Abstract
To examine whether there are any characteristics of women or their initial tumors that might be useful for tailoring surveillance recommendations to optimize outcomes. We followed 17,286 women for up to 5 years after an initial diagnosis of ductal carcinoma in situ (DCIS) or early stage (I/II) invasive breast cancer diagnosed between 1996 and 2006. We calculated rates per 1,000 women years of recurrences and second breast primaries relative to demographics, risk factors, and characteristics of initial diagnosis: stage, treatment, mode of initial diagnosis. Nearly 4% had a second breast cancer event (314 recurrences and 344 second breast primaries). Women who used adjuvant hormonal therapy or were ≥80 years had the lowest rates of second events. Factors associated with higher recurrence and second primary rates included: initial DCIS or stage IIB, estrogen/progesterone receptor-negative, younger women (<50 years). Women with a family history or greater breast density had higher second primary rates, and women who received breast conserving surgery without radiation had higher recurrence rates. Roughly one-third of recurrences (37.6%) and second primaries (36.3%) were not screen-detected. Initial mode of diagnosis was a predictor of second events after adjusting for age, stage, primary treatment, and breast density. A recent negative mammogram should not falsely reassure physicians or women with new breast symptoms or changes because one-third of second cancers were interval cancers. This study does not provide any evidence in support of changing surveillance intervals for different subgroups.
Keywords: Carcinoma, Ductal, Breast, Recurrence, Neoplasm recurrence, Local, Neoplasms, Second primary, Ultrasonography, Mammary, Diagnostic imaging, Breast neoplasms, Mammography
Background
Breast cancer survivors remain at high risk of a second breast cancer for many years after initial diagnosis [1, 2]. Surveillance mammography aims to detect second breast cancers at earlier stages to decrease morbidity and ultimately improve survival [3]. Annual surveillance mammograms and clinical breast examination are recommended for women starting 1 year after initial diagnosis and no earlier than 6-months after radiation therapy is completed [4].
There are demonstrated benefits of surveillance mammography on reducing breast cancer mortality [5–7]. In a recent study, surveillance mammography reduced breast cancer mortality only for women with local recurrences and did not affect regional or distant recurrences; this provides compelling evidence that early detection of local recurrences drives mortality benefits [6].
We examined factors associated with surveillance mammography among survivors of early stage breast cancer in the Breast Cancer Surveillance Consortium (BCSC). We describe time to recurrence and second primary breast cancers by initial tumor characteristics, risk factors (including breast density), and mode of initial and subsequent detection.
Methods
This cohort study used pooled BCSC data from four registries: Group Health, Vermont Breast Cancer Surveillance System, New Hampshire Mammography Network, and New Mexico Mammography Project [8, 9]. Participating registries collect information on mammograms in their defined catchment areas and link women to pathology and state tumor registries or regional SEER programs to obtain population-based cancer data. These registries collect demographic and breast cancer risk factor data from a self-reported questionnaire completed at each mammogram.
The central statistical coordinating center (SCC) analyzed the data. Each registry and the SCC have received Institutional Review Board approval for either active or passive consenting processes or a waiver of consent to enroll participants, link data, and perform analytic studies. All procedures are Health Insurance Portability and Accountability Act compliant and all registries and the SCC have received a Federal Certificate of Confidentiality and other protection for the identities of women, physicians, and facilities that are subjects of this research [10].
We included women who had a mammogram and were diagnosed with in situ or early stage (I/II) [11] breast cancer between 1996 and 2006 (N = 25,640). Starting from the initial breast cancer diagnosis, we determined whether any further breast cancer diagnoses occurred within the next 6-months. If so, we used the most invasive diagnosis in that period. We collected stage (0/I/IIA/IIB) [11], nodal status, estrogen (ER) and progesterone receptor (PR) status, and primary treatment from tumor registries.
We excluded women with evidence of prior breast cancer (n = 2108), unknown laterality or bilateral first cancer (n = 769), second breast cancer diagnoses ≤6 months (n = 55), missing stage or stage ≥III (n = 3436) or no definitive surgery (n = 1298), missing second cancer laterality (n = 25), and <6 months of follow-up after initial diagnosis (n = 663). This left a final sample of 17,286.
