Abstract
This report updates patient and primary graft survival statistics for the liver transplantation program at the University of Pittsburgh for 1984 through 1990. Minimum follow-up for all patients is one year and only patients who received a primary liver graft in Pittsburgh are included. Recipients of multiple organ or multivisceral transplants, patients who received a primary graft at another center and were sent to Pittsburgh for rescue with FK506 or for retransplantation, and patients who underwent an extended resection (“cluster operation”) for cancer were excluded.
METHODS
DEMOGRAPHICS
Time Periods and Treatment Protocols
The liver transplant program at the University of Pittsburgh was established in 1981. Data are presented for three time periods (Table 1) which correspond to major changes in treatment protocols. In the first three years of the program, protocols for immunosuppression with cyclosporine (CsA) and prednisone (Pred) were refined, the veno-venous bypass technique for management of the anhepatic phase of the recipient operation was developed, and the techniques of multiple organ procurement from cadaveric donors using Euro-Collins and ice slush preservation were standardized. In 1984, we began using OKT3 monoclonal antibody (Orthoclone OKT3®, Ortho Pharmaceuticals) for treatment of steroid resistant acute allograft rejection, after Cosimi and his associates at the Massachusetts General Hospital demonstrated its effectiveness for the treatment of acute renal allograft rejection. These protocols remained the essentials of patient management in Pittsburgh for the period from 1984 through September, 1987, during which time 787 patients received a primary liver transplant. Of these patients, 573 (72.8%) ultimately received only one graft, 163 (20.7%) received two grafts, and 51 (6.5%) required three or more grafts. As of December 31, 1991, there were 480 (61.0%) surviving patients including 396 (50.3%) with a functioning first graft.
Table 1.
Liver Transplantation – University of Pittsburgh
| Period | N | % | |
|---|---|---|---|
| 1/84–9/87 | CsA, vein bypass, OKT-3 | 787 | 37.7% |
| 10/87–12/88 | UW solution | 481 | 23.0% |
| 1/89–12/90 | FK 506 | 822 | 39.3% |
| Total | 2090 | 100.0% | |
In October, 1987, we began the regular use of the University of Wisconsin solution instead of Euro-Collins for liver preservation. Between October, 1987 and December, 1988 there were 481 recipients of a primary liver graft in Pittsburgh under CsA-Pred therapy.
Among these patients, 390 (81.1 %) received only one graft, 70 (14.6%) received two grafts, and 21 (4.4%) received three or more grafts. As of December 31, 1991, there were 331 (68.8%) surviving patients including 292 (60.7%) with functioning first grafts.
Experimental trials with FK506 began in 1989. From January, 1989 through December, 1990, 822 patients received liver transplants, including 684 (83.2%) who required only one graft to date, 120 (14.6%) who received a second graft, and 18 (2.2%) who have received three or more grafts. As of December 31, 1991, there were 650 (79.1%) surviving patients including 577 (70.2%) surviving with their first graft.
In the entire series of 2,090 patients, 1,633 (78.1%) were transplanted using the CsA-Pred regimen (Table 2), but 272 were eventually switched to FK506, including 41 of 54 patients who were transplanted under CsA-Pred in 1990 as part of a randomized trial comparing CsA-Pred to FK506. Another 103 of the 311 patients in period 3 who were transplanted under CsA-Pred, but were not part of the randomized trial, were also converted to FK506. Approximately 5 to 10 percent of the patients per year who received a primary graft under CsA-Pred during periods 1 and 2 have been converted to FK 506.
Table 2.
Immunosuppressive protocol
| Period 1 | Period 2 | Period 3 | Total | |
|---|---|---|---|---|
| Primary FK 506 | 399 | 399 19.1% |
||
| Rescue FK 506 | 99 12.6% |
29 6.0% |
103 12.5% |
231 11.1% |
| Randomized trial* | 112 13.6% |
112 5.4% |
||
| Primary CsA | 688 87.4% |
452 94.0% |
208 25.3% |
1348 64.5% |
| Total | 787 | 481 | 822 | 2090 |
Includes 54 patients randomized to CsA and 58 patients randomized to FK 506.
Age Groups and Body Weight
The distribution of patients according to age is shown in Table 3. There has been an increase in the proportion of elderly patients (over 60 years of age) accepted for transplantation since 1984 from 5.7% of the patients in period 1 to 16.0% of the patients in period 3. The proportion of infants receiving transplants has remained fairly constant. Survival rates for 208 small patients (under 12 kg) and 21 obese patients (over 115 kg) were compared with survival rates for 1,383 adults between 50 and 115 kg..
Table 3.
