com) mixed with xylazine 12.38 mg/mL (Intervet, Skovlunde, Denmark; www.intervet.dk), ketamine 14.29 mg/mL (Intervet), and methadone 2.38 mg/mL (Nycomed, Roskilde, Denmark; www.nycomed.com)]. The pigs underwent endotracheal intubation and were artificially ventilated with 2–3% isoflurane (Abbott, Solna, Sweden; www.abbott.se) in combination with oxygen. Stroke volume (300 mL/stroke) and respiratory frequency (12/min) were kept constant throughout. In each case the left pupil was treated with topical drops consisting of a combination of 0.4% oxybuprocain (SAD, Copenhagen, Denmark), 10% Metaoxedrin (SAD), 0.5% Mydriacyl (Alcon, Belgium, www.alcon.com/belgium), 1% atropine (SAD), and 5% povidone–iodine (SAD). At surgery, the central and posterior vitreous was removed together with the posterior hyaloid membrane using a three-port pars plana vitrectomy.
Our prior work has indicated that progenitor cells rarely integrate into the retina in the absence of active retinal disease or injury (Young et al., 2000). To promote the integration of grafted cells into the host retina, a number of different lesions were created in the left eye of the recipients prior to transplantation. These included laser burns applied by green argon endolaser in a grid pattern to the area centralis, subretinal scraping of the area centralis and performed through a retinotomy, as well as a pressure lesion, generated by elevating intraocular pressure to a level 5 mmHg below mean arterial pressure for a period of 2 h via monitored infusion of physiologic saline to the ocular anterior chamber. In three cases, laser lesions and pressure lesions were combined in the same eye (Table 2). Thereafter, a retinal bleb was elevated in the area centralis by injection of 0.25–0.5 mL 0.9% NaCl through a 41-gauge needle. Endodiathermy was applied to the detached retina prior to enlargement of the retinotomy for transplantation.
Table 2.
Transplantation of GFP-Transgenic CNS Progenitor Cells to the Retina of 4-Month-Old Allorecipients
| Pig no. | Survival time | Retinal pretreatment | Surviving GFP cells | Localization of GFP cells |
|---|---|---|---|---|
| A. | ||||
| 170 | 9 days | Laser | +++ | SR, V |
| 171 | 9 days | Laser + pressure | +++ | SR, V |
| 172 | 9 days | Pressure | +++ | SR, R, V |
| 168 | 3 weeks | Laser + pressure | +++ | SR, R, V |
| 173 | 3 weeks | Laser | ++ | SR |
| 174 | 3 weeks | Pressure | + | SR, V |
| B. | ||||
| 132 | 2 weeks | Laser | +++ | SR, Ro, R, V |
| 133 | 2 weeks | Scraped | ++ | C, SR, Ro |
| 134 | 2 weeks | Laser | +++ | SR |
| 175 | 3 weeks | Laser + pressure | +++ | SR, R, V |
| 128 | 5 weeks | Laser | +++ | SR, R |
| 129 | 5 weeks | Scraped | +++ | SR, R |
| 135 | 5 weeks | Scraped | +++ | SR, V |
| 176 | 5 weeks | Laser | +++ | SR, R |
| 177 | 5 weeks | Laser | ++ | SR |
| 178 | 5 weeks | Laser | +++ | SR, R |
| 130 | 10 weeks | Laser | + | SR |
| 131 | 10 weeks | Scraped | +++ | SR, R |
| 167 | 10 weeks | Laser | + | R |
| 169 | 10 weeks | Laser | +++ | SR, R |
| 179 | 10 weeks | Laser | +++ | SR |
Donor cells were derived from either (A) forebrain or (B) neural retina of fetal GFP-transgenic pigs.
Cell survival is estimated compared to number of injected cells as follows: +++ = is consistent with an order of magnitude decrement in cell number, ++ = an additional order of magnitude decrement, and += yet another order of magnitude decrement.
Abbreviations used: C = choroid, SR = subretinal, Ro = rosettes present, R = retina, V = vitreous.
GFP+ progenitor cells were injected into the retinal bleb, either as a single cell suspension using a 27-gauge needle or as aggregated “neurospheres” using a 20-gauge needle. The single cell suspension and neurosphere injections both contained approximately 2 × 107 cells. Immediate reflux of some cells into the vitreous cavity was frequently observed; therefore, a small air bubble was placed in the subretinal bleb under the retinotomy to prevent further reflux after withdrawal of the needle. Chloramfenicol (SAD) was given locally at the completion of surgery to avoid infection. Postsurgically, pigs were examined by ophthalmoscopy on a weekly basis.
