Abstract
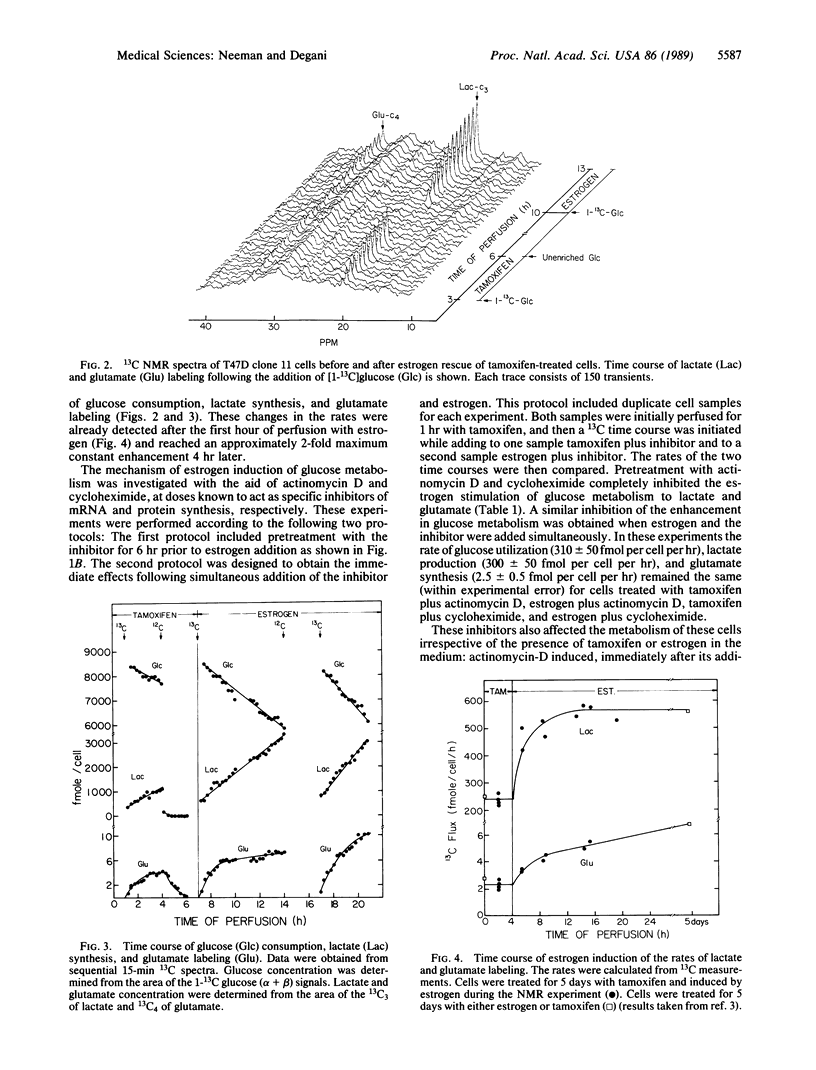
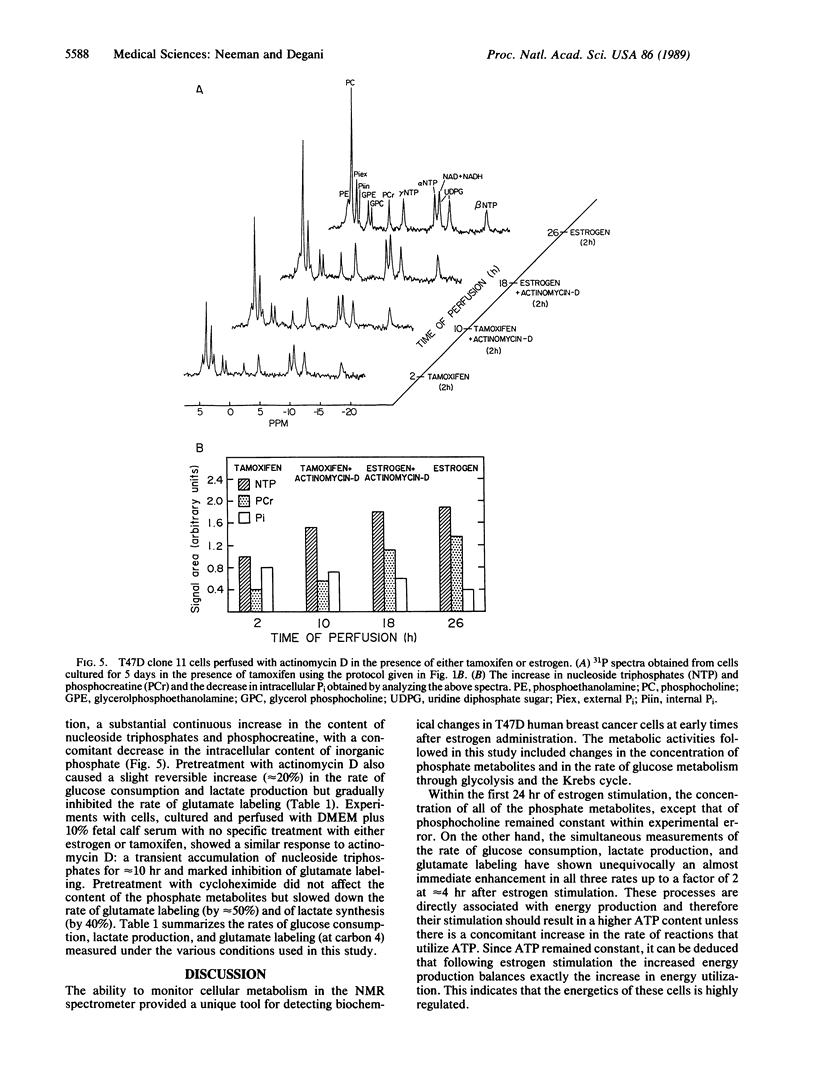
Metabolic changes following estrogen stimulation and the inhibition of these changes in the presence of actinomycin D and cycloheximide were monitored continuously in perfused human breast cancer T47D clone 11 cells with 31P and 13C NMR techniques. The experiments were performed by estrogen rescue of tamoxifen-treated cells. Immediately after perfusion with estrogen-containing medium, a continuous enhancement in the rates of glucose consumption, lactate production by glycolysis, and glutamate synthesis by the Krebs cycle occurred with a persistent 2-fold increase at 4 hr. The content of phosphocholine had increased by 10% to 30% within the first hour of estrogen stimulation, but the content of the other observed phosphate metabolites as well as the pH remained unchanged. Pretreatment with either actinomycin D or cycloheximide, at concentrations known to inhibit mRNA and protein synthesis, respectively, and simultaneous treatment with estrogen and each inhibitor prevented the estrogen-induced changes in glucose metabolism. This suggested that the observed estrogen stimulation required synthesis of mRNA and protein. These inhibitors also modulated several metabolic activities that were not related to estrogen stimulation. The observed changes in the in vivo kinetics of glucose metabolism may provide a means for the early detection of the response of human breast cancer cells to estrogen versus tamoxifen treatment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Edwards D. P., McGuire W. L. Estrogen regulation of specific proteins as a mode of hormone action in human breast cancer. Biomembranes. 1983;11:389–414. [PubMed] [Google Scholar]
- Aitken S. C., Lippman M. E., Kasid A., Schoenberg D. R. Relationship between the expression of estrogen-regulated genes and estrogen-stimulated proliferation of MCF-7 mammary tumor cells. Cancer Res. 1985 Jun;45(6):2608–2615. [PubMed] [Google Scholar]
- Arafah B. M., Griffin P., Gordon N. H., Pearson O. H. Influence of tamoxifen and estradiol on the growth of human breast cancer cells in vitro. Cancer Res. 1986 Jul;46(7):3268–3272. [PubMed] [Google Scholar]