Age Groups
| Period 1 | Period 2 | Period 3 | Total | |
|---|---|---|---|---|
| Infants (under 2 yrs) | 63 8.0% |
45 9.4% |
59 7.2% |
167 8.0% |
| Children (2–11 yrs) | 171 21.7% |
40 8.3% |
46 5.6% |
257 12.3% |
| Adolescents (12–17 yrs) | 37 4.7% |
9 1.9% |
23 2.8% |
69 3.3% |
| Adults (18–59 yrs) | 471 59.8% |
333 69.2% |
562 68.4% |
1366 65.4% |
| Seniors (over 60 yrs) | 45 5.7% |
54 11.2% |
132 16.0% |
231 11.1% |
| Total | 787 | 481 | 822 | 2090 |
Medical Urgency
Table 4 presents the distribution of patients in periods 2 and 3 according to patient condition at the time of transplantation. There has been a substantial increase in the percentage of patients at this center in critical condition at the time of transplantation.
Table 4.
Medical urgency
| Period 2 | Period 3 | Total | |
|---|---|---|---|
| Elective | 26 5.4% |
17 2.1% |
43 3.3% |
| Out of hospital | 94 19.6% |
89 10.8% |
183 14.1% |
| In hospital | 216 45.1% |
304 37.0% |
520 40.0% |
| Critical | 143 29.9% |
412 50.1% |
555 42.7% |
| Total | 479 | 822 | 1301 |
Primary liver disease
Distribution of the most common primary indications for liver transplantation for each period is shown in Table 5. The most significant changes were the relative decrease in the percentage of adult patients with primary biliary cirrhosis and the increase in the percentage of patients with alcoholic cirrhosis.
Table 5.
Most common primary liver diseases
| Period 1 | Period 2 | Period 3 | Total | |||||
|---|---|---|---|---|---|---|---|---|
| % | % | % | % | |||||
| Fulminant hepatic failure | 36 | 4.6 | 33 | 6.9 | 54 | 2.9 | 93 | 4.5 |
| Postnecrotic cirrhosis | 222 | 28.2 | 140 | 29.1 | 280 | 34.1 | 642 | 30.7 |
| Cryptogenic cirrhosis* | 166 | 21.1 | 83 | 17.3 | 177 | 21.5 | 426 | 20.4 |
| HBsAg+ cirrhosis | 27 | 3.4 | 34 | 7.1 | 53 | 6.4 | 114 | 5.5 |
| Autoimmune CAH | 17 | 2.2 | 16 | 3.3 | 25 | 3.0 | 58 | 2.8 |
| Alcoholic cirrhosis | 39 | 5.0 | 69 | 4.3 | 167 | 20.3 | 275 | 13.2 |
| Biliary atresia | 152 | 19.3 | 52 | 10.8 | 81 | 9.9 | 285 | 13.6 |
| Primary biliary cirrhosis | 123 | 15.6 | 59 | 12.3 | 66 | 8.0 | 248 | 11.9 |
| Sclerosing cholangitis | 55 | 7.0 | 36 | 7.5 | 55 | 6.8 | 146 | 7 |
| Genetic disorders | 72 | 9.1 | 27 | 5.6 | 33 | 4.0 | 132 | 6.3 |
| Alpha-1-antitrypsin deficiency | 40 | 5.1 | 13 | 2.7 | 18 | 2.2 | 71 | 3.4 |
| Wilson’s disease | 13 | 1.7 | 7 | 1.5 | 5 | 0.6 | 25 | 1.2 |
| Primary liver cancer | 20 | 3.2 | 32 | 6.7 | 58 | 7.1 | 110 | 5.3 |
includes all non-A, non-B forms of viral hepatitis and cryptogenic cirrhosis in HBsAg-patients
STATISTICAL METHODS
Actuarial survival
Survival out to 5 years after transplantation is presented in a series of actuarial survival plots. Most of the cohorts analyzed for this report are large (over 100 patients) and therefore suitable for analysis by the life-table method in which survival is determined over predefined time intervals (1, 3, 6, and every 6 mos thereafter out to 60 mos). Life-table data are presented as a plot of the estimated survivor function (the probability that a subject will survive to at least a specified time or longer) and as a plot of the cumulative hazard function (negative natural logarithm of the estimated survivor function). In the few series in which the number of cases was small, we used the product-limit (Kaplan-Meier) method of computation for the survivor function.
Observed (actual) Survival
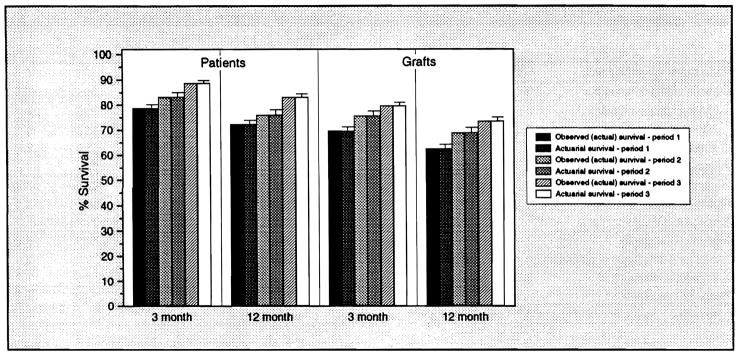
This year the National Organ Sharing Network (UNOS) will release center-specific survival data (observed 3- and 12-month patient and primary graft survival rates) for transplantations performed between October 1, 1987 and December 31, 1989. The observed survival rate is simply the proportion of patients who survive to a specified cut point. Since follow-up for our series was complete and all patients were followed for at least 1 year, the 3- and 12-month estimated survivor functions determined by life-table calculation and by observed survival rate calculation should be identical. Figure 1 compares observed and actuarial patient and primary graft survival rates for each of the 3 periods at 3 and 12 months.