Histology and immunohistochemistry
Eyes were enucleated under anesthesia at 9 days (n = 3), 2 weeks (n = 3), 3 weeks (n = 4), 5 weeks (n = 6), and 10 weeks (n = 5) posttransplantation (Table 2). Following enucleation, pigs were killed using 2–4 g intravenous pentobarbital (Pentobarbital 200 mg/mL, KVL, Copenhagen, Denmark). Globes were placed in 4% paraformaldehyde (PFA) for 10–20 min, the anterior segment and lens removed, and posterior segment postfixed for 2 h in 4% PFA, with subsequent rinsing in rising concentrations of sucrose containing Sörensen's phosphate buffer. For each globe, a horizontal cut was made from temporal retinal margin to 2–3 mm nasal to the optic disc, so as to include the temporal ciliary margin, area centralis, and optic disc. Tissues were embedded in gelatin and sectioned at 12 μm on a cryostat. Every 15th section was examined by epifluorescence microscopy for GFP+ cells and every 10th stained with H&E.
Sections for immunolabeling were incubated with primary antisera for 16–18 h in a moist chamber at 4°C, rinsed in 0.1 M phosphate-buffered saline (PBS) with 0.25% Triton X-100, then incubated with secondary Texas Red-conjugated antibodies (1:200, Jackson Immunoresearch, West Grove, PA) for 1–2 h at room temperature in the dark. Nonoperated eyes served as untreated controls and there were additional negative controls from operated eyes in which incubation with primary antisera was omitted. Specimens were examined using an epifluorescence microscope and colocalization of GFP and Texas Red-labeled primary antibodies was assessed by superimposition of separate digital images of each fluorochrome.
Results
Fetal GFP-transgenic pigs could be distinguished from nontransgenic littermates by their yellowish appearance under ambient room lighting or, more definitively, by their striking appearance under illumination with fluorescent light of 480 nm (Fig. 1A). Fetal pigs showed evidence of widespread GFP expression, although the fluorescence did not appear to be uniformly distributed. In some cases, illumination of the intact fetal eye produced a green reflex, suggestive of substantial GFP within the developing retina (Fig. 1B). On closer examination, the observed level of endogenous GFP-associated fluorescence exhibited by fetal tissues was also variable and appeared to reflect a mosaic pattern of expression, as previously reported for these animals (Carter et al., 2002) and illustrated here in the mature retina (Fig. 1C).
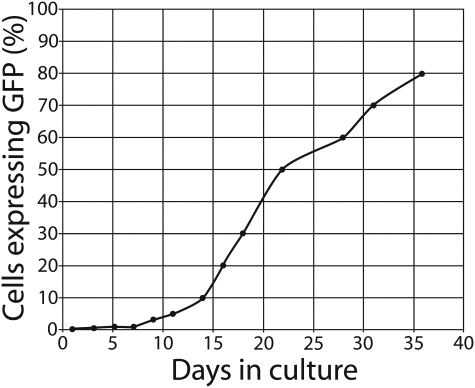
Following tissue dissection and dissociation of tissue from the brain, neural retina, and mincing of the isolated corneoscleral limbus, viable cellular populations were obtained from each of these tissue types (Fig. 1D–F). Within cultured cell populations, the percentage of strongly GFP+ profiles was frequently low yet repeated passaging of cells consistently resulted in increasing levels of endogenous GFP fluorescence. For cells derived from the retina, the degree of GFP upregulation was striking. In the case of fetal retinal cells, less than 1% of the profiles expressed detectable GFP during initial plating, whereas this figure rose to 80% over the initial 36-day period in culture (Fig. 2).
FIG. 2.
GFP expression by retinal cells during the initial culture period. The percentage of cells exhibiting GFP fluorescence increased markedly over the initial 36 days in culture. GFP-positive cells were quite rare in primary retinal isolates from fetal GFP-transgenic pigs, and remained below 10% of the total population until culture day 14. Subsequently, the percentage of GFP-positive cells increased rapidly, reaching 50% on day 22 and 80% on day 36, the latest time point examined.
The morphology of cultured cellular populations varied with site of origin. Except for their green fluorescence, progenitors from GFP-transgenic pigs exhibited the features typical of such cells. Progenitor cells derived from the CNS (Fig. 1D,E) were indistinguishable from those previously described from the porcine brain [24] and neural retina (Klassen et al., 2007). Those derived from the corneo-scleral limbal region gave rise to a continuous monolayer of epithelioid morphology (Fig. 1F), consistent with other cells of this type from other species, including humans (Cotsarelis et al., 1989; Schermer et al., 1986; Tseng, 1989; Zieske et al., 1992).
