Abstract
Introduction
Individuals who have deep and periventricular white matter hyperintensities may have a higher risk for suicidal behavior. There are mixed results in the literature regarding whether unipolar or bipolar patients who have attempted suicide have more MRI findings of deep white matter hyperintensities (DWMH) or periventricular hyperintensities (PVH) relative to those who have no history of suicide attempts.
Methods
We performed a meta-analysis of studies examining white matter hyperintensities (WMH) in mood disorder patients with and without a history of suicide attempts.
Results
Four studies and a total of 173 patients who attempted suicide and 183 who did not attempt were included. A significantly higher number of attempters were found to have hyperintensities than non-attempters. Unipolar depressed patients who were attempters had 1.9 times more DWMH and 2.1 times more PVH than those who were non-attempters. Bipolar patients who were attempters had 5.4 times more PVH than those who were non-attempters. Taken together, unipolar and bipolar patients who were attempters had 2.8 times more DWMH and 4.5 times more PVH than those who were not attempters.
Conclusions
These findings raise the possibility that WMH are biological substrates of symptoms that lead to suicidal behavior.
Keywords: meta-analysis, MRI, deep white matter hyperintensities, periventricular white matter hyperintensities, suicide, unipolar depression, bipolar depression
Introduction
As the 11th leading cause of death among all age groups and the 2nd among adults between the ages of 25 and 34, suicide represents a major health problem.1 Approximately 13.5% of the U.S. population have experienced suicidal thoughts or wishes at some point in their lives,2 and 2.7% have made a suicide attempt with the intent to die.3 Globally, suicide accounted for 16 deaths for every 100,000 persons in the year 2000. Although reported suicide rates have been higher among the male elderly, rates among young people have increased to such an extent that they are at present the group at highest risk in a third of developed and developing countries.4 Ten to 15% of bipolar disorder patients and 2-12% of the patients with major depressive disorder die by suicide.5,6
Despite efforts from different research fields aiming a significant reduction of completed and attempted suicide, studies addressing prediction and prevention issues have yielded disappointing results. Until the middle of the past century, suicide prevention approaches were based largely on clinical experience, but most recent approaches to suicide prevention are based on scientifically sound empirical evidence.7 Considerable evidence from postmortem studies has suggested abnormalities in the brain serotonin system of individuals who died by suicide. It has been demonstrated that serotonin receptor changes associated with major depression could be distinguished from receptor changes associated with suicide.8 Also, low 5-hydroxyindoleacetic acid (5HIAA) concentrations, a serotonin metabolite, as well as higher levels of platelet 5HT2A receptors have been demonstrated in the CSF of depressed patients with a history of suicide attempts.9,10 Furthermore, it has also been suggested that a genetic susceptibility may play a role in suicide, as patients with a family history of suicide present an increased risk for both suicide attempts and completed suicide.11
Sophisticated neuroimaging techniques provide another evidenced-based approach to study suicide and have been widely applied to the study of affective disorders. Structural magnetic resonance imaging (sMRI) alterations such as subcortical and periventricular white matter hyperintensities (WMH) have been demonstrated in older depressed patients.12,13,14 Interestingly, these abnormalities have also been described in younger depressed patients, but they appear to be restricted to suicidal subjects in this age group.15,16
WMH represent ependymal loss and differing degrees of myelination and appear as high intensity signal lesions on T2-weighted MRI sequence, which may be classified as deep white matter hyperintensities (DWMH) and periventricular hyperintensities (PVH) depending on localization.17,18 Although it is generally agreed that etiology is related to vascular events, little is known about pathophysiological processes underlying these lesions. It has been proposed that, depending on their localization, WMH might cause disruption of neural circuits involved in different emotional processes, resulting in increased vulnerability to psychiatric morbidity.15,17 WMH are common in depressed and nondepressed subjects, and research suggests that their location is the critical factor for the pathogenesis of depression.19 According to Soares and Mann,20 white matter lesions may interrupt fibers in regions considered important for mood regulation, resulting in disconnection among those regions, which may result in depression. Additionally, Iosifescu et al.21 suggested that subcortical WMH in the left hemisphere (but not in other brain areas) may be associated with poor response to antidepressant treatment in major depression.