Figure 1.
Comparison of 3- and 12-month observed (actual) and actuarial (life-table method) patient and primary graft survival rates. Since there is complete follow-up and all patients were followed for at least one year, the survival rates determined by the two methods are the same.
Statistical significance
Statistical significance between observed survival rates was determined using the chi-square statistic. For comparison of life-table survival curves, Breslow, Mantel-Cox, Peto, and Tarone-Ware statistics were calculated. Differences were considered statistically significant if p was less than 0.05.
RESULTS
Time Periods
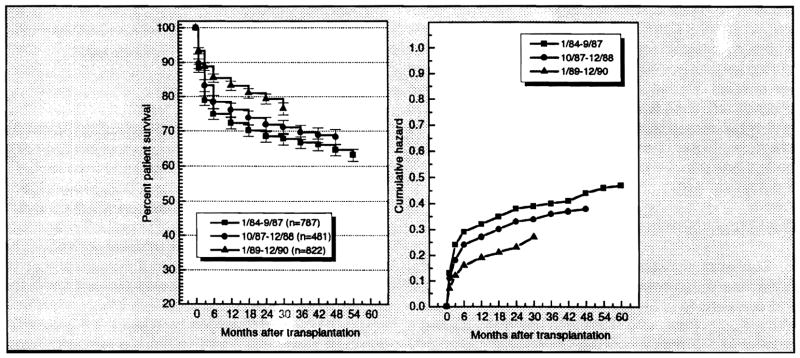
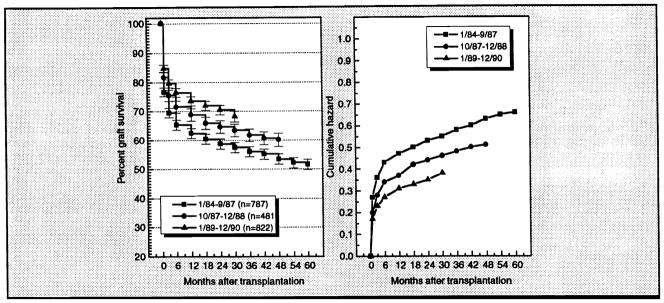
There has been a significant improvement in patient survival over the periods analyzed. Figures 2 and 3 present the life-table analysis plots for patient and primary graft survival by period. Table 6 shows the observed 3- and 12-month patient and primary graft survival rates. The life-table plots demonstrate that most of the mortality after liver transplantation (12–21%) occurs within the first 3-months posttransplantation. The 3-month observed patient survival rate improved from 78.9% in period 1 to 88.7% in period 3. The 12-month patient survival rate improved from 72.3% in period 1 to 83.1% in period 3.
Figure 2.
Left: Estimated survivor function (life-table method) for patient survival according to time period during which transplantation was performed. Right: Cumulative hazard function for patient survival by time period.
Figure 3.
Left: Estimated survivor function (life-table method) for primary graft survival according to time period during which transplantation was performed. Right: Cumulative hazard function for primary graft survival by time period.
Table 6.
Observed patient and graft survival rates by period
| Period 1 | Period 2 | Period 3 | ||||
|---|---|---|---|---|---|---|
| Surviving | Lost | Surviving | Lost | Surviving | Lost | |
| 3 -month patient* | 621 78.9% |
166 21.1% |
400 83.2% |
81 16.8% |
729 88.7% |
93 11.3% |
| 12-month patient* | 569 72.3% |
218 27.7% |
366 76.1% |
115 23.9% |
683 83.1% |
139 16.9% |
| 3-month first graft* | 548 69.6% |
239 30.4% |
363 75.5% |
118 24.5% |
654 79.6% |
168 20.4% |
| 12-month first graft* | 491 62.4% |
296 37.6% |
331 68.8% |
150 31.2% |
604 73.5% |
218 26.5% |
p < 0.001
Immunosuppressive Protocol
Table 7 presents the observed survival rates for 399 patients who received a transplant under an FK506 protocol, 391 historical control patients from period 2 who received transplants under a CsA protocol, and 161 patients converted from CsA to FK506. Survival rates under the FK506 protocol improved over those obtained for the historical CsA protocol controls. The differences in patient survival at 3 months and at 12 months between the FK506 treated patients and the CsA treated historical controls are highly significant (p < 0.01). The differences between the 2 groups for first graft survival at 3 and 12 months were significant (p < 0.02).
Table 7.
