Abstract
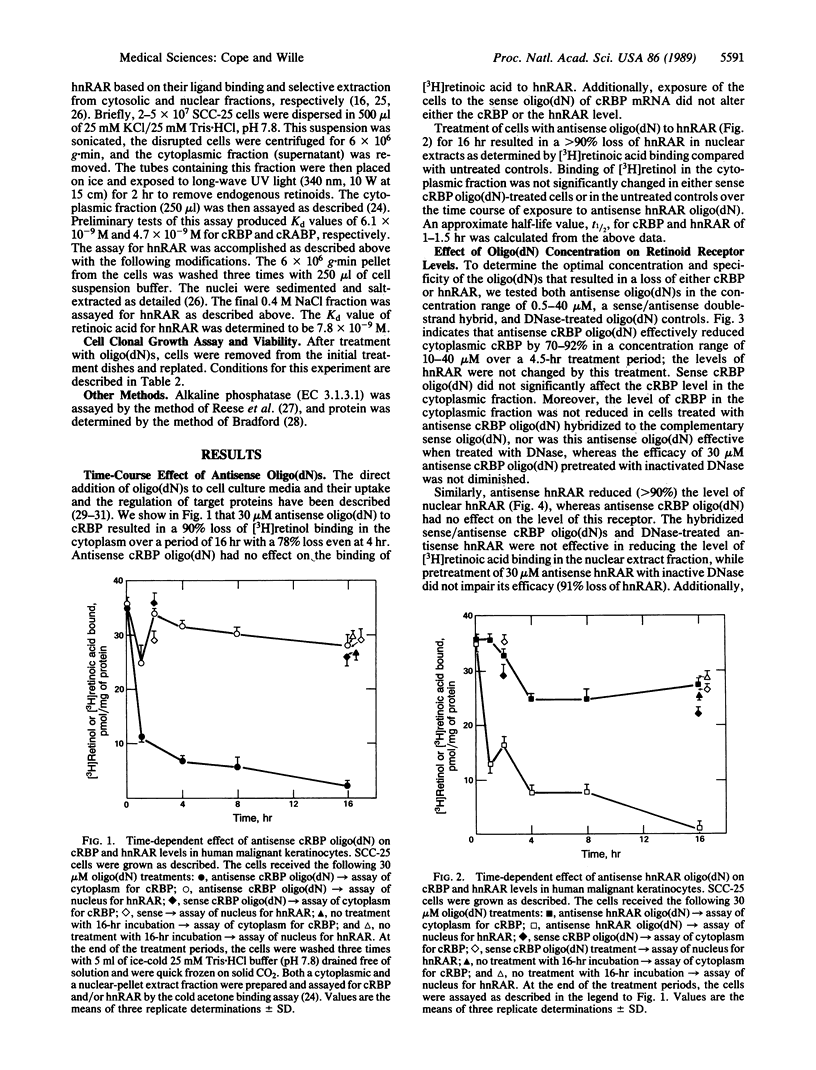
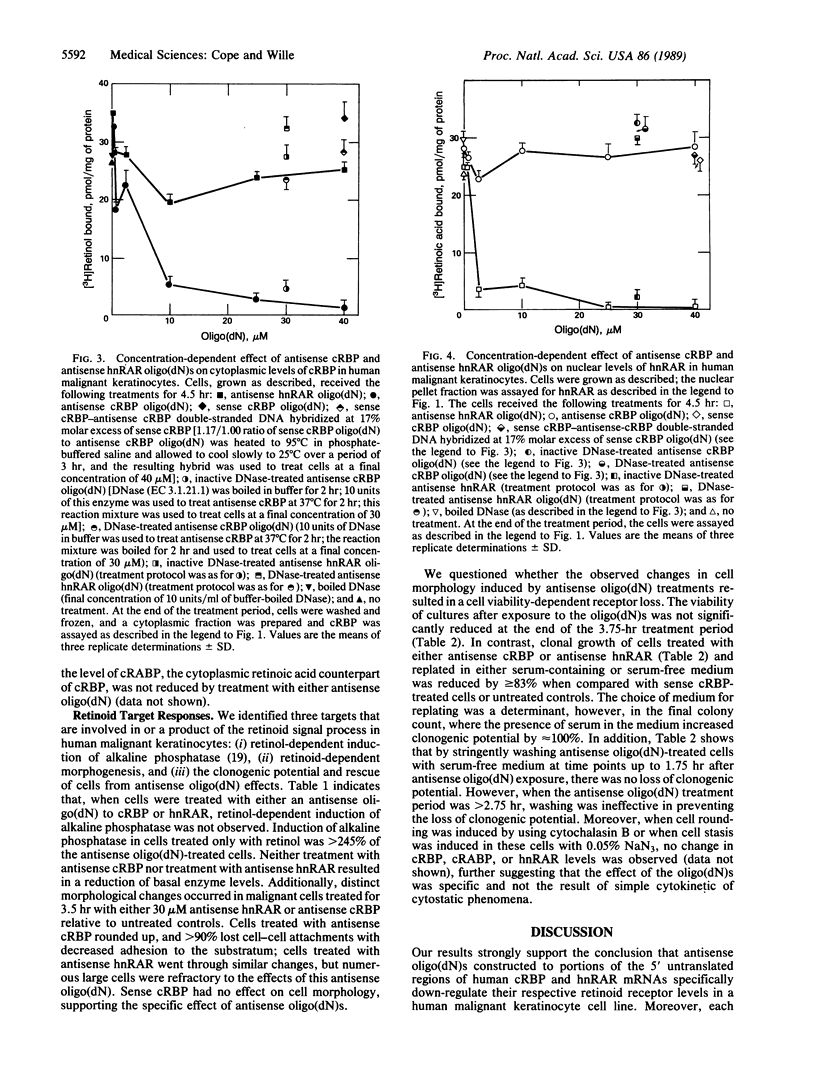
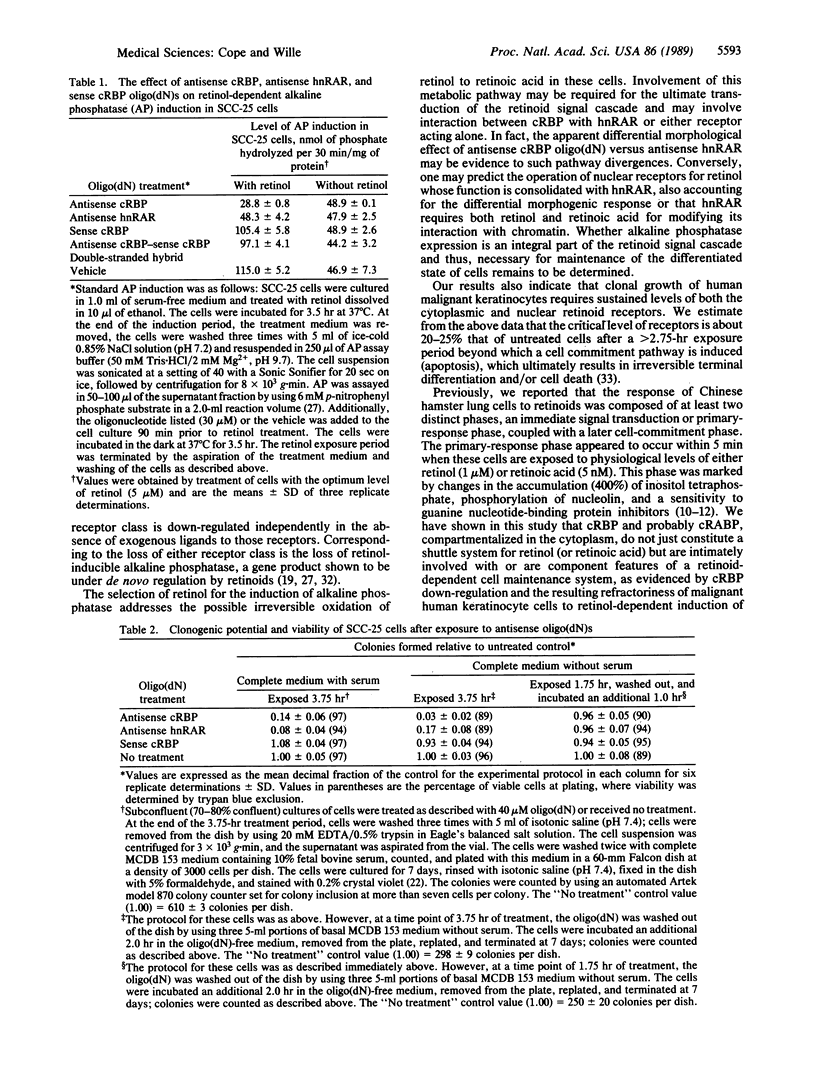
Antisense oligodeoxynucleotides [oligo(dN)s] corresponding to human cellular retinol-binding protein I (cRBP) and human nuclear retinoic acid receptor alpha (hnRAR) were synthesized. Exposure of human malignant keratinocytes to these oligo(dN)s significantly attenuated the level of cytoplasmic cRBP and hnRAR in a concentration- and time-dependent manner. Further, the induction of alkaline phosphatase by retinol in these cells was blocked by treatment with 30 microM antisense oligo(dN) to cRBP or hnRAR but not by 30 microM of sense oligo(dN) to cRBP. Antisense oligo(dN) treatments concomitantly induced cell rounding, loss of cell-cell attachment, and cell adhesion to the substratum. By contrast, treatment of cells with an anticytokinetic agent, cytochalasin B, or with a cytostatic concentration of sodium azide failed to reduce cytoplasmic cRBP or hnRAR from nuclear extracts, even though antisense oligo(dN)-like changes in cell morphology were observed. Treatment of the cells for greater than 2.75 hr with 20-40 microM of either antisense oligo(dN) also led to the loss of clonogenic potential. These results show that both cytoplasmic and nuclear receptors for retinoids are important in the transduction of a retinoid signal response critical to cellular growth and differentiation. Our findings also suggest that defined genes, which are specified by retinoids and their receptors, may account for the pleiotropic effect of vitamin A compounds.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashendel C. L., Boutwell R. K. Direct measurement of specific binding of highly lipophilic phorbol diester to mouse epidermal membranes using cold acetone. Biochem Biophys Res Commun. 1981 Mar 31;99(2):543–549. doi: 10.1016/0006-291x(81)91779-4. [DOI] [PubMed] [Google Scholar]
