Abstract
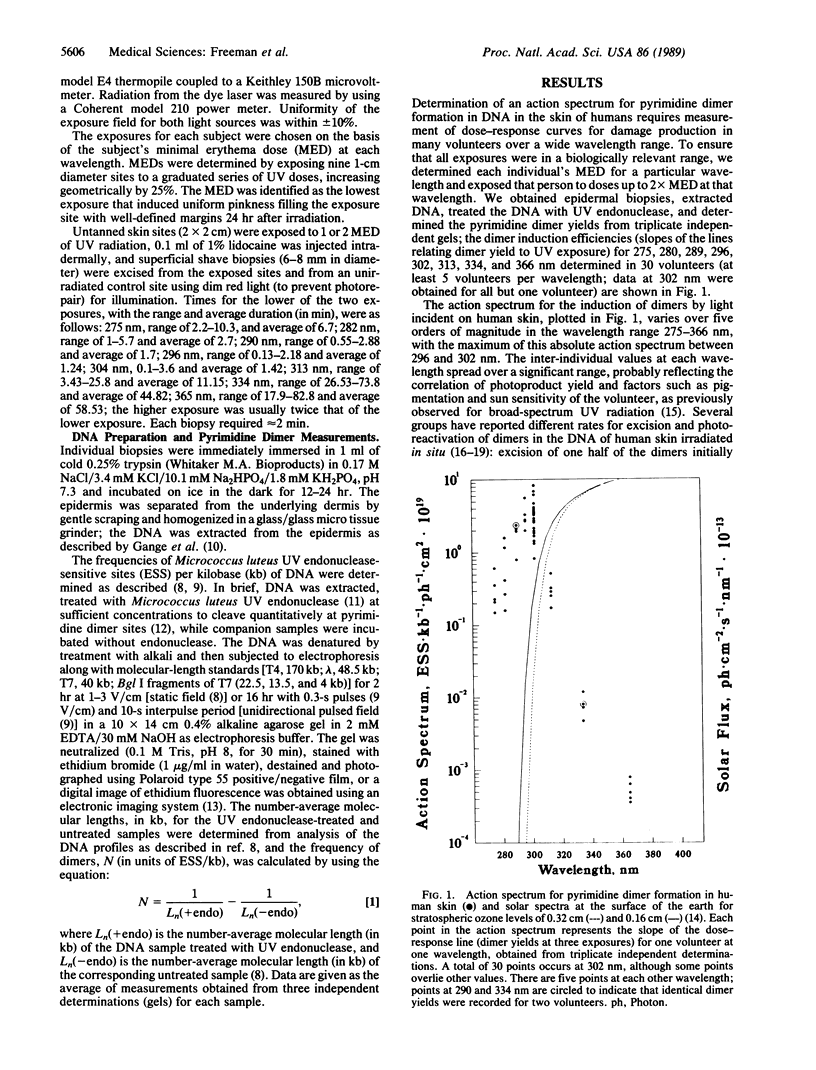
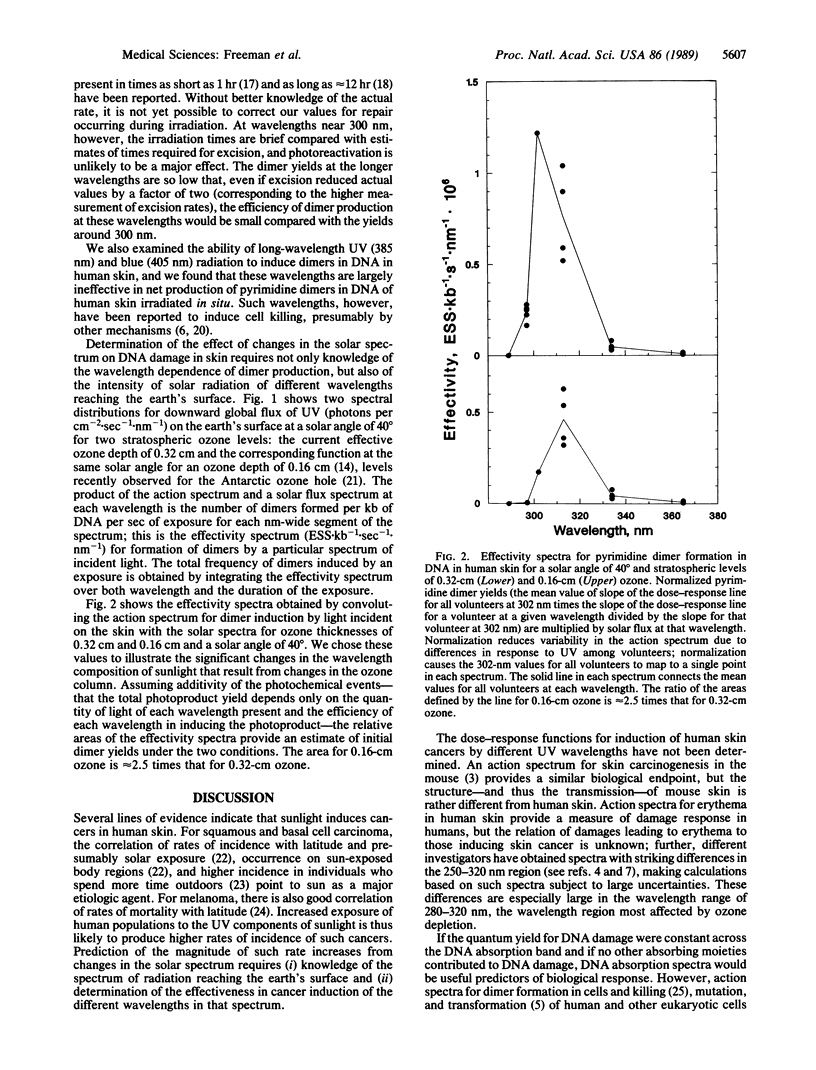
The UV components of sunlight are believed to be a major cause of human skin cancer, and DNA is thought to be the principal molecular target. Alterations of the intensity and wavelength distribution of solar UV radiation reaching the surface of the earth, for example by depletion of stratospheric ozone, will change the effectiveness of solar radiation in damaging DNA in human skin. Evaluation of the magnitude of such effects requires knowledge of the altered sunlight spectrum and of the action spectrum for damaging DNA in human skin. We have determined an action spectrum for the frequency of pyrimidine dimer formation induced in the DNA of human skin per unit dose of UV incident on the skin surface. The peak of this action spectrum is near 300 nm and decreases rapidly at both longer and shorter wavelengths. The decrease in our action spectrum for wavelengths less than 300 nm is attributed to the absorption of the upper layers of the skin, an in situ effect that is inherently included in our measurements. Convolution of the dimer action spectrum with the solar spectra corresponding to a solar angle of 40 degrees under current levels of stratospheric ozone (0.32-cm O3 layer) and those for 50% ozone depletion (0.16-cm O3 layer), indicate about a 2.5-fold increase in dimer formation. If the action spectrum for DNA damage that results in skin cancer resembles that for dimer induction in skin, our results, combined with epidemiological data, suggest that a 50% decrease in stratospheric ozone would increase the incidence of nonmelanoma skin cancers among white males in Seattle, Washington, by 7.5- to 8-fold, to a higher incidence than is presently seen in the corresponding population of Albuquerque, New Mexico.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed F. E., Setlow R. B. Different rate-limiting steps in excision repair of ultraviolet- and N-acetoxy-2-acetylaminofluorene-damaged DNA in normal human fibroblasts. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1548–1552. doi: 10.1073/pnas.74.4.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger D. S. The sunburning ultraviolet meter: design and performance. Photochem Photobiol. 1976 Dec;24(6):587–593. doi: 10.1111/j.1751-1097.1976.tb06877.x. [DOI] [PubMed] [Google Scholar]
