Abstract
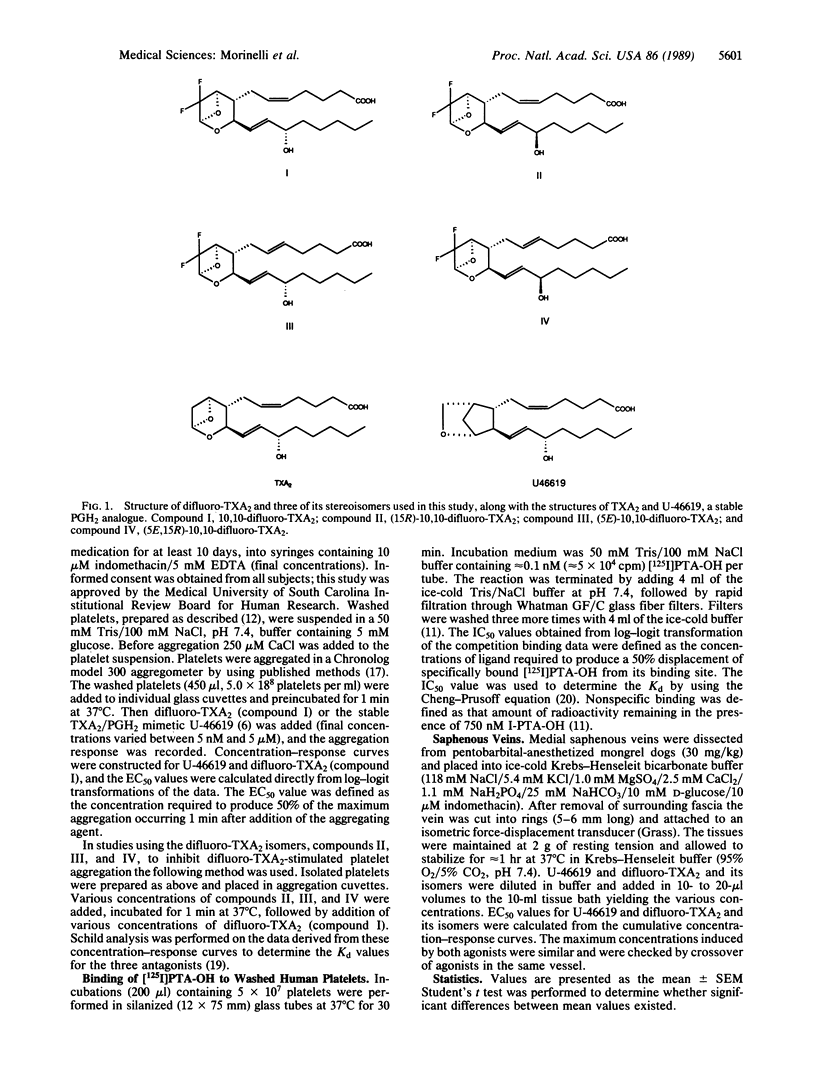
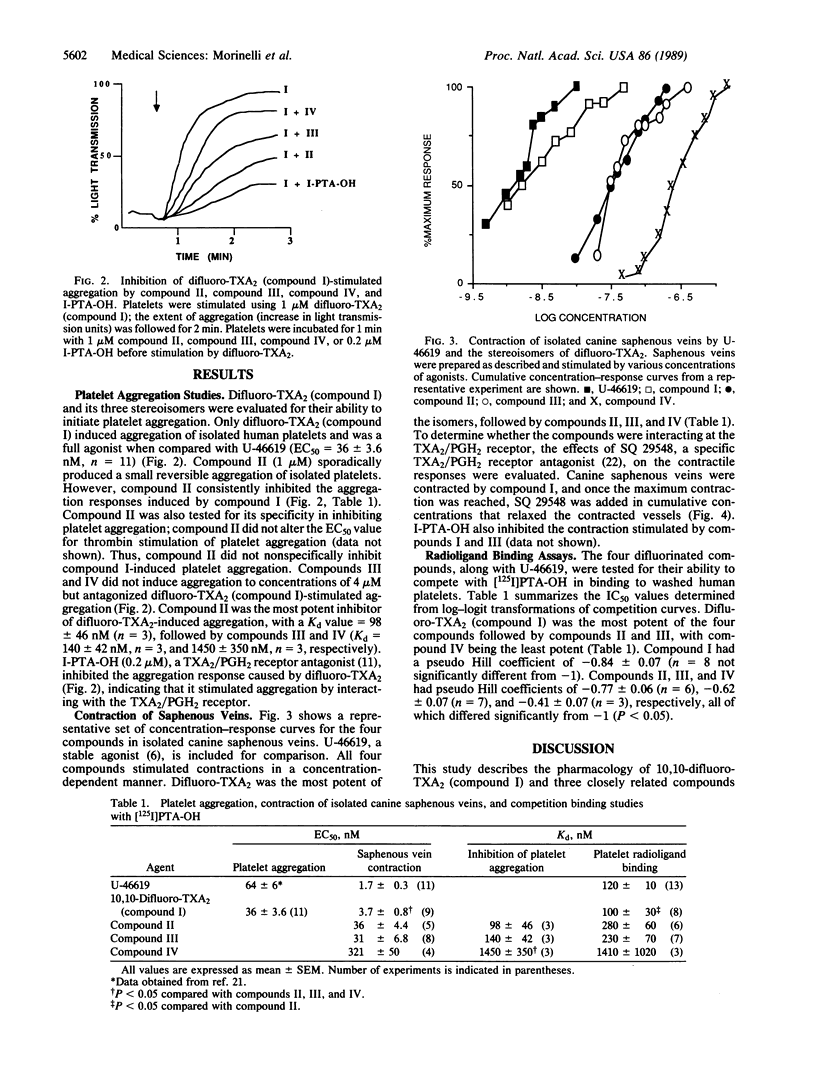
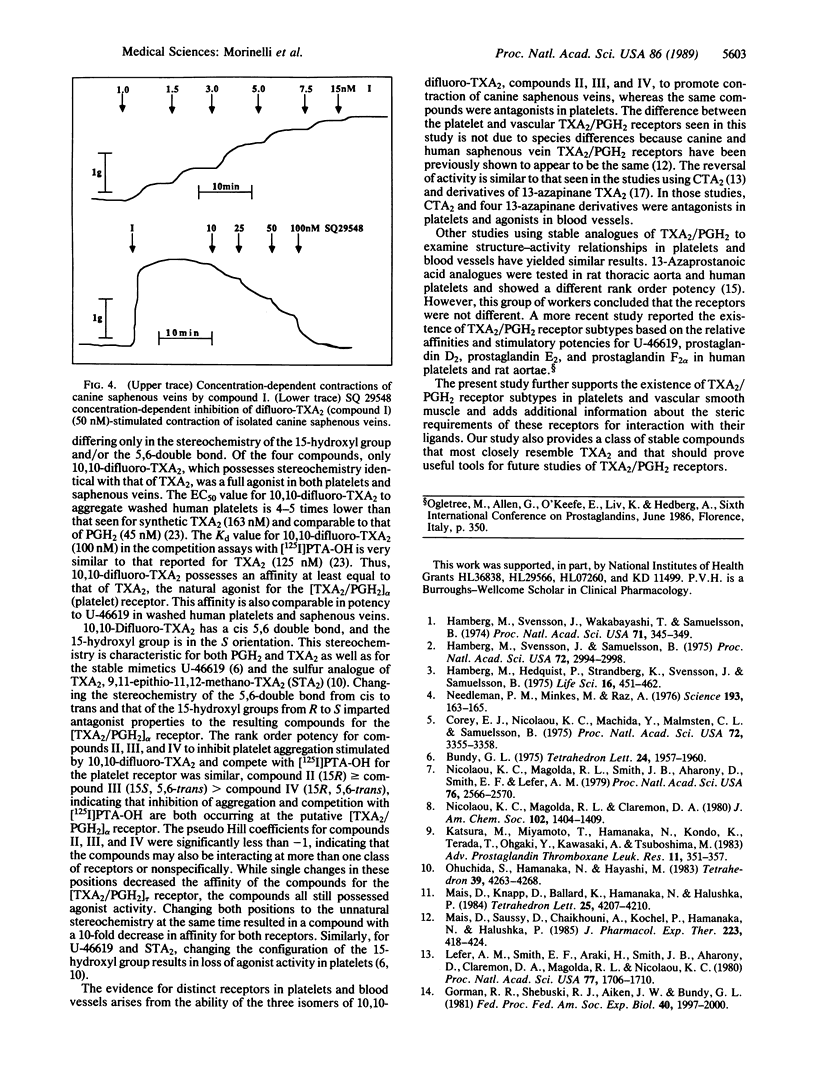
The present study reports on the selective effects on human platelets and canine saphenous veins of four stable difluorinated analogues and thromboxane A2 (TXA2), in which the characteristic 2,6-dioxa[3.1.1]bicycloheptane structure of TXA2 has been retained. The four compounds differ in their stereochemistry of the 5,6 double bond and/or the 15-hydroxyl group. Only 10,10-difluoro-TXA2 (compound I) with the natural stereochemistry of TXA2 was an agonist in both platelets and canine saphenous veins (EC50 = 36 +/- 3.6 nM and 3.7 +/- 0.8 nM, respectively). (15R)-10,10-Difluoro-TXA2 (compound II), (5E)-10,10-difluoro-TXA2 (compound III), and (5E,15R)-10,10-difluoro-TXA2 (compound IV) were antagonists of platelet aggregation stimulated by compound I (Kd = 98 +/- 46 nM, 140 +/- 42 nM, and 1450 +/- 350 nM, respectively). However, compounds II, III, and IV stimulated contraction of canine saphenous veins (EC50 = 36 +/- 4.4 nM, 31 +/- 6.8 nM, and 321 +/- 50 nM, respectively). All four compounds could displace the TXA2/prostaglandin H2 antagonist 9,11-dimethylmethano-11,12-methano-16-(3- 125I-4-hydroxyphenyl)-13,14-dihydro-13-aza-15 alpha beta-omega-tetranor-TXA2 from its platelet receptor (Kd values = 100 +/- 30 nM, compound I; 280 +/- 60 nM, compound II; 230 +/- 70 nM, compound III; and 1410 +/- 1020 nM, compound IV). These results support the existence of two subtypes of TXA2/prostaglandin H2 receptors and