A limited number of studies have examined MRI WMH in depressed patients who have attempted suicide. The first study examining the relation between hyperintensities and suicidality was conducted by Ahearn et al.,22 who found more subcortical grey matter hyperintensities in unipolar patients with a history of suicide attempt compared with patients without such a history. This group, and subsequently other investigators, also investigated pathological white matter hyperintensities in unipolar and/or bipolar patients who had attempted suicide.15,16,17,18 As there have been mixed results in investigators’ attempts to understand the role of pathological hyperintensities in suicidal behavior, and predicting those at risk for suicide remains difficult, more research on this issue is warranted. The main objective of this meta-analysis was to investigate the relation between WMH and suicidality in patients with unipolar or bipolar depression in the studies on this topic that have been published to date.
Methods
We performed a search of the Medline, PsycInfo, Biological Abstracts and Web of Science databases using the following expressions: “white matter ” and “suicid*”. To ensure that no study was missed, a free-text search was carried out using the expression “white matter hyperintensities”. All publications available up to July 2009 were considered. After performing the electronic search, the references of all studies considered relevant were carefully examined, including meta-analyses and reviews, in order to locate any additional articles not identified in the above-mentioned search.
To be included in this review, a study had to meet the following criteria: 1) to be published irrespective of the language; 2) to be carried out in a population predominantly diagnosed with affective disorders (i.e., unipolar or bipolar disorders); 3) to use sMRI to assess white matter hyperintensities and 4) to present retrospective or prospective outcome data (for example, proportion of patients who attempted suicide vs. patients who did not).
To obtain the potentially relevant studies, three independent reviewers (CS, IRO, and MCG) evaluated the abstracts identified in the literature search. Next, the same three reviewers, working independently, decided which of those papers fulfilled the inclusion criteria. Disagreement at any stage was resolved by consensus. For each study investigated, a data collection form was used, and these data were obtained independently by the three reviewers.
Statistical analyses were performed using Comprehensive Meta-Analysis Software, version 2.2.048, November 7, 2008.23 For each study, odds ratios and 95% confidence intervals were calculated. We used a random effects model that weighted the studies according to the inverse variance and calculated the odds ratio and the corresponding confidence interval. A random effects model was chosen due to its being more conservative than a fixed effect model.
A Q test of homogeneity or “combinability” for the odds ratios was calculated to ascertain whether pooling was viable among the selected studies (p> 0.05). Data from the included studies were broken down into 2×2 contingency tables, presenting the proportion of patients who attempted suicide compared to those who did not attempt suicide. The number of studies (with null results) needed to overturn the conclusions (fail-safe n) when a significant result was found was also calculated using WinPepi software.24 The fail-safe n is used to address the problem posed by unpublished non significant results residing in the “file drawers” of the researchers conducting relevant studies. It estimates how many studies with null results must be in the file drawers to overturn the results of the combined significant test. The higher the significance, the more negative studies are necessary to counteract such result, and the higher the fail-safe n, the more confidence exists in the finding.25
Results
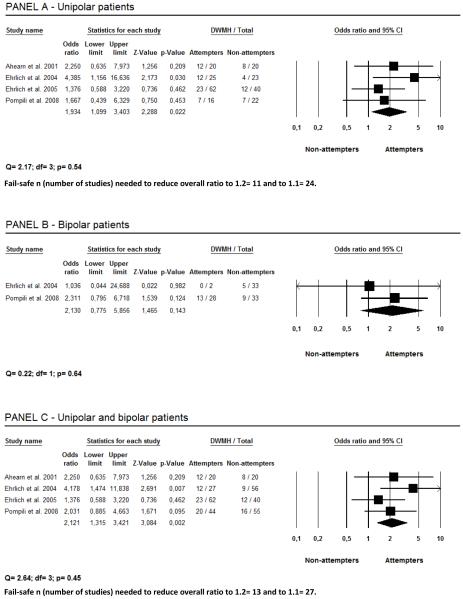
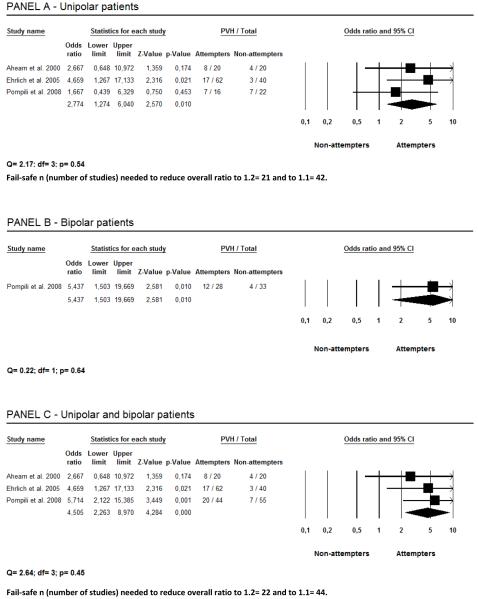
Studies examining the prevalence of WMH in depressed and bipolar patients who attempted suicide and those who did not are shown in Table 1. Of the five articles that fulfilled inclusion criteria for this meta-analysis, two did not contain all the information that would permit calculations.18,22 Non published data on WMH from the study by Ahearn et al.18 were obtained from the authors (including D.C.S.). One of us (M.P.) provided data from the Pompili et al. study.18 One article17 was excluded in order to avoid double inclusion because some patients were investigated again in a more recent paper.18 Thus, four studies provided comparative data from patients with DWMH and PVH who attempted suicide vs. those who did not.15,16,18,22 A test for heterogeneity of the odds ratio of the four studies revealed that the groups were not significantly different for either DWMH (p>0.45; total group= 228 subjects) or PVH (p> 0.56; total group= 180 subjects).