- Carrier W. L., Setlow R. B. Endonuclease from Micrococcus luteus which has activity toward ultraviolet-irradiated deoxyribonucleic acid: purification and properties. J Bacteriol. 1970 Apr;102(1):178–186. doi: 10.1128/jb.102.1.178-186.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G. L., Peak M. J., Peak J. G., Haseltine W. A. Action spectrum for the formation of endonuclease-sensitive sites and (6-4) photoproducts induced in a DNA fragment by ultraviolet radiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1986 Oct;50(4):641–648. doi: 10.1080/09553008614551041. [DOI] [PubMed] [Google Scholar]
- Cole C. A., Forbes P. D., Davies R. E. An action spectrum for UV photocarcinogenesis. Photochem Photobiol. 1986 Mar;43(3):275–284. doi: 10.1111/j.1751-1097.1986.tb05605.x. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio S. M., Slazinski L., Whetstone J. W., Lowney E. Excision repair of UV-induced pyrimidine dimers in human skin in vivo. J Invest Dermatol. 1981 Sep;77(3):311–313. doi: 10.1111/1523-1747.ep12482484. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio S. M., Whetstone J. W., Slazinski L., Lowney E. Photorepair of pyrimidine dimers in human skin in vivo. Photochem Photobiol. 1981 Oct;34(4):461–464. [PubMed] [Google Scholar]
- Freeman S. E., Blackett A. D., Monteleone D. C., Setlow R. B., Sutherland B. M., Sutherland J. C. Quantitation of radiation-, chemical-, or enzyme-induced single strand breaks in nonradioactive DNA by alkaline gel electrophoresis: application to pyrimidine dimers. Anal Biochem. 1986 Oct;158(1):119–129. doi: 10.1016/0003-2697(86)90599-3. [DOI] [PubMed] [Google Scholar]
- Freeman S. E., Gange R. W., Matzinger E. A., Sutherland B. M. Higher pyrimidine dimer yields in skin of normal humans with higher UVB sensitivity. J Invest Dermatol. 1986 Jan;86(1):34–36. doi: 10.1111/1523-1747.ep12283768. [DOI] [PubMed] [Google Scholar]
- Freeman S. E. Variations in excision repair of UVB-induced pyrimidine dimers in DNA of human skin in situ. J Invest Dermatol. 1988 Jun;90(6):814–817. doi: 10.1111/1523-1747.ep12462039. [DOI] [PubMed] [Google Scholar]
- Gange R. W., Blackett A. D., Matzinger E. A., Sutherland B. M., Kochevar I. E. Comparative protection efficiency of UVA- and UVB-induced tans against erythema and formation of endonuclease-sensitive sites in DNA by UVB in human skin. J Invest Dermatol. 1985 Oct;85(4):362–364. doi: 10.1111/1523-1747.ep12276983. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L., Rosenstein B. S. The use of specific radioimmunoassay to determine action spectra for the photolysis of (6-4) photoproducts. Photochem Photobiol. 1987 Jun;45(6):781–786. doi: 10.1111/j.1751-1097.1987.tb07882.x. [DOI] [PubMed] [Google Scholar]