All women were followed for 5 years from initial diagnosis until the first of the following: second breast cancer, death, or the last day of available information from the cancer registry. We identified 658 second DCIS and invasive breast cancers. Laterality and histology (ductal, lobular or mixed, not otherwise specified or other) of the first and second invasive cancers were compared to distinguish between recurrences and new primaries. Recurrences were unilateral diagnoses in the ipsilateral breast and had to share the same histology as the primary tumor (n = 314). We defined 344 second events as second primaries, including 15 bilateral second events, 290 contralateral events, and 39 ipsilateral events whose histology differed from that of the initial tumor.
Self-administered questionnaires were used to collect race/ethnicity, self-reported lump before mammogram, menopausal status (last reported ≤6 months before first and second diagnosis), and first-degree breast cancer family history reported before initial diagnosis. Breast density was recorded by the interpreting radiologist using the Breast Imaging Reporting and Data Systems [12] four density categories and was included only if recorded less than or equal to 2 years before the mammogram used for initial detection mode.
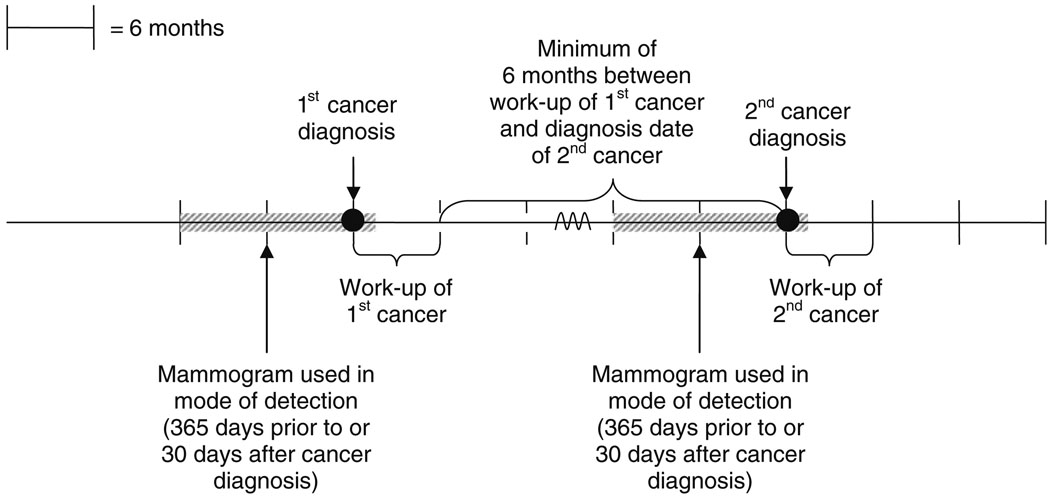
We used established methods combining mammogram indication and results to classify detection mode at each diagnosis [13–15]. Mammograms were classified as screening (routine screening without evidence of a self-reported lump ≤30 days before the mammogram leading to the 1st diagnosis and with a radiologists’ indication for exam noted as screening) [13] or diagnostic (additional evaluation of a recent mammogram, short-interval follow-up, evaluation of a breast problem including self-reported presence of a breast lump by patient or provider ≤30 days before diagnosis) [16]. We used the earliest screening exam ≤365 days before diagnosis to define detection mode. If the registry had no screening mammograms, we used the earliest diagnostic mammogram ≤365 days before the diagnosis (Fig. 1) [17]. If no mammogram was found ≤365 days before diagnosis, we used the first diagnostic mammogram ≤30 days after diagnosis, because diagnosis dates in tumor registries often represent the first evidence of cancer.
Fig. 1.
Mammograms used to define detection mode for first and second cancer events. Most patient risk factors were based on responses to questionnaires completed within 6 months of the mammogram used for initial mode of detection. Breast density was recorded by the interpreting radiologist and was only included in the analysis if it was recorded within ≤2 years of the mammogram used for initial mode of detection. Example Assume a woman has her first breast cancer diagnosed on 1 January 2007. To determine the mode of detection we search for a mammogram from 1 January 2006–1 January 2007. If none is found then we also look ahead until 1 February 2007. Assume work-up of her first cancer finishes on 15 February 2007. We then begin looking for a second breast cancer diagnosis 6 months later (after 16 August 2007)
Detection mode was defined as: screen-positive, screen-negative (interval cancer following a negative screening exam), diagnostic-positive, or diagnostic-negative, using standard definitions [13–15, 18]. Mammograms with final BI-RADS® assessments of 4 or 5 were considered “positive,” as were BI-RADS® assessments of 3 or 0 with a biopsy recommendation, surgical consult or fine needle aspiration. All others were considered “negative.” The same strategy was used for the second diagnosis, with the added restriction that the mammogram had to follow the first cancer workup.