Observed patient and graft survival rates - immunosuppression
| Primary FK 506 | Historical CsA* | After FK 506 rescue** | ||||
|---|---|---|---|---|---|---|
| Surviving | Lost | Surviving | Lost | Surviving | Lost | |
| 3-month patient | 358 89.7% |
41 10.3% |
318 81.3% |
73 18.7% |
151 93.8% |
10 6.2% |
| 12-month patient | 333 83.5% |
66 16.5% |
291 74.4% |
100 25.6% |
138 85.7% |
23 14.3% |
| 3-month first graft | 327 82.0% |
72 18.0% |
294 75.2% |
97 24.8% |
144 89.4% |
17 10.6% |
| 12-month first graft | 304 76.2% |
95 23.8% |
266 68.0% |
125 32.0% |
132 82.0% |
29 18.0% |
391 patients treated with CsA-Pred in period 2 (10/87–12/88)
Observed survival rates after rescue with FK 506
The rescue series included all patients who retained their first grafts and were converted to FK506 prior to January 1, 1991. The observed survival rates were calculated from the time of crossover to FK506. There is a high rate of successful patient and graft rescue out to 12 months after crossover.
Results of the randomized trial comparing FK506 and CsA were presented recently by Fung et al1 and are not included here.
Age Group and Body Weight
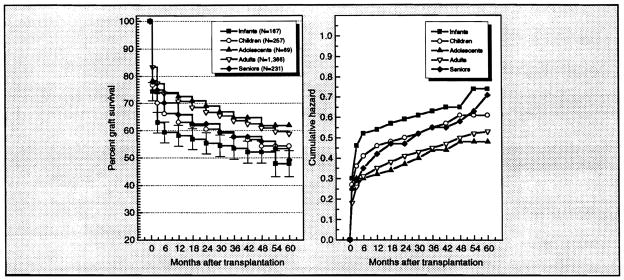
There were significant differences in survival rates among patients in different age groups and significant improvements in survival rates for most age groups across the 3 time periods. Figures 4 and 5 present the life-table plots for patient and primary graft survival by age groups. Table 8 shows the observed 3- and 12-month patient and primary graft survival rates. Survival rates for infants have been generally lower than for older children and adults, but in period 3, infant survival rates attained parity. Early survival rates for patients over 60 compared favorably with those for younger adult patients, but, as would be expected, for older patients there was a modest increase in the rate of patient loss over the longer term.
Figure 4.
Left: Estimated survivor function (life-table method) for patient survival according to age group during which transplantation was performed. Right: Cumulative hazard function for primary graft survival by age group.
Figure 5.
Left: Estimated survivor function (life-table method) for primary survival according to age group. Right: Cumulative hazard function for primary graft survival by age group.
Table 8.
Observed patient and graft survival rates by age
| Infant (under 2 yrs) |
Child (2–11 yrs) |
Adolescent (12–17 yrs) |
Adult (18–59 yrs) |
Senior (over 60 yrs) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Surviving | Lost | Surviving | Lost | Surviving | Lost | Surviving | Lost | Surviving | Lost | |
| 3-month patient | ||||||||||
| Period 1 | 42 (66.7%) | 21 (33.3%) | 135 (78.9%) | 36 (21.1%) | 30 (81.1%) | 7 (18.9%) | 382 (81.1%) | 89 (18.9%) | 32 (71.1%) | 13 (28.9%) |
| Period 2 | 32 (71.1%) | 13 (28.9%) | 35 (87.5%) | 5 (12.5%) | 6 (66.7%) | 3 (33.3%) | 281 (84.4%) | 52 (15.6%) | 46 (85.2%) | 8 (14.8%) |
| Period 3 | 52 (88.1%) | 7 (11.9%) | 41 (89.1%) | 5 (10.9%) | 21 (91.3%) | 2 (8.7%) | 504 (89.7%) | 58 (10.3%) | 111 (84.1%) | 21 (15.9%) |
| 12-month patient | ||||||||||
| Period 1 | 38 (60.3%) | 25 (39.7%) | 120 (70.2%) | 51 (29.8%) | 28 (75.7%) | 9 (24.3%) | 357 (75.8%) | 114 (24.2%) | 26 (57.8%) | 19 (42.2%) |
| Period 2 | 28 (62.2%) | 17 (37.8%) | 35 (87.5%) | 5 (12.5%) | 6 (66.7%) | 3 (33.3%) | 255 (76.6%) | 78 (23.4%) | 42 (77.8%) | 12 (22.2%) |
| Period 3 | 50 (84.7%) | 9 (15.3%) | 40 (87.0%) | 6 (13.0%) | 21 (91.3%) | 2 (8.7%) | 471 (83.8%) | 91 (16.2%) | 101 (76.5%) | 31 (23.5%) |