Phenotypic marker expression by GFP-transgenic cells also corresponded to the pattern seen in our previous studies of porcine CNS progenitors (Klassen et al., 2007; Schwartz et al., 2005). In both brain and retinal cultures we replicated widespread expression of nestin, vimentin, Sox2, Ki-67, NCAM, as well as more circumscribed expression of CD15, DCX, GFAP, PSA-NCAM, synapsin I, and β-III tubulin (see supplemental data). AQP4 expression was replicated in brain cultures (Schwartz et al., 2005) but not evaluated in retinal cultures. Retinal cultures contained a subpopulation of recoverin-expressing cells, as previously reported (Klassen et al., 2007). More detailed examination of brain-derived cultures showed that expression of the neurodevelopmental marker Sox2 was particularly widespread, as was the proliferation marker Ki-67, albeit to a lesser degree, with considerable overlap in the distribution of these two markers of phenotypic immaturity. In contrast, expression of the marker GFAP was restricted, with little evidence of either Sox2 or Ki-67 coexpression by these cells (Fig. 3A). These findings are consistent with cultures predominantly consisting of immature progenitor cells, but also containing restricted sub-populations of more mature cells of neural lineage, as we have previously reported.
Supplemental Data
| Marker | Forebrain | Retina |
|---|---|---|
| Nestin | X | X |
| Vimentin | X | X |
| Sox2 | X | X |
| Ki-67 | X | X |
| NCAM | X | X |
| CD15 | x | x |
| DCX | x | x |
| GFAP | x | x |
| PSA-NCAM | x | x |
| synapsin 1 | x | x |
| Beta-3 tubulin | x | X |
| AQP4 | x | not tested |
FIG. 3.
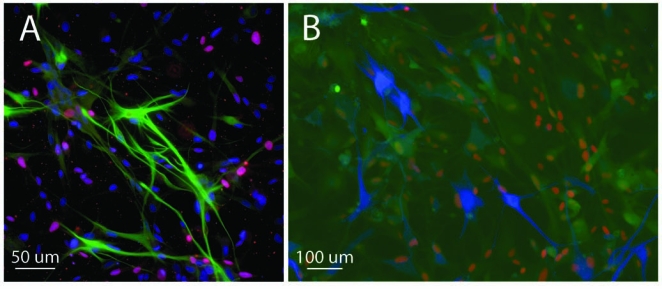
Expression of markers by GFP-transgenic brain progenitor cultures. Cultures derived from the fetal forebrain showed widespread nuclear expression of the neurodevelopmental marker Sox2, shown here in either blue (A) or red (B), as well as the proliferation marker Ki-67, shown in red (A), with colabeling for these markers shown in varying intermediate (purplish) shades (A). A subpopulation of cellular profiles was positive for GFAP, seen here as either green (A) or blue (B), and these tended not to colabel for Sox2 or Ki-67. Examination of endogenous GFP expression, shown in green in (B), revealed variable levels of transgene expression in this culture with many, but not all, of the Sox2–expressing profiles also GFP+ (coexpression shown in yellow-orange shades). There was little evidence for GFP expression by the subpopulation of GFAP+ (blue) profiles (B) in culture. (See paper online for Fig. 3 in color)
In the present study it was possible to further evaluate the pattern of immunoreactivity with particular attention to the degree of correspondence between the expression of specific phenotypic markers and the GFP transgene. Comparison of the majority Sox2-expressing population in brain-derived cultures with the minority GFAP-expressing subpopulation revealed that many, but not all, of the Sox2-expressing cells were also GFP+, whereas there was little evidence for GFP expression by GFAP+ cells (Fig. 3B). Because GFAP is more often associated with mature cell types than is Sox2, the lack of endogenous GFP fluorescence in GFAP+ cells raises the possibility of active downregulation of transgene expression by cells as they mature.
Following transplantation to the subretinal space of pigs with a variety of retinal lesions, GFP+ profiles were subsequently identified in all recipient animals (n = 21). Surviving cells were seen in the vitreous, retina, subretinal space, retinal pigment epithelium (RPE), and in one instance in the choroid (Table 2 and Figs. 4 and 5). Transplanted progenitors were able to integrate morphologically into host cellular layers and prominent GFP expression allowed visualization of fine cellular processes extending into the surrounding parenchyma. Integration into the neural retina was seen in 11 out of 21 recipients, including [1/3] that received brain-derived cells ([2/6]) and a majority ([9/15]) of those that had received retina-derived cells (Table 2). GFP+ brain progenitors could be seen migrating into the retina from either the vitreal surface (Fig. 4D–F) or the subretinal space (Fig. 4I). In two cases, GFP+ retinal progenitors formed characteristic rosettes suggestive of photoreceptor differentiation, as we previously reported with non-GFP porcine retinal progenitor cells (Klassen et al., 2007). Radial integration into the ONL and INL was also seen (Fig. 4I–K), although the phenotypic status of these was not established. Survival and retinal integration were replicated with each of the different types of retinal lesions, namely subretinal scraping, laser photocoagulation, transient elevated intraocular pressure, and laser combined with pressure (Table 2). Retinal integration was enhanced in the vicinity of injury (Fig. 4L–N), as we have previously reported in a number of mammalian models (Klassen et al., 2004; Warfvinge et al., 2005; Young et al.).