- Barkai U., Gubler M. L., Sherman M. I. Retinoid-binding proteins and retinoid-induced differentiation of embryonal carcinoma cells. Prog Clin Biol Res. 1986;226:205–213. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brand N., Petkovich M., Krust A., Chambon P., de Thé H., Marchio A., Tiollais P., Dejean A. Identification of a second human retinoic acid receptor. Nature. 1988 Apr 28;332(6167):850–853. doi: 10.1038/332850a0. [DOI] [PubMed] [Google Scholar]
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Colantuoni V., Cortese R., Nilsson M., Lundvall J., Båvik C. O., Eriksson U., Peterson P. A., Sundelin J. Cloning and sequencing of a full length cDNA corresponding to human cellular retinol-binding protein. Biochem Biophys Res Commun. 1985 Jul 16;130(1):431–439. doi: 10.1016/0006-291x(85)90435-8. [DOI] [PubMed] [Google Scholar]
- Cope F. O., Knox K. L., Hall R. C., Jr Rat testes interstitial cell nuclei exhibit three distinct receptors for retinoic acid. Experientia. 1984 Mar 15;40(3):276–277. doi: 10.1007/BF01947580. [DOI] [PubMed] [Google Scholar]
- Crow J. A., Ong D. E., Chytil F. Specificity of cellular retinol-binding protein in the transfer of retinol to nuclei and chromatin. Arch Biochem Biophys. 1987 Apr;254(1):372–375. doi: 10.1016/0003-9861(87)90113-5. [DOI] [PubMed] [Google Scholar]
- Eriksson U., Hansson E., Nilsson M., Jönsson K. H., Sundelin J., Peterson P. A. Increased levels of several retinoid binding proteins resulting from retinoic acid-induced differentiation of F9 cells. Cancer Res. 1986 Feb;46(2):717–722. [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Giguère V., Ong E. S., Evans R. M., Tabin C. J. Spatial and temporal expression of the retinoic acid receptor in the regenerating amphibian limb. Nature. 1989 Feb 9;337(6207):566–569. doi: 10.1038/337566a0. [DOI] [PubMed] [Google Scholar]
- Goodchild J., Agrawal S., Civeira M. P., Sarin P. S., Sun D., Zamecnik P. C. Inhibition of human immunodeficiency virus replication by antisense oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5507–5511. doi: 10.1073/pnas.85.15.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeble S., Maden M. Retinoic acid-binding protein in the axolotl: distribution in mature tissues and time of appearance during limb regeneration. Dev Biol. 1986 Oct;117(2):435–441. doi: 10.1016/0012-1606(86)90312-x. [DOI] [PubMed] [Google Scholar]