Table 1.
Characteristics of MRI studies of hyperintensities in unipolar and bipolar patients with and without a history of suicide attempts
| Study |
Depression Type |
SA/NSA (N) |
Depression Diagnostic Criteria |
Mean Age, Years SA/NSA |
MRI Magnet Strength |
Slice Thickness (mm) |
Lesion Classification System |
|---|---|---|---|---|---|---|---|
| Ahearn et al.22 |
Unipolar | 20/20 | DSM-IV | 66.0/66.4 | 1.5 T | 2 | Boyko scale Coffey scale |
| Ehrlich et al.15 |
Unipolar and bipolar |
43/110 | DSM-IV | 15.1 (total sample) |
1.5 T | 5 | Coffey scale (modified) |
| Ehrlich et al.16 |
Unipolar | 62/40 | DSM-IV | 26.7 (total sample) |
? | 5/3a | Fazekas (modified) |
| Pompili et al.18 |
Unipolar and bipolar |
44/55 | DSM-IV-TR | 45.6/47.3 | 1.5 T | 5 | Fazekas (modified) |
SA = suicide attempts; NSA = no suicide attempts.
The first number applies to axial acquisition and the second number to coronal acquisition.
As shown in Figure 1, suicide attempters were more likely to have DWMH in comparison with non-attempters. The result of this random effects meta-analysis shows that unipolar depressed patients who presented with a history of suicide attempts had 1.9 times more DWMH than those without such a history (Panel A). Only two studies provided data on DWMH in bipolar patients, showing no significant difference between attempters and non-attempters (Panel B). However, when data on unipolar and bipolar patients were taken together subjects who presented with a history of suicide attempts had 2.3 times more DWMH than those who did not attempt suicide (Panel C).
Figure 1.
Deep white matter hiperintensities (DWMH) and their relationship with suicidality.
Figure 2 also shows that suicide attempters were more likely to have PVH in comparison with non-attempters. Unipolar depressed patients who presented with a history of suicide attempts had 2.8 times more PVH than those without such a history (Panel A). Panel B shows that bipolar patients who were attempters had 5.4 times more PVH than non-attempters. Taken together, unipolar and bipolar patients who presented a history of suicide attempts had 4.5 times more PVH than those who did not attempt suicide (Panel C).
Figure 2.
Periventricular hiperintensities (PVH) and their relationship with suicidality.
Discussion
To our knowledge, this is the first meta-analysis of sMRI studies examining WMH in patients with a history of suicide attempts. These results show a higher risk of suicidality in patients with such findings in comparison with controls (i.e., matched patients without DWMH or PVH). The present results suggest that WMH are biological alterations associated with suicidality in both unipolar and bipolar patients. Several studies have demonstrated that WMH have increased prevalence in medical conditions that have also been associated with increased suicide risk, such as cardiovascular disease and diabetes mellitus.26,27 In addition, increased frequency of WMH has also been reported in patients with hypertension.28 These findings raise the possibility that WMH interact with vascular risk to play a role in suicidal behavior in a yet unknown way.
WMH appear on T2-weighted and fluid-attenuated inversion recovery (FLAIR) MRI sequences as lesions of increased signal intensity. Although it is almost universally accepted that they are caused by vascular events resulting in recurrent episodes of hypoperfusion, the pathological meaning of WMH remains unclear. WMH have been associated with different impairments, including neurocognitive deficits,29 medical diseases,30 gait and balance problems,31 urinary dysfunction,32 and depression.16,33 Interestingly, different studies have found a negative association between the prevalence of past suicide attempts and age even in the presence of WMH, suggesting that WMH associated with suicidality may be etiologically different from those associated with cardiovascular risk factors.