emphasize the importance of the stereochemical requirements of these TXA2 analogues for interaction with these receptors. These stable fluorinated TXA2 analogues should prove useful tools for the further characterization of these and other TXA2/prostaglandin H2 receptors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Corey E. J., Nicolaou K. C., Machida Y., Malmsten C. L., Samuelsson B. Synthesis and biological properties of a 9,11-azo-prostanoid: highly active biochemical mimic of prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3355–3358. doi: 10.1073/pnas.72.9.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman R. R., Shebuski R. J., Aiken J. W., Bundy G. L. Analysis of the biological activity of azoprostanoids in human platelets. Fed Proc. 1981 May 15;40(7):1997–2000. [PubMed] [Google Scholar]
- Halushka P. V., Kochel P. J., Mais D. E. Binding of thromboxane A2/prostaglandin H2 agonists to human platelets. Br J Pharmacol. 1987 May;91(1):223–227. doi: 10.1111/j.1476-5381.1987.tb09002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Hedqvist P., Strandberg K., Svensson J., Samuelsson B. Prostaglandin endoperoxides IV. Effects on smooth muscle. Life Sci. 1975 Feb 1;16(3):451–462. doi: 10.1016/0024-3205(75)90266-0. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Wakabayashi T., Samuelsson B. Isolation and structure of two prostaglandin endoperoxides that cause platelet aggregation. Proc Natl Acad Sci U S A. 1974 Feb;71(2):345–349. doi: 10.1073/pnas.71.