- Kongsuwan K., Webb E., Housiaux P., Adams J. M. Expression of multiple homeobox genes within diverse mammalian haemopoietic lineages. EMBO J. 1988 Jul;7(7):2131–2138. doi: 10.1002/j.1460-2075.1988.tb03052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavilio F., Simeone A., Boncinelli E., Andrews P. W. Activation of four homeobox gene clusters in human embryonal carcinoma cells induced to differentiate by retinoic acid. Differentiation. 1988;37(1):73–79. doi: 10.1111/j.1432-0436.1988.tb00798.x. [DOI] [PubMed] [Google Scholar]
- Mavilio F., Simeone A., Giampaolo A., Faiella A., Zappavigna V., Acampora D., Poiana G., Russo G., Peschle C., Boncinelli E. Differential and stage-related expression in embryonic tissues of a new human homoeobox gene. Nature. 1986 Dec 18;324(6098):664–668. doi: 10.1038/324664a0. [DOI] [PubMed] [Google Scholar]
- McCormick A. M., Shubeita H. E., Stocum D. L. Cellular retinoic acid binding protein: detection and quantitation in regenerating axolotl limbs. J Exp Zool. 1988 Mar;245(3):270–276. doi: 10.1002/jez.1402450307. [DOI] [PubMed] [Google Scholar]
- Ng K. W., Gummer P. R., Michelangeli V. P., Bateman J. F., Mascara T., Cole W. G., Martin T. J. Regulation of alkaline phosphatase expression in a neonatal rat clonal calvarial cell strain by retinoic acid. J Bone Miner Res. 1988 Feb;3(1):53–61. doi: 10.1002/jbmr.5650030109. [DOI] [PubMed] [Google Scholar]
- Reese D. H., Fiorentino G. J., Claflin A. J., Malinin T. I., Politano V. A. Rapid induction of alkaline phosphatase activity by retinoic acid. Biochem Biophys Res Commun. 1981 Sep 16;102(1):315–321. doi: 10.1016/0006-291x(81)91523-0. [DOI] [PubMed] [Google Scholar]
- Reese D. H., Gordon B., Gratzner H. G., Claflin A. J., Malinin T. I., Block N. L., Politano V. A. Effect of retinoic acid on the growth and morphology of a prostatic adenocarcinoma cell line cloned for the retinoid inducibility of alkaline phosphatase. Cancer Res. 1983 Nov;43(11):5443–5450. [PubMed] [Google Scholar]