| 3-month first graft | ||||||||||
| Period 1 | 33 (52.4%) | 30 (47.6%) | 114 (66.7%) | 57 (33.3%) | 25 (67.6%) | 12 (32.4%) | 347 (73.7%) | 124 (26.3) | 29 (64.4%) | 16 (35.6%) |
| Period 2 | 27 (60.0%) | 18 (40.0%) | 31 (77.5%) | 9 (22.5%) | 6 (66.7%) | 3 (33.3%) | 253 (76.0%) | 80 (24.0) | 46 (85.2%) | 8 (14.8%) |
| Period 3 | 46 (78.0%) | 13(22.0%) | 35 (76.1%) | 11 (23.9%) | 20 (87.0%) | 3 (13.0%) | 455 (81.0%) | 107 (19.0) | 98 (74.2%) | 34 (25.8%) |
| 12-month first graft | ||||||||||
| Period 1 | 28 (44.4%) | 35 (55.6%) | 97 (56.7%) | 74 (43.3%) | 24 (64.9%) | 13(35.1%) | 319(67.7%) | 152 (32.2%) | 23 (51.1%) | 22 (48.9%) |
| Period 2 | 25 (55.6%) | 20 (44.4%) | 31 (77.5%) | 9 (22.5%) | 6 (66.7%) | 3 (33.3%) | 228 (68.5%) | 105 (31.5%) | 41 (75.9%) | 13 (24.1%) |
| Period 3 | 44 (74.6%) | 15(25.4%) | 34 (73.9%) | 12 (26.1%) | 20 (87.0%) | 3 (13.0%) | 418 (74.4%) | 144 (25.6%) | 88 (66.7%) | 44 (33.3%) |
In Figure 6 the estimated survivor and cumulative hazard functions (Kaplan-Meier method) were stratified into 3 weight groups, under 12 kg (small), 50–115 kg (normal adults), and over 115 kg (obese). Survival rates for small and obese patients were significantly lower than those for normal weight adult patients.
Figure 6.
Left: Estimated survivor function (Kaplan-Meier method) for patient survival according to body weight. Right: Cumulative hazard function for patient survival by age group.
Medical Urgency
Table 9 presents the observed patient and graft survival rates and Figure 7 presents the estimated patient survivor and cumulative hazard functions after liver transplantation according to patient condition the time of transplantation. A significant penalty is demonstrated for patients in high urgency status at the time of transplantation.
Table 9.
Observed patient and graft survival rates by medical urgency
| Elective | Out of hospital | In hospital | Critical | |||||
|---|---|---|---|---|---|---|---|---|
| Surviving | Lost | Surviving | Lost | Surviving | Lost | Surviving | Lost | |
| 3-month patient | 56 98.2% |
1 1.8% |
171 88.6% |
22 11.4% |
482 90.6% |
50 9.4% |
481 81.9% |
102 18.1% |
| 12-month patient | 54 94.7% |
3 5.3% |
167 86.5% |
26 13.5% |
451 84.8% |
81 15.2% |
415 73.7% |
148 26.3% |
| 3-month first graft | 54 94.7% |
3 5.3% |
154 79.8% |
39 20.2% |
440 82.7% |
92 17.3% |
403 71.6% |
160 28.4% |
| 12-month first graft | 49 86.0% |
8 14.0% |
148 76.7% |
45 23.3% |
398 74.8% |
134 25.2% |
366 65.0% |
197 35.0% |
Based on 1,345 patients from periods 2 and 3.
Figure 7.
Left: Estimated survivor function (life-table method) for patient survival according to medical urgency at the time of transplantation. Right: Cumulative hazard function for patient survival according to medical urgency.
Primary Liver Disease
Tables 10 through 12 show the observed 3- and 12-month patient and primary graft survival rates for each time period according to primary liver disease. Figures 8 and 9 illustrate the estimated survivor and cumulative hazard functions for patient and primary graft survival for patients with postnecrotic cirrhosis (excluding HBsAg+ patients), adult cholestatic liver disease (primary biliary cirrhosis and primary sclerosing cholangitis), biliary atresias, and alcoholic cirrhosis. Figures 10 and 11 present the life-table functions for patient and primary graft survival for patients with fulminant failure, chronic active hepatitis B (HBsAg+ patients), and primary liver cancer (mostly hepatomas).
Table 10.