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huzoor-Akbar, Mukhopadhyay A., Anderson K. S., Navran S. S., Romstedt K., Miller D. D., Feller D. R. Antagonism of prostaglandin-mediated responses in platelets and vascular smooth muscle by 13-azaprostanoic acid analogs. Evidence for selective blockade of thromboxane A2 responses. Biochem Pharmacol. 1985 Mar 1;34(5):641–647. doi: 10.1016/0006-2952(85)90258-8. [DOI] [PubMed] [Google Scholar]
- Katsura M., Miyamoto T., Hamanaka N., Kondo K., Terada T., Ohgaki Y., Kawasaki A., Tsuboshima M. In vitro and in vivo effects of new powerful thromboxane antagonists (3-alkylamino pinane derivatives). Adv Prostaglandin Thromboxane Leukot Res. 1983;11:351–357. [PubMed] [Google Scholar]
- Lefer A. M., Smith E. F., 3rd, Araki H., Smith J. B., Aharony D., Claremon D. A., Magolda R. L., Nicolaou K. C. Dissociation of vasoconstrictor and platelet aggregatory activities of thromboxane by carbocyclic thromboxane A2, a stable analog of thromboxane A2. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1706–1710. doi: 10.1073/pnas.77.3.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mais D. E., DeHoll D., Sightler H., Halushka P. V. Different pharmacologic activities for 13-azapinane thromboxane A2 analogs in platelets and blood vessels. Eur J Pharmacol. 1988 Apr 13;148(3):309–315. doi: 10.1016/0014-2999(88)90108-2. [DOI] [PubMed] [Google Scholar]
- Mais D. E., Dunlap C., Hamanaka N., Halushka P. V. Further studies on the effects of epimers of thromboxane A2 antagonists on platelets and veins. Eur J Pharmacol. 1985 Apr 23;111(1):125–128. doi: 10.1016/0014-2999(85)90121-9. [DOI] [PubMed] [Google Scholar]