- Sumikawa K., Miledi R. Repression of nicotinic acetylcholine receptor expression by antisense RNAs and an oligonucleotide. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1302–1306. doi: 10.1073/pnas.85.4.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaller C., Eichele G. Identification and spatial distribution of retinoids in the developing chick limb bud. Nature. 1987 Jun 18;327(6123):625–628. doi: 10.1038/327625a0. [DOI] [PubMed] [Google Scholar]
- Tomei L. D., Kanter P., Wenner C. E. Inhibition of radiation-induced apoptosis in vitro by tumor promoters. Biochem Biophys Res Commun. 1988 Aug 30;155(1):324–331. doi: 10.1016/s0006-291x(88)81088-x. [DOI] [PubMed] [Google Scholar]
- Walder R. Y., Walder J. A. Role of RNase H in hybrid-arrested translation by antisense oligonucleotides. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5011–5015. doi: 10.1073/pnas.85.14.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom E. L., Bacon T. A., Gonzalez A., Freeman D. L., Lyman G. H., Wickstrom E. Human promyelocytic leukemia HL-60 cell proliferation and c-myc protein expression are inhibited by an antisense pentadecadeoxynucleotide targeted against c-myc mRNA. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1028–1032. doi: 10.1073/pnas.85.4.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille J. J., Jr, Pittelkow M. R., Scott R. E. Normal and transformed human prokeratinocytes express divergent effects of a tumor promoter on cell cycle-mediated control of proliferation and differentiation. Carcinogenesis. 1985 Aug;6(8):1181–1187. doi: 10.1093/carcin/6.8.1181. [DOI] [PubMed] [Google Scholar]
- Zile M. H., Cullum M. E., Roltsch I. A., DeHoog J. V., Welsch C. W. Effect of moderate vitamin A supplementation and lack of dietary vitamin A on the development of mammary tumors in female rats treated with low carcinogenic dose levels of 7,12-dimethylbenz(a)anthracene. Cancer Res. 1986 Jul;46(7):3495–3503. [PubMed] [Google Scholar]
- de Thé H., Marchio A., Tiollais P., Dejean A. A novel steroid thyroid hormone receptor-related gene inappropriately expressed in human hepatocellular carcinoma. Nature. 1987 Dec 17;330(6149):667–670. doi: 10.1038/330667a0. [DOI] [PubMed] [Google Scholar]