FIG. 4.
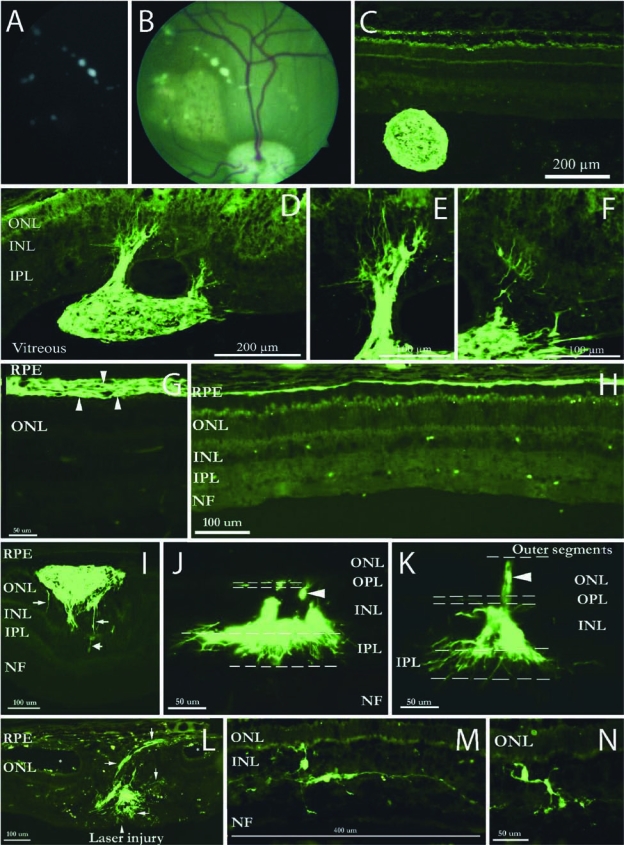
Transplantation and integration of GFP-transgenic progenitors in the eye. Following transplantation, spherical aggregates could be seen in the vitreous cavity, shown here for brain-derived cells and viewed both without back-illumination (A) and with the fundus in the background (B). Preretinal aggregates were confirmed histologically as GFP+ spheres (C). GFP+ spheres adhered to the vitreal surface of the retina, resulting in migration of GFP+ profiles into the host tissue and elaboration of fine processes (D–F). Retinal-derived donor cells survived in the subretinal space (G, arrowheads) and integrated into the RPE layer (H) as well as the neural retina (I–N). Integrating cells frequently displayed a radial orientation, seen here as vertical (I, arrows; J, arrowhead; K, arrowhead), and extended processes within the plexiform layers, especially the IPL (J,K). GFP+ profiles showed a tropism for areas of injury (L, arrows), including that produced by laser photocoagulation (L, centered on arrowhead), with asterisks indicating retinal lacunae peripheral to the central axis of the laser-induced damage (L). Individual profiles within the INL displayed both radial and horizontal orientations (M,N). (See paper online for Fig. 4 in color.)
FIG. 5.