Observed patient and primary graft survival rates for period 1 (1/84/−9/87)
| 3-month patient (%) |
12-month patient (%) |
3-month graft (%) |
12-month graft (%) |
|||||
|---|---|---|---|---|---|---|---|---|
| Surviving | Lost | Surviving | Lost | Surviving | Lost | Surviving | Lost | |
| Fulminant failure | 24 (66.7) | 12(33.3) | 22 (61.1) | 14 (38.9) | 17 (47.2) | 19 (52.8) | 13 (36.1) | 23 (63.9) |
| Postnecrotic cirrhosis | 179 (80.6) | 43 (19.4) | 169 (76.1) | 53 (23.9) | 158 (71.2) | 64 (28.8) | 145 (65.3) | 77 (34.7) |
| Cryptogenic cirrhosis* | 138 (83.1) | 28 (16.9) | 131 (78.9) | 35 (21.1) | 120 (72.3) | 46 (27.7) | 112 (67.5) | 54 (32.5) |
| HBsAg+ cirrhosis | 19 (70.4) | 8 (29.6) | 16 (59.3) | 11 (40.7) | 18 (66.7) | 9 (33.3) | 14 (51.9) | 13 (48.1) |
| Autoimmune CAH | 14 (82.4) | 3 (17.6) | 14 (82.4) | 3 (17.6) | 13 (76.5) | 4 (23.5) | 12 (70.6) | 5 (29.4) |
| Alcoholic cirrhosis | 32 (82.1) | 7 (17.9) | 30 (76.9) | 9 (23.1) | 30 (76.9) | 9 (23.1) | 28 (71.8) | 11 (28.2) |
| Biliary atresia | 113 (74.3) | 39 (25.7) | 99 (65.1) | 53 (34.9) | 92 (60.5) | 60 (39.5) | 77 (50.7) | 75 (49.3) |
| Primary biliary cirrhosis | 102 (82.9) | 21 (17.1) | 93 (75.6) | 30 (24.4) | 93 (75.6) | 30 (24.4) | 81 (65.9) | 42 (34.1) |
| Sclerosing cholangitis | 48 (87.3) | 7 (12.7) | 46 (83.6) | 9 (16.4) | 42 (76.4) | 13 (23.6) | 42 (76.4) | 13 (23.6) |
| Genetic disorders | 56 (77.8) | 16 (22.2) | 55 (76.4) | 17 (23.6) | 54 (75.0) | 18 (25.0) | 54 (75.0) | 18 (25.0) |
| Alpha-1-antitrypsin deficiency | 32 (80.0) | 8 (20.0) | 32 (80.0) | 8 (20.0) | 32 (80.0) | 8 (20.0) | 32 (80.0) | 8 (20.0) |
| Wilson’s disease | 10 (76.9) | 3 (23.1) | 10 (76.9) | 3 (23.1) | 9 (69.2) | 4 (30.8) | 9 (69.2) | 4 (30.8) |
| Primary liver cancer | 16 (80.0) | 4 (20.0) | 10 (50.0) | 10 (50.0) | 14 (70.0) | 6 (30.0) | 10 (50.0) | 10 (50.0) |
includes all non-A, non-B forms of viral hepatitis and cryptogenic cirrhosis in HBsAg- patients
Table 12.
Observed patient and primary graft survival rates for period 3 (1/89–12/90)
| 3-month patient (%) |
12-month patient (%) |
3-month graft (%) |
12-month graft (%) |
|||||
|---|---|---|---|---|---|---|---|---|
| Surviving | Lost | Surviving | Lost | Surviving | Lost | Surviving | Lost | |
| Fulminant failure | 19 (79.2) | 5 (20.8) | 19 (79.2) | 5 (20.8) | 15 (62.5) | 9 (37.5) | 14 (58.3) | 10 (41.7) |
| Postnecrotic cirrhosis | 249 (88.9) | 31 (11.1) | 230 (82.1) | 50 (17.9) | 225 (80.4) | 55 (19.6) | 207 (73.9) | 73 (26.1) |
| Cryptogenic cirrhosis* | 158 (89.3) | 19 (10.7) | 144 (81.4) | 33 (19.6) | 139 (78.5) | 38 (21.5) | 130 (73.5) | 47 (26.5) |
| HBsAg+ cirrhosis | 49 (92.5) | 4 (7.5) | 46 (86.6) | 7 (13.2) | 47 (88.7) | 6 (11.3) | 43 (81.1) | 10 (18.9) |
| Autoimmune CAH | 22 (88.0) | 3 (12.0) | 21 (84.0) | 4 (16.0) | 20 (80.0) | 5 (20.0) | 18 (72.0) | 7 (28.0) |
| Alcoholic cirrhosis | 144 (86.2) | 23 (13.8) | 138 (82.6) | 29 (17.4) | 134 (80.2) | 33 (19.8) | 126 (75.4) | 41 (24.6) |
| Biliary atresia | 73 (90.1) | 8 (9.9) | 72 (88.9) | 9 (11.1) | 65 (80.2) | 16 (19.8) | 63 (77.8) | 18 (22.2) |
| Primary biliary cirrhosis | 59 (89.4) | 7 (10.6) | 54 (81.8) | 12 (18.2) | 49 (74.2) | 17 (25.8) | 46 (69.7) | 20 (30.3) |
| Sclerosing cholangitis | 55 (100) | 0 (0) | 55 (98.2) | 1 (1.8) | 47 (85.5) | 8 (14.5) | 44 (80.0) | 11 (20.0) |
| Genetic disorders | 30 (90.9) | 3 (9.1) | 28 (84.8) | 5 (15.2) | 29 (87.9) | 4 (12.1) | 27 (81.8) | 6 (18.2) |
| Alpha-1-antitrypsin deficiency | 16 (88.9) | 2 (11.1) | 14 (77.8) | 4 (22.2) | 16 (88.9) | 2 (11.1) | 14 (77.8) | 4 (22.2) |
| Wilson’s disease | 5 (100) | 0 (0) | 5 (100) | 0 (0) | 4 (80.0) | 1 (20.0) | 4 (80.0) | 1 (20.0) |
| Primary liver cancer | 51 (87.9) | 7 (12.1) | 41 (70.7) | 17 (29.3) | 47 (81.0) | 11 (19.0) | 37 (63.8) | 21 (36.2) |
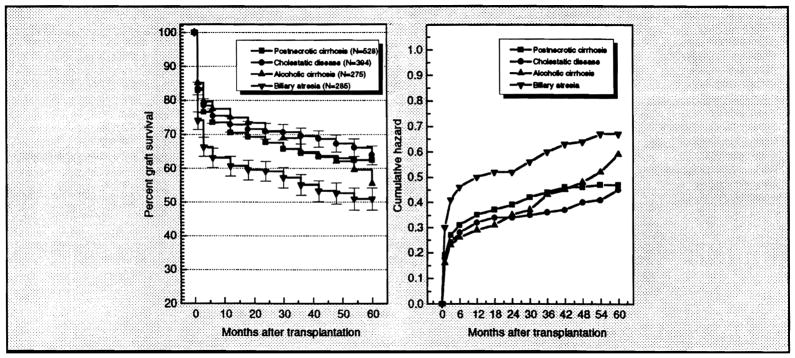
Figure 8.