The mechanism by which patients with WMH may be at a higher risk for suicide has not been elucidated. Taylor et al.19 have suggested that WMH possibly disrupt anatomic pathways involved in mood regulation. These disruptions involve key areas responsible for mood regulation which include frontal cortex, amygdala-hippocampus complex, thalamus, basal ganglia and the extensive connections between these areas.20 Mood regulation abnormalities could confer a biological alteration which, in combination with environmental stressors, results in suicidal behavior.18
This meta-analysis is limited by the small number of studies available for inclusion (n= 4). However, fail-safe ns were quite high, particularly concerning PVH in suicidal patients, suggesting that these findings are robust. Another possible limitation is the lack of information on issues as the effects of age, gender, comorbid disorders, disability, and cognitive impairment with disinhibition/impulsivity produced by the disconnection syndrome due to WMH. These are important variables to control for in future studies in order to determine the precise amount of variance that WMH accounts for in a history of suicide attempts.
In conclusion, results of this meta-analysis suggest that DWMH and PVH in unipolar and bipolar disorders might be a helpful biological marker of suicidality, as suggested by the authors of original studies (e.g., Pompili et al.18). However, a causal relationship cannot yet be established based on available evidence. Additionally, mechanisms by which patients with WMH are at a greater risk for suicide behavior remain unclear. Studies examining more specific localization of WMH may help identify the neural substrates associated with suicidality. Future prospective studies examining participants at high risk for suicidal behavior may be especially instructive.
Focus points.
Individuals who have deep and periventricular WMH may have a higher risk for suicidal behavior.
WMH are biological substrates of symptoms that lead to suicidal behavior.
Mechanisms by which subjects with WMH are at a greater risk for suicide behavior remain unclear.
WMH may interrupt fibers in regions considered important for mood regulation, which may result in depression.
Studies examining more specific localization of WMH may help identify the neural substrates associated with suicidality.
Acknowledgments
Thanks are due to Dr. Eileen Ahearn who kindly provided data on WMH not shown in their original publication,22 and to Dr. Josué Bacaltchuk for important suggestions.
Biographies and Disclosure Information
Miss Grangeon and Mrs. Seixas are post-graduate students in the Department of Neurosciences and Mental Health, Federal University of Bahia, Salvador, Brazil. Dr. Quarantini is clinical psychiatrist in the University Hospital Professor Edgard Santos, Federal University of Bahia, Salvador, Brazil. Dr. Miranda-Scippa is associate professor in the Department of Neurosciences and Mental Health, Federal University of Bahia, Salvador, Brazil. Dr. Pompili is in the Department of Psychiatry, Sant’Andrea Hospital, Sapienza University of Rome, Italy. Dr. Steffens is in the Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Durham, NC, USA. Dr. Wenzel is a clinical associate in the Department of Psychiatry, University of Pennsylvania, Philadelphia, USA. Dr. Lacerda is in the Department of Psychiatry, Federal University of São Paulo, São Paulo, Instituto Sinapse de Neurociências Clínicas, and the Center for Research and Clinical Trials Sinapse-Bairral, Brazil. Dr. de Oliveira is professor of psychiatry in the Department of Neurosciences and Mental Health at the Federal University of Bahia in Brazil.
Footnotes
Disclosure Information for Maria Conceição Grangeon for the past 12 months:
Nothing to disclose.
Disclosure Information for Camila Seixas for the past 12 months:
Nothing to disclose.
Disclosure Information for Lucas C. Quarantini for the past 12 months:
Nothing to disclose.
Disclosure Information for Angela Miranda-Scippa for the past 12 months:
Dr. Miranda-Scippa has received honoraria from AstraZeneca, Eli Lilly, Torrent and Wyeth.
Disclosure Information for Maurizio Pompili for the past 12 months:
Dr. Pompili has been a consultant or has been engaged in research with Eli Lilly and Organon Corporations.
Disclosure Information for David C. Steffens for the past 12 months:
Dr. Steffens is a consultant to TransForm Pharmaceuticals, Inc, has received financial support from Bristol-Myers Squibb for travel expenses to present at a national meeting, and receives support for research from the National Institute of Mental Health.
Disclosure Information for Amy Wenzel for the past 12 months:
Nothing to Disclose.