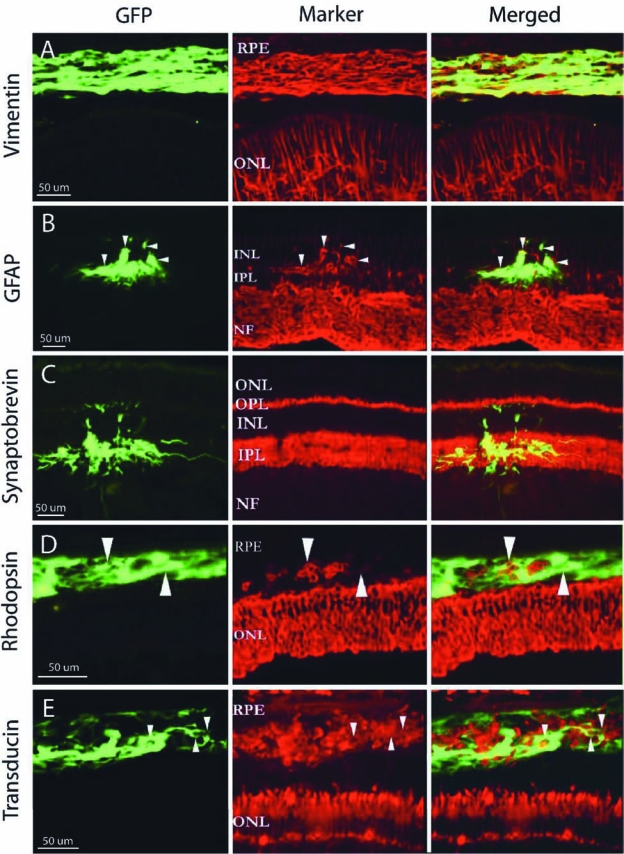
Marker expression by grafted GFP-transgenic retinal progenitors. Following transplantation, GFP+ porcine RPCs in the subretinal space broadly expressed vimentin (A), while others located in the INL colabeled with GFAP (B), although labeling of donor profiles with this marker was limited (arrowheads). GFP+ processes exhibited a tropism for the synaptic layers of the retina and colabeled extensively with synaptobrevin within them (C). Grafted cells expressed the photoreceptor markers rhodopsin (D) and transducin (E) in the subretinal space, although this appeared to be associated with limited GFP coexpression for both markers (arrowheads). (See paper online for Fig. 5 in color.)
Evaluation of marker expression in vivo revealed that GFP+ retinal-derived cells in the subretinal space exhibited widespread colabeling for vimentin (Fig. 5A–C). Ramifying GFP+ processes within the inner plexiform layer (IPL) showed extensive colabeling with synaptobrevin, while those extending into the cellular layers on either side of the IPL did not (Fig. 5D–F). Grafted retinal-derived cells in the subretinal space also expressed the photoreceptor markers transducin (Fig. 5G–I) and rhodopsin (Fig. 5J–O), although the degree of GFP colabeling was more limited than with the other markers. There was not evidence of immune cell infiltration of the grafts at any time point; however, there was more variability in the overall amount of GFP+ profiles seen at the latest time points examined, namely 3 weeks for brain-derived cells and 10 weeks for retina-derived cells (Table 2).
Discussion
Identification and visualization of grafted donor cells remains a pivotal methodological challenge for the field of stem cell transplantation. The standard for comparison in this regard is the use of cells derived from transgenic animals capable of germ-line transmission of the GFP (or other fluorescent protein) transgene. While tremendous progress has been made with respect to the generation of transgenic fluorescent mice, this technology has only recently become available for large animal studies. Here we show that progenitors can be cultured from the brain, retina, and corneolimbal region of fetal GFP-transgenic pigs, thereby expanding on previous work in this model reporting the isolation of progenitor cells from peripheral blood (Price et al., 2006). In addition, we demonstrate here the utility of using GFP-transgenic porcine progenitor cells in experiments involving transplantation to the injured retina of allogeneic recipients.
Characterization of cultured cells from the CNS of fetal GFP-transgenic pigs revealed a strong correspondence with previously obtained cell types from the brain (Schwartz et al., 2005) and retina (Klassen et al., 2007) of nontransgenic domestic pigs. These similarities included morphological features, proliferative activity, and expression of immature and mature markers. The corneo-limbal cultures generated confluent monolayers of flat cells of epithelioid appearance, consistent with the presence of limbal progenitor cells within these cultures, although further work will be necessary to verify the identity and potential of these cells. From the different porcine progenitor cell types investigated here, the only difference between GFP-transgenic and nontransgenic cells appears to be expression of the fluorescent reporter gene. Although future investigations may reveal other differences, the relative normalcy of the GFP-transgenic cells should not be unexpected considering that the cells were obtained from a lineage of viable transgenic animals rather than as a result of extensive in vitro modification of cell lines.
After transplantation to the posterior vitreous or subretinal space, the cells maintained endogenous GFP expression sufficient to allow positive identification of grafted cells as they migrated into, and took up residence within, the host neural retina. In addition, it was possible to visualize the morphology of fine cellular processes extending from these cells within the context of the surrounding retinal cytoarchitecture. Our previous work in the pig allograft model, which had relied exclusively on the use of vital dyes to label grafted progenitor cells, was only able to show very limited evidence in this regard (Klassen et al., 2007). Importantly, we are now able to show that the processes of grafted porcine retinal progenitor cells exhibit a notable degree of respect for the laminar organization of the retina, preferentially ramifying toward the outer plexiform layer (OPL) and within the IPL of the host.