- Mais D. E., Saussy D. L., Jr, Chaikhouni A., Kochel P. J., Knapp D. R., Hamanaka N., Halushka P. V. Pharmacologic characterization of human and canine thromboxane A2/prostaglandin H2 receptors in platelets and blood vessels: evidence for different receptors. J Pharmacol Exp Ther. 1985 May;233(2):418–424. [PubMed] [Google Scholar]
- Mayeux P. R., Morton H. E., Gillard J., Lord A., Morinelli T. A., Boehm A., Mais D. E., Halushka P. V. The affinities of prostaglandin H2 and thromboxane A2 for their receptor are similar in washed human platelets. Biochem Biophys Res Commun. 1988 Dec 15;157(2):733–739. doi: 10.1016/s0006-291x(88)80311-5. [DOI] [PubMed] [Google Scholar]
- Needleman P., Minkes M., Raz A. Thromboxanes: selective biosynthesis and distinct biological properties. Science. 1976 Jul 9;193(4248):163–165. doi: 10.1126/science.945611. [DOI] [PubMed] [Google Scholar]
- Nicolaou K. C., Magolda R. L., Smith J. B., Aharony D., Smith E. F., Lefer A. M. Synthesis and biological properties of pinane-thromboxane A2, a selective inhibitor of coronary artery constriction, platelet aggregation, and thromboxane formation. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2566–2570. doi: 10.1073/pnas.76.6.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogletree M. L., Harris D. N., Greenberg R., Haslanger M. F., Nakane M. Pharmacological actions of SQ 29,548, a novel selective thromboxane antagonist. J Pharmacol Exp Ther. 1985 Aug;234(2):435–441. [PubMed] [Google Scholar]