Disclosure Information for Acioly L. T. Lacerda for the past 12 months:
Dr. Lacerda has received grant support/honoraria form Aché, AstraZeneca, Eli Lilly, Boehringer Ingelheim, Bristol-Myers-Squibb, Janssen-Cilag, Solvay Farma, Moksha8, Roche and Wyeth.
Disclosure Information for Irismar Reis de Oliveira for the past 12 months:
Dr. de Oliveira has received grant support/honoraria from AstraZeneca, Bristol-Myers-Squibb, Eli Lilly, Janssen-Cilag, Pfizer and Roche.
References
- 1.Centers for Disease Control and Prevention [Accessed January 3rd, 2009];Web-Based Injury Statistics Query and Reporting System (WISQARS) Available at: http://www.cdc.gov/ncipc/dvp/Suicide/suicide_data_sheet.pdf.
- 2.Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch Gen Psychiatry. 1999;56(7):617–626. doi: 10.1001/archpsyc.56.7.617. [DOI] [PubMed] [Google Scholar]
- 3.Nock MK, Kessler RC. Prevalence of and risk factors for suicide attempts versus suicide gestures: analysis of the National Comorbidity Survey. J Abnorm Psychol. 2006;115(3):616–623. doi: 10.1037/0021-843X.115.3.616. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization [Accessed January 3rd, 2009]; Available at: http://www.who.int/mental_health/prevention/suicide/suicideprevent/en/
- 5.Bostwick JM, Pankratz VS. Affective disorders and suicide risk: a reexamination. Am J Psychiatry. 2000;157(12):1925–1932. doi: 10.1176/appi.ajp.157.12.1925. [DOI] [PubMed] [Google Scholar]
- 6.Sinclair JM, Harriss L, Baldwin DS, King EA. Suicide in depressive disorders: a retrospective case-control study of 127 suicides. J Affect Disord. 2005;87(1):107–113. doi: 10.1016/j.jad.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Wenzel A, Brown GK, Beck AT. Cognitive therapy for suicidal patients: Scientific and clinical applications. American Psychological Association; Washington DC: 2009. pp. 3–12. [Google Scholar]
- 8.Mann JJ. What does brain imaging tell us about the predisposition to suicidal behavior. Crisis. 2005;26(3):101–103. doi: 10.1027/0227-5910.26.3.101. [DOI] [PubMed] [Google Scholar]
- 9.Pandey GN, Pandey SC, Janicak PG, Marks RC, Davis JM. Platelet serotonin-2 receptor binding sites in depression and suicide. Biol Psychiatry. 1990;28(3):215–222. doi: 10.1016/0006-3223(90)90576-n. [DOI] [PubMed] [Google Scholar]
- 10.Pandey GN, Pandey SC, Dwivedi Y, Sharma RP, Janicak PG, Davis JM. Platelet serotonin-2A receptors: a potential biological marker for suicidal behavior. Am J Psychiatry. 1995;152(6):850–855. doi: 10.1176/ajp.152.6.850. [DOI] [PubMed] [Google Scholar]
- 11.Currier D, Mann JJ. Stress, genes and the biology of suicidal behavior. Psychiatr Clin North Am. 2008;31(2):247–269. doi: 10.1016/j.psc.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coffey CE, Figiel GS, Djang WT, Weiner RD. Subcortical hyperintensity on magnetic resonance imaging: a comparison of normal and depressed elderly subjects. Am J Psychiatry. 1990;147(2):187–189. doi: 10.1176/ajp.147.2.187. [DOI] [PubMed] [Google Scholar]
- 13.Rabins PV, Pearlson GD, Aylward E, Kumar AJ, Dowell K. Cortical magnetic resonance imaging changes in elderly inpatients with major depression. Am J Psychiatry. 1992;148(5):617–620. doi: 10.1176/ajp.148.5.617. [DOI] [PubMed] [Google Scholar]
- 14.Krishnan KR, McDonald WM, Doraiswamy PM, et al. Neuroanatomical substrates of depression in the elderly. Eur Arch Psychiatry Clin Neurosci. 1993;243(1):41–46. doi: 10.1007/BF02191522. [DOI] [PubMed] [Google Scholar]
- 15.Ehrlich S, Noam GG, Lyoo IK, Kwon BJ, Clark MA, Renshaw PF. White matter hyperintensities and their associations with suicidality in psychiatrically hospitalized children and adolescents. J Am Acad Child Adolesc Psychiatry. 2004;43(6):770–776. doi: 10.1097/01.chi.0000120020.48166.93. [DOI] [PubMed] [Google Scholar]