GFP expression also allowed for more detailed examination of marker coexpression by grafted cells than was possible in our previous study. In particular, synaptobrevin was seen to strongly colocalize with GFP+ processes, specifically within the host plexiform layers where synapses are normally found. GFP+ profiles in the cellular layers were negative for this synaptic marker. These data suggest differentiation of grafted cells along the neuronal lineage and raise the possibility of at least some degree of directed synaptic integration into the local retinal circuitry. The present study also verified the expression of photoreceptor markers by grafted cells within the subretinal space, as we reported previously in both mouse (Klassen et al., 2004) and pig (Klassen et al., 2007) retinal progenitor allograft models. In addition, we found preliminary evidence indicating that the porcine cells are also capable of integrating into the ONL and assuming an appropriate radial orientation within that layer, as previously seen in mouse studies (Klassen et al., 2004; MacLaren et al., 2006). Whether these same cells also develop outer segments and express photoreceptor markers is not yet clear.
An additional consideration is variability in expression of the GFP reporter gene itself. It was previously known that the strain of GFP-transgenic pig used here for donor cell derivation exhibits a mosaic transgene expression pattern (Carter et al., 2002). That being the case, it was unclear from the outset whether CNS progenitor cells cultured from fetal tissue would successfully express GFP at all. Interestingly, although our initial cultures contained relatively few GFP+ profiles, the prevalence of transgene expressing cells increased to a very significant extent with repeated passaging in culture to the point where GFP+ cells dominated the cultures. This enrichment for GFP+ cells appeared to result from increased expression of the GFP transgene in previously nonexpressing cells, possibly associated with an abrogation of endogenous transgene suppression. Although tentative, this conclusion is based on the rapid rate of change in fluorescence and the neighbor–neighbor relationship of GFP+ cells in the cultures, which did not suggest strictly clonal origins. Furthermore, close inspection of the immunolabeling data from the present study reveals some additional findings in this respect. In particular, there appeared be greater coexpression of GFP in association with the immature, albeit nonspecific, progenitor markers vimentin and Ki-67, and noticeably decreased expression of GFP in association with more mature markers GFAP, rhodopsin, and transducin. Most likely, the observed variability in transgene expression relates to the use of a CMV promoter, which appears to function optimally for cells in culture, but is suppressed by differentiated cells of the retina. Initial results from a more recently developed GFP-transgenic line (NT92) in which the GFP gene is driven by a β-actin promoter indicate that more uniform and sustained expression is attainable (Prather et al., unpublished data). Since the GFP signal appears to diminish upon differentiation, the number of GFP+ cells detected after 10 weeks is likely to underrepresent the number of cells that actually survived at that time point. Similarly, it is possible that the morphological evidence presented here underrepresents the degree and extent of progenitor cell integration obtained in the retina, specifically in reference to cells differentiating into radially oriented photoreceptor cells within the ONL.
In the present study, endogenous GFP expression allowed us to demonstrate that cultured progenitor cells can survive transplantation to the allogeneic retina for at least 10 weeks, the latest time point examined. This doubles our previously reported survival time of 5 weeks in the pig (Klassen et al., 2007; Warfvinge et al., 2005) and can be attributed to the advantages of using GFP+ allogeneic cells as donor material. Furthermore, even at 10 weeks there was no evidence of rejection such as perivascular cuffing or cellular infiltration of either the choroid or the grafts, suggesting that longer survival times are achievable and that expression of a modified jellyfish protein (GFP) by donor cells is relatively well tolerated in the microenvironment of the pig retina. Nevertheless, because the overall number of GFP+ cells could be interpreted as diminishing at 3 weeks for the brain progenitors and at 10 weeks for the retinal progenitors, the possibility of a more gradual attrition of donor cell cannot be ruled out. This apparent decrease in cell viability might actually reflect decreased levels of GFP expression as the cells differentiate or simply variability between samples.
Conclusion
The ability to culture progenitor populations from the pig eye and brain, combined with the availability of a GFP-transgenic pig from which to source these cells, improve considerably the utility of this large animal model in the context of developing regenerative approaches to the retina and elsewhere. Successful progenitor cell transplantation in the pig eye provides an important demonstration that the results previously obtained in rodents can be reproduced in large mammals. Further studies are indicated wherein the advantageous donor cells developed here are combined with more specific porcine models of ocular disease (Petters et al., 1997), as well as refined methods of subretinal delivery such as biodegradable polymer scaffolds (Klassen, 2006; Lavik et al., 2005; Tao et al., 2007; Tomita et al., 2005; Warfvinge et al., 2005).