- Rosenstein B. S., Mitchell D. L. Action spectra for the induction of pyrimidine(6-4)pyrimidone photoproducts and cyclobutane pyrimidine dimers in normal human skin fibroblasts. Photochem Photobiol. 1987 Jun;45(6):775–780. doi: 10.1111/j.1751-1097.1987.tb07881.x. [DOI] [PubMed] [Google Scholar]
- Setlow R. B. The wavelengths in sunlight effective in producing skin cancer: a theoretical analysis. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3363–3366. doi: 10.1073/pnas.71.9.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland B. M., Delihas N. C., Oliver R. P., Sutherland J. C. Action spectra for ultraviolet light-induced transformation of human cells to anchorage-independent growth. Cancer Res. 1981 Jun;41(6):2211–2214. [PubMed] [Google Scholar]
- Sutherland B. M., Harber L. C., Kochevar I. E. Pyrimidine dimer formation and repair in human skin. Cancer Res. 1980 Sep;40(9):3181–3185. [PubMed] [Google Scholar]
- Sutherland J. C., Griffin K. P. Absorption spectrum of DNA for wavelengths greater than 300 nm. Radiat Res. 1981 Jun;86(3):399–409. [PubMed] [Google Scholar]
- Sutherland J. C., Lin B., Monteleone D. C., Mugavero J., Sutherland B. M., Trunk J. Electronic imaging system for direct and rapid quantitation of fluorescence from electrophoretic gels: application to ethidium bromide-stained DNA. Anal Biochem. 1987 Jun;163(2):446–457. doi: 10.1016/0003-2697(87)90247-8. [DOI] [PubMed] [Google Scholar]
- Sutherland J. C., Monteleone D. C., Mugavero J. H., Trunk J. Unidirectional pulsed-field electrophoresis of single- and double-stranded DNA in agarose gels: analytical expressions relating mobility and molecular length and their application in the measurement of strand breaks. Anal Biochem. 1987 May 1;162(2):511–520. doi: 10.1016/0003-2697(87)90427-1. [DOI] [PubMed] [Google Scholar]
- Tyrrell R. M., Pidoux M. Action spectra for human skin cells: estimates of the relative cytotoxicity of the middle ultraviolet, near ultraviolet, and violet regions of sunlight on epidermal keratinocytes. Cancer Res. 1987 Apr 1;47(7):1825–1829. [PubMed] [Google Scholar]