- 16.Ehrlich S, Breeze JL, Hesdorffer DC, et al. White matter hyperintensities and their association with suicidality in depressed young adults. J Affect Disord. 2005;86(2-3):281–287. doi: 10.1016/j.jad.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Pompili M, Ehrlich S, De Pisa E, et al. White matter hyperintensities and their associations with suicidality in patients with major affective disorders. Eur Arch Psychiatry Clin Neurosci. 2007;257(8):494–499. doi: 10.1007/s00406-007-0755-x. [DOI] [PubMed] [Google Scholar]
- 18.Pompili M, Innamorati M, Mann JJ, et al. Periventricular white matter hyperintensities as predictors of suicide attempts in bipolar disorders and unipolar depression. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1501–1507. doi: 10.1016/j.pnpbp.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Taylor WD, Payne ME, Krishnan KR, et al. Evidence of white matter tract disruption in MRI hyperintensities. Biol Psychiatry. 2001;50(3):179–183. doi: 10.1016/s0006-3223(01)01160-x. [DOI] [PubMed] [Google Scholar]
- 20.Soares JC, Mann JJ. The anatomy of mood disorders – review of structural neuroimaging studies. Biol Psychiatry. 1997;41(1):86–106. doi: 10.1016/s0006-3223(96)00006-6. [DOI] [PubMed] [Google Scholar]
- 21.Iosifescu DV, Renshaw PF, Lyoo IK, et al. Brain white-matter hyperintensities and treatment outcome in major depressive disorder. Br J Psychiatry. 2006;188(2):180–185. doi: 10.1192/bjp.188.2.180. [DOI] [PubMed] [Google Scholar]
- 22.Ahearn EP, Jamison KR, Steffens DC, et al. MRI correlates of suicide attempt history in unipolar depression. Biol Psychiatry. 2001;50(4):266–270. doi: 10.1016/s0006-3223(01)01098-8. [DOI] [PubMed] [Google Scholar]
- 23.Borenstein M, Hedges L, Higgins J, Rothstein H. [Accessed September 5th, 2009];Comprehensive meta-analysis. Available at: http://www.meta-analysis.com/
- 24.Abramson JH. [Accessed September 5th, 2009];WINPEPI (PEPI-for-Windows), computer programs for epidemiologists. doi: 10.1186/1742-5573-1-6. Available at: http://www.brixtonhealth.com/pepi4windows.html. [DOI] [PMC free article] [PubMed]
- 25.Hedges LV, Olkin I. Statistical methods for meta-analysis. Academic Press; Orlando: 1985. pp. 285–309. [Google Scholar]
- 26.Goldston DB, Kovacs M, Ho VY, Parrone PL, Stiffler L. Suicidal ideation and suicide attempts among youth with insulin-dependent diabetes mellitus. J Am Acad Child Adolesc Psychiatry. 1994;33(2):240–246. doi: 10.1097/00004583-199402000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Placido A, Sposito AC. Association between suicide and cardiovascular disease: Time series of 27 years. Int J Cardiol. 2009;135(2):261–262. doi: 10.1016/j.ijcard.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 28.Levitan H. Suicidal trends in patients with asthma and hypertension. A chart study. Psychother Psychosom. 1983;39(3):165–170. doi: 10.1159/000287737. [DOI] [PubMed] [Google Scholar]
- 29.Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14(2):224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- 30.Sachdev PS, Wen W, Christensen H, Jorm AF. White matter hyperintensities are related to physical disability and poor motor function. J Neurol Neurosurg Psychiatry. 2005;76(3):362–367. doi: 10.1136/jnnp.2004.042945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Starr JM, Leaper SA, Murray AD, et al. Brain white matter lesions detected by magnetic resonance [correction of resonance] imaging are associated with balance and gait speed. J Neurol Neurosurg Psychiatry. 2003;74(1):94–98. doi: 10.1136/jnnp.74.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakakibara R, Hattori T, Uchiyama T, Yamanishi T. Urinary function in elderly people with and without leukoaraiosis: relation to cognitive and gait function. J Neurol Neurosurg Psychiatry. 1999;67(5):658–660. doi: 10.1136/jnnp.67.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheline YI, Price JL, Vaishnavi SN, et al. Regional white matter hyperintensity burden in automated segmentation distinguishes late-life depressed subjects from comparison subjects matched for vascular risk factors. Am J Psychiatry. 2008;165(4):524–532. doi: 10.1176/appi.ajp.2007.07010175. [DOI] [PMC free article] [PubMed] [Google Scholar]