Acknowledgments
The authors would like to thank Tasneem Zahir, Boback Ziaeian, Teresa Almeda, Hubert Nethercott, and Maria Voss Kyhn for assistance with technical aspects of this project, and Max Aguilera-Hellweg for generously providing the GFP pig photograph used. This work was supported by the Lincy Foundation, Gail and Richard Siegal Foundation, Discovery Eye Foundation, CHOC Foundation, Guilds, and Padrinos (HK, PHS), Larry Hoag Foundation (HK), 2nd ONCE International Award for New Technologies for the Blind (KW); the Crown Princess Margareta's Committee for the Blind (KW), the Swedish Association of the Visually Impaired (KW), the Swedish Science Council (Medicine) (KW), the Minda de Gunzburg Research Center for Retinal Transplantation (MJY), and the NIH: R01RR13438 and U42RR18877 (RSP), NS044060 (HK), EY09595 (MJY).
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- Brustle O. Jones K.N. Learish R.D., et al. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;285:754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- Carter D.B. Lai L. Park K.W., et al. Phenotyping of transgenic cloned piglets. Cloning Stem Cells. 2002;4:131–145. doi: 10.1089/153623002320253319. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G. Cheng S.Z. Dong G., et al. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- Couly G.F. Coltey P.M. Le Douarin N.M. The developmental fate of the cephalic mesoderm in quail-chick chimeras. Development. 1992;114:1–15. doi: 10.1242/dev.114.1.1. [DOI] [PubMed] [Google Scholar]
- Devgan V. Rao M.R. Seshagiri P.B. Impact of embryonic expression of enhanced green fluorescent protein on early mouse development. Biochem. Biophys. Res. Commun. 2004;313:1030–1036. doi: 10.1016/j.bbrc.2003.11.184. [DOI] [PubMed] [Google Scholar]
- Guo J.K. Cheng E.C. Wang L., et al. The commonly used beta-actin-GFP transgenic mouse strain develops a distinct type of glomerulosclerosis. Transgenic Res. 2007;16:829–834. doi: 10.1007/s11248-007-9107-x. [DOI] [PubMed] [Google Scholar]
- Huang W.Y. Aramburu J. Douglas P.S., et al. Transgenic expression of green fluorescence protein can cause dilated cardiomyopathy. Nat. Med. 2000;6:482–483. doi: 10.1038/74914. [DOI] [PubMed] [Google Scholar]
- Klassen H. Transplantation of cultured progenitor cells to the mammalian retina. Expert Opin. Biol. Ther. 2006;6:443–451. doi: 10.1517/14712598.6.5.443. [DOI] [PubMed] [Google Scholar]
- Klassen H. Lund R. Parameters of retinal graft-mediated responses are related to underlying target innervation. Brain Res. 1990;533:181–191. doi: 10.1016/0006-8993(90)91338-h. [DOI] [PubMed] [Google Scholar]
- Klassen H.J. Ng T.F. Kurimoto Y., et al. Multipotent retinal progenitors express developmental markers, differentiate into retinal neurons, and preserve light-mediated behavior. Invest. Ophthalmol. Vis. Sci. 2004;45:4167–4173. doi: 10.1167/iovs.04-0511. [DOI] [PubMed] [Google Scholar]
- Klassen H. Kiilgaard J. Zahir T., et al. Progenitor cells from the porcine neural retina express photoreceptor markers after transplantation to the subretinal space of allorecipients. Stem Cells. 2007;25:1222–1230. doi: 10.1634/stemcells.2006-0541. [DOI] [PubMed] [Google Scholar]
- Kuehn M.H. Kim C.Y. Ostojic J., et al. Retinal synthesis and deposition of complement components induced by ocular hypertension. Exp. Eye Res. 2006;83:620–628. doi: 10.1016/j.exer.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Lavik E. Klassen H. Warfvinge K., et al. Fabrication of degradable polymer scaffolds to direct the integration and differentiation of retinal progenitors. Biomaterials. 2005;26:3187–3196. doi: 10.1016/j.biomaterials.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Liu H.S. Jan M.S. Chou C.K., et al. Is green fluorescent protein toxic to the living cells? Biochem. Biophys. Res. Commun. 1999;260:712–717. doi: 10.1006/bbrc.1999.0954. [DOI] [PubMed] [Google Scholar]
- MacLaren R.E. Pearson R.A. MacNeil A., et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- Martinez-Serrano A. Lundberg C. Horellou P., et al. CNS-derived neural progenitor cells for gene transfer of nerve growth factor to the adult rat brain: complete rescue of axotomized cholinergic neurons after transplantation into the septum. J. Neurosci. 1995;15:5668–5680. doi: 10.1523/JNEUROSCI.15-08-05668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostenfeld T. Horn P. Aardal C., et al. Mouse epidermal growth factor-responsive neural precursor cells increase the survival and functional capacity of embryonic rat dopamine neurons in vitro. Neuroreport. 1999;10:1985–1992. doi: 10.1097/00001756-199906230-00035. [DOI] [PubMed] [Google Scholar]