- Berthois Y., Katzenellenbogen J. A., Katzenellenbogen B. S. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. P., Adams D. J., Savage N., McGuire W. L. Estrogen induced synthesis of specific proteins in human breast cancer cells. Biochem Biophys Res Commun. 1980 Apr 14;93(3):804–812. doi: 10.1016/0006-291x(80)91148-1. [DOI] [PubMed] [Google Scholar]
- Evelhoch J. L., Keller N. A., Corbett T. H. Response-specific adriamycin sensitivity markers provided by in vivo 31P nuclear magnetic resonance spectroscopy in murine mammary adenocarcinomas. Cancer Res. 1987 Jul 1;47(13):3396–3401. [PubMed] [Google Scholar]
- Fantini J., Galons J. P., Marvaldi J., Cozzone P. J., Canioni P. Growth of a human colonic adenocarcinoma cell line (HT 29) on microcarrier beads: metabolic studies by 31phosphorus nuclear magnetic resonance spectroscopy. Int J Cancer. 1987 Feb 15;39(2):255–260. doi: 10.1002/ijc.2910390222. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., McGuire W. L. Nuclear estrogen receptors. Effect of inhibitors on processing and steady state levels. J Biol Chem. 1980 Oct 25;255(20):9699–9705. [PubMed] [Google Scholar]
- Huff K. K., Lippman M. E. Hormonal control of plasminogen activator secretion in ZR-75-1 human breast cancer cells in culture. Endocrinology. 1984 May;114(5):1702–1710. doi: 10.1210/endo-114-5-1702. [DOI] [PubMed] [Google Scholar]
- Laszlo J., Miller D. S., McCarty K. S., Hochstein P. Actinomycin D: inhibition of respiration and glycolysis. Science. 1966 Feb 25;151(3713):1007–1010. doi: 10.1126/science.151.3713.1007. [DOI] [PubMed] [Google Scholar]
- Lima G., Shiu R. P. Role of polyamines in estradiol-induced growth of human breast cancer cells. Cancer Res. 1985 Jun;45(6):2466–2470. [PubMed] [Google Scholar]
- Neeman M., Degani H. Metabolic studies of estrogen- and tamoxifen-treated human breast cancer cells by nuclear magnetic resonance spectroscopy. Cancer Res. 1989 Feb 1;49(3):589–594. [PubMed] [Google Scholar]
- Neeman M., Rushkin E., Kadouri A., Degani H. Adaptation of culture methods for NMR studies of anchorage-dependent cells. Magn Reson Med. 1988 Jun;7(2):236–242. doi: 10.1002/mrm.1910070212. [DOI] [PubMed] [Google Scholar]
- Neeman M., Rushkin E., Kaye A. M., Degani H. 31P-NMR studies of phosphate transfer rates in T47D human breast cancer cells. Biochim Biophys Acta. 1987 Sep 14;930(2):179–192. doi: 10.1016/0167-4889(87)90030-9. [DOI] [PubMed] [Google Scholar]