Left: Estimated survivor function (life-table method) for patient survival for liver recipients receiving transplants for postnecrotic cirrhosis (excluding HBsAg+ patients), adult cholestatic diseases (primary biliary cirrhosis or primary sclerosing. cholangitis), biliary atresia, and alcoholic cirrhosis. Right: Cumulative hazard function for patient survival for these primary liver conditions.
Figure 9.
Left: Estimated survivor function (life-table method) for primary graft survival for liver recipients receiving transplants for postnecrotic cirrhosis (excluding HBsAg+ patients), adult cholestatic diseases (primary biliary cirrhosis or primary sclerosing. cholangitis), biliary atresia, and alcoholic cirrhosis. Right: Cumulative hazard function for primary graft survival for these primary liver conditions.
Figure 10.
Left: Estimated survivor function (life-table method) for patient survival for liver recipients receiving transplants for fulminant hepatic failure, chronic active hepatitis B (HBsAg+ patients), and primary liver cancer. Right: Cumulative hazard function for patient survival for these primary liver conditions.
Figure 11.
Left: Estimated survivor function (life-table method) for primary graft survival for liver recipients receiving transplants for fulminant hepatic failure, chronic active hepatitis B (HBsAg+ patients), and primary liver cancer. Right: Cumulative hazard function for primary graft survival for these primary liver conditions.
As mentioned earlier, overall results for the most recent period have improved significantly over earlier results. The most dramatic improvements in survival rates were seen in patients receiving liver transplants for fulminant hepatic failure, chronic active hepatitis B, biliary atresia, and primary sclerosing cholangitis. In the case of fulminant hepatic failure, new protocols for monitoring of intracerebral blood flow and prevention of excess intracranial pressure were probably responsible for the improved results. In biliary atresia, technical improvements in arterial reconstruction, increased use of reduced grafts to find organs for smaller children, and improved immunosuppression with FK506 protocols may have all contributed to the improvements. For chronic active hepatitis B, immunoprophylaxis, including both active and passive immunization, and a reduced requirement for steroids in patients receiving FK506 may have a beneficial effect. Earlier referral of patients, decreasing technical difficulties in performing liver transplantation in patients with prior surgery (including biliary diversion or prior bowel resection), and improvements in immunosuppression have probably contributed to the improved results for patients with sclerosing cholangitis.
We continue to offer liver transplantation to patients with alcoholic cirrhosis and to obtain results which compare favorably to those obtained in patients with other forms of postnecrotic cirrhosis.
New protocols for liver transplantation for primary cancer which have been instituted in our program have not yet been analyzed and are not presented here. Iwatsuki et al2 recently summarized our experience for 1980–1989 with hepatocellular carcinoma in a report which favorably compared results for liver transplantation with hepatic resection. The reader is advised to consult this paper for additional information.
CONCLUSION
Survival after liver transplantation continues to improve, reflecting continuing advances in surgical methods, organ preservation, and immunosuppressive protocols. At the present time, the Health Care Financing Administration (Medicare) has approved liver transplantation for biliary atresia in children and for postnecrotic cirrhosis (except for HBsAg+ patients), alcoholic cirrhosis, primary sclerosing cholangitis, primary biliary cirrhosis, alpha-1-antitrypsin deficiency, Wilson’s disease, and hemochromatosis in adults. Liver transplantation for fulminant hepatic failure and for chronic active hepatitis B has not yet been approved. Given the improved results now being obtained, expansion of the approved indications for liver transplantation to include fulminant hepatic failure and chronic active hepatitis B seems warranted.
SUMMARY.