- Palmer T.D. Takahashi J. Gage F.H. The adult rat hippocampus contains primordial neural stem cells. Mol. Cell. Neurosci. 1997;8:389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- Park K.W. Kühholzer B. Lai L., et al. Development and expression of the green fluorescent protein in porcine embryos derived from nuclear transfer of transgenic granulosa-derived cells. Anim. Reprod. Sci. 2001;68:111–120. doi: 10.1016/s0378-4320(01)00138-5. [DOI] [PubMed] [Google Scholar]
- Petters R.M. Alexander C.A. Wells K.D., et al. Genetically engineered large animal model for studying cone photoreceptor survival and degeneration in retinitis pigmentosa. Nat. Biotechnol. 1997;15:965–970. doi: 10.1038/nbt1097-965. [DOI] [PubMed] [Google Scholar]
- Price E.M. Prather R.S. Foley C.M. Multipotent adult progenitor cell lines originating from the peripheral blood of green fluorescent protein transgenic swine. Stem Cells Dev. 2006;15:507–522. doi: 10.1089/scd.2006.15.507. [DOI] [PubMed] [Google Scholar]
- Reynolds B.A. Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Rubio F. Kokaia Z. Arco A., et al. BDNF gene transfer to the mammalian brain using CNS-derived neural precursors. Gene Ther. 1999;6:1851–1866. doi: 10.1038/sj.gt.3301028. [DOI] [PubMed] [Google Scholar]
- Ryder E.F. Snyder E.Y. Cepko C.L. Establishment and characterization of multipotent neural cell lines using retrovirus vector-mediated oncogene transfer. J. Neurobiol. 1990;21:356–375. doi: 10.1002/neu.480210209. [DOI] [PubMed] [Google Scholar]
- Schermer A. Galvin S. Sun T.T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J. Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz P. Bryant P. Fuja T., et al. Isolation and characterization of neural progenitor cells from post-mortem human cortex. J. Neurosci. Res. 2003;74:838–851. doi: 10.1002/jnr.10854. [DOI] [PubMed] [Google Scholar]
- Schwartz P. Nethercott H. Kirov I., et al. Expression of neurodevelopmental markers by cultured porcine neural precursor cells. Stem Cells. 2005;23:1286–1294. doi: 10.1634/stemcells.2004-0306. [DOI] [PubMed] [Google Scholar]
- Swenson E.S. Price J.G. Brazelton T, et al. Limitations of green fluorescent protein as a cell lineage marker. Stem Cells. 2007;25:2593–2600. doi: 10.1634/stemcells.2007-0241. [DOI] [PubMed] [Google Scholar]
- Tao S. Young C. Redenti S., et al. Survival, migration and differentiation of retinal progenitor cells transplanted on micro-machined poly(methyl methacrylate) scaffolds to the subretinal space. Lab Chip. 2007;7:695–701. doi: 10.1039/b618583e. [DOI] [PubMed] [Google Scholar]
- Terada N. Hamazaki T. Oka M., et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- Tomita M. Lavik E. Klassen H., et al. Biodegradable polymer composite grafts promote the survival and differentiation of retinal progenitor cells. Stem Cells. 2005;23:1579–1588. doi: 10.1634/stemcells.2005-0111. [DOI] [PubMed] [Google Scholar]
- Tseng S.C. Concept and application of limbal stem cells. Eye. 1989;3:141–157. doi: 10.1038/eye.1989.22. [DOI] [PubMed] [Google Scholar]
- Warfvinge K. Kiilgaard J. Lavik E., et al. Retinal progenitor cell xenografts to the pig retina: morphological integration and cytochemical differentiation. Arch. Ophthalmol. 2005;123:1385–1393. doi: 10.1001/archopht.123.10.1385. [DOI] [PubMed] [Google Scholar]
- Warfvinge K. Kiilgaard J. Klassen H., et al. Retinal progenitor cell xenografts to the pig retina: immunological reactions. Cell Transplant. 2006;15:603–612. doi: 10.3727/000000006783981594. [DOI] [PubMed] [Google Scholar]
- Young M.J. Ray J. Whiteley S.J., et al. Neuronal differentiation and morphological integration of hippocampal progenitor cells transplanted to the retina of immature and mature dystrophic rats. Mol. Cell. Neurosci. 2000;16:197–205. doi: 10.1006/mcne.2000.0869. [DOI] [PubMed] [Google Scholar]
- Zieske J.D. Bukusoglu G. Yankauckas M.A. Characterization of a potential marker of corneal epithelial stem cells. Invest. Ophthalmol. Vis. Sci. 1992;33:143–152. [PubMed] [Google Scholar]