- Osborne C. K., Boldt D. H., Estrada P. Human breast cancer cell cycle synchronization by estrogens and antiestrogens in culture. Cancer Res. 1984 Apr;44(4):1433–1439. [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Inhibition of RNA synthesis by actinomycin D: characteristic dose-response of different RNA species. J Cell Physiol. 1970 Oct;76(2):127–139. doi: 10.1002/jcp.1040760202. [DOI] [PubMed] [Google Scholar]
- Pourreau-Schneider N., Berthois Y., Gandilhon P., Cau P., Martin P. M. Early alterations at the plasma membrane of breast cancer cell lines in response to estradiol and hydroxytamoxifen. Mol Cell Endocrinol. 1986 Nov;48(1):77–88. doi: 10.1016/0303-7207(86)90168-1. [DOI] [PubMed] [Google Scholar]
- Shafie S. M. Estrogen and the growth of breast cancer: new evidence suggests indirect action. Science. 1980 Aug 8;209(4457):701–702. doi: 10.1126/science.6994231. [DOI] [PubMed] [Google Scholar]
- Soto A. M., Sonnenschein C. Mechanism of estrogen action on cellular proliferation: evidence for indirect and negative control on cloned breast tumor cells. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1097–1103. doi: 10.1016/0006-291x(84)91204-x. [DOI] [PubMed] [Google Scholar]
- Spooner P. M., Gorski J. Early estrogen effects on lipid metabolism in rat uterus. Endocrinology. 1972 Nov;91(5):1273–1283. doi: 10.1210/endo-91-5-1273. [DOI] [PubMed] [Google Scholar]
- Steen R. G., Tamargo R. J., McGovern K. A., Rajan S. S., Brem H., Wehrle J. P., Glickson J. D. In vivo 31P nuclear magnetic resonance spectroscopy of subcutaneous 9L gliosarcoma: effects of tumor growth and treatment with 1,3-bis(2-chloroethyl)-1-nitrosourea on tumor bioenergetics and histology. Cancer Res. 1988 Feb 1;48(3):676–681. [PubMed] [Google Scholar]
- Strobl J. S., Kasid A., Huff K. K., Lippman M. E. Kinetic alterations in estrogen receptors associated with estrogen receptor processing in human breast cancer cells. Endocrinology. 1984 Sep;115(3):1116–1124. doi: 10.1210/endo-115-3-1116. [DOI] [PubMed] [Google Scholar]
- Vignon F., Terqui M., Westley B., Derocq D., Rochefort H. Effects of plasma estrogen sulfates in mammary cancer cells. Endocrinology. 1980 Apr;106(4):1079–1086. doi: 10.1210/endo-106-4-1079. [DOI] [PubMed] [Google Scholar]
- Warden C. H., Friedkin M. Regulation of phosphatidylcholine biosynthesis by mitogenic growth factors. Biochim Biophys Acta. 1984 Mar 7;792(3):270–280. doi: 10.1016/0005-2760(84)90194-2. [DOI] [PubMed] [Google Scholar]