Patient and primary graft survival for 2,090 patients who received primary liver transplants at the University of Pittsburgh from 1984 through 1990 are presented. Observed (actual) 3- and 12-month patient and first graft survival rates were compared for three time periods: 1) January, 1984 — September, 1987 (cyclosporine, OKT3, and Euro-Collins preservation period); 2) October, 1987 — December, 1988 (University of Wisconsin solution preservation period); and 3) January, 1989 — December, 1990 (FK506 period). Data for results according to age group, medical urgency, and primary diagnosis are provided. In addition, estimated survivor and cumulative hazard functions (life-table method) for patient and first graft survival out to 60 months after transplantation are presented.
Overall results have improved significantly in recent experience. Most notable are the improved results seen in liver transplantation for patients with biliary atresia (especially in infants), primary sclerosing cholangitis, fulminant hepatic failure, and chronic active hepatitis B. For all but a few conditions, most of the mortality after liver transplantation occurs in the first three months after surgery. Less than 2% of patients are lost in each 6-month interval beyond the first 6 months after transplantation.
Outcome is related to patient condition at the time of transplantation. Observed patient survival rates at 3 and at 12 months for patients called in to the hospital to receive a graft were 88.6% and 86.5%, respectively, compared to 81.9% and 73.7% for patients in critical condition. The continuing shortage of organs for transplantation, which often forces patients to wait longer for an organ than they can afford to, continues to impose a significant penalty.
Table 11.
Observed patient and primary graft survival rates for period 2 (10/87–12/88)
| 3-month patient (%) |
12-month patient (%) |
3-month graft (%) |
12-month graft (%) |
|||||
|---|---|---|---|---|---|---|---|---|
| Surviving | Lost | Surviving | Lost | Surviving | Lost | Surviving | Lost | |
| Fulminant failure | 21 (63.6) | 12 (36.4) | 19 (57.6) | 14 (42.3) | 20 (60.6) | 13 (39.4) | 19 (57.6) | 14 (42.4) |
| Postnecrotic cirrhosis | 117 (83.6) | 23 (16.4) | 103 (73.6) | 37 (26.4) | 110 (78.6) | 30 (21.4) | 94 (67.1) | 46 (32.9) |
| Cryptogenic cirrhosis* | 72 (86.7) | 11 (13.3) | 63 (75.9) | 20 (24.1) | 66 (79.5) | 17 (20.5) | 57 (68.7) | 26 (31.3) |
| HBsAg+ cirrhosis | 24 (70.6) | 10 (29.4) | 19 (55.1) | 15 (44.1) | 24 (70.6) | 10 (29.4) | 17 (50.0) | 17 (50.0) |
| Autoimmune CAH | 15 (93.8) | 1 (6.3) | 15 (93.8) | 1 (6.3) | 14 (87.5) | 2 (12.5) | 14 (87.5) | 2 (12.5) |
| Alcoholic cirrhosis | 61 (88.4) | 8 (11.6) | 59 (85.5) | 10 (14.5) | 55 (79.7) | 14 (20.3) | 52 (75.4) | 17 (24.6) |
| Biliary atresia | 39 (75.0) | 13 (25.0) | 37 (71.2) | 15 (28.8) | 33 (63.5) | 19 (36.5) | 33 (63.5) | 19 (36.5) |
| Primary biliary cirrhosis | 54 (91.5) | 5 (8.5) | 51 (86.4) | 8 (13.6) | 49 (83.1) | 10 (16.9) | 47 (79.7) | 12 (20.3) |
| Sclerosing cholangitis | 32 (88.9) | 4 (11.1) | 30 (83.3) | 6 (16.7) | 29 (80.6) | 7 (19.4) | 27 (75.0) | 9 (25.0) |
| Genetic disorders | 23 (85.2) | 4 (14.8) | 21 (77.8) | 6 (22.2) | 21 (77.8) | 6 (22.2) | 20 (62.5) | 7 (25.9) |
| Apha-1-antltrypsin deficiency | 12 (92.3) | 1 (7.7) | 11 (84.6) | 2 (15.4) | 11 (84.6) | 2 (15.4) | 10 (76.9) | 3 (23.1) |
| Wilson’s disease | 5 (71.4) | 2 (28.6) | 5 (71.4) | 2 (28.6) | 5 (71.4) | 2 (28.6) | 5 (71.4) | 2 (28.6) |
| Primary liver cancer | 26 (81.3) | 6 (18.8) | 22 (68.6) | 10 (31.3) | 24 (75.0) | 8 (25.0) | 20 (62.5) | 12 (37.5) |
References
- 1.Fung J, Abu-Elmagd K, Jain A, et al. A randomized trial of primary liver transplantation under immunosuppression with FK 506 vs cyclosporine. Transplant Proc. 1991;23:2977. [PMC free article] [PubMed] [Google Scholar]
- 2.Iwatsuki S, Starzl TE, Sheahan DG, et al. Hepatic resection versus transplantation for hepatocellular carcinoma. Ann Surg. 1991;214:221. doi: 10.1097/00000658-199109000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]