Analysis
Frequency distributions were computed for characteristics of women and first cancers. These were examined separately for three groups, those without a second cancer, those with a recurrence, and those with a second primary cancer. Cross-tabulations of initial detection mode and self-reported lump by detection mode of the second cancer were done separately by second breast event.
A surveillance mammogram was defined as any mammogram ≥6 months after the work-up of the first cancer and before the second cancer. Kaplan–Meier curves were used to summarize the relations between first cancer characteristics and surveillance mammography receipt.
We computed recurrence and new primary rates/1,000 woman years with 95% confidence intervals (CI) calculated on the Poisson distribution. We plotted the Nelson–Aalen estimate of the cumulative hazard function separately for each second events for certain characteristics. We also plotted an estimate of the hazard function for second events to detect any incidence peak.
We fit multivariable models using Cox regression to examine factors associated with second events; different models were created for each risk factor (breast density, initial detection mode, family history) and then one with all risk factors included. All models adjusted for stage, adjuvant therapy, age at diagnosis, and registry. We used SAS 9.0 for most analyses and generated hazard plots and multivariable models in Stata v.9.0.
Results
Stage I included 48.2% of the cohort, followed by stage II (33.1%), and DCIS (18.8%) (Table 1). Nearly 4% of the 17,286 women were diagnosed with a recurrence (N = 314) or second primary (N = 344). Among second cancers, 4% were stage III/IV, 21% were stage II, 33% were stage I, 22% were DCIS, and staging was unavailable for 19%.
Table 1.
Characteristics of women with early stage breast cancer (0, I, II) and their outcomes
| Total (17,286) |
Women with no second breast cancer (N = 16,628) |
Breast cancer recurrencea total (N = 314) |
Second primary breast cancer total (N = 344) |
|||||
|---|---|---|---|---|---|---|---|---|
| N | Row % | N | Row % | N | Row % | |||
| Characteristics of 1st cancer diagnosis | ||||||||
| Stage at 1st diagnosis | ||||||||
| 0 | 3,243 | 18.8 | 3,071 | 94.7 | 92 | 2.8 | 80 | 2.5 |
| I | 8,327 | 48.2 | 8,050 | 96.7 | 110 | 1.3 | 167 | 2.0 |
| IIA | 3,797 | 22.0 | 3,680 | 96.9 | 68 | 1.8 | 49 | 1.3 |
| IIB | 1,919 | 11.1 | 1,827 | 95.2 | 44 | 2.3 | 48 | 2.5 |
| Nodal status | ||||||||
| No invasion | 10,565 | 61.1 | 10,215 | 96.7 | 157 | 1.5 | 193 | 1.8 |
| Invasion | 3,476 | 20.1 | 3,340 | 96.1 | 65 | 1.9 | 71 | 2.0 |
| Unknown | 3,245 | 18.8 | 3,073 | 94.7 | 92 | 2.8 | 80 | 2.5 |
| Hormone receptor | ||||||||
| ER−/PR− | 2,097 | 12.1 | 1,994 | 95.1 | 60 | 2.9 | 43 | 2.1 |
| ER−/PR+ | 215 | 1.2 | 205 | 95.3 | 8 | 3.7 | 2 | 0.9 |
| ER+/PR− | 1,310 | 7.6 | 1,270 | 96.9 | 24 | 1.8 | 16 | 1.2 |
| ER+/PR+ | 9,131 | 52.8 | 8,861 | 97.0 | 109 | 1.2 | 161 | 1.8 |
| Unknown | 4,533 | 26.2 | 4,298 | 94.8 | 113 | 2.5 | 122 | 2.7 |
| Primary surgery | ||||||||
| BCS without radiation therapy | 3,150 | 18.2 | 2,971 | 94.3 | 120 | 3.8 | 59 | 1.9 |
| BCS with radiation therapy | 7,962 | 46.1 | 7,689 | 96.6 | 118 | 1.5 | 155 | 1.9 |
| Mastectomy | 6,174 | 35.7 | 5,968 | 96.7 | 76 | 1.2 | 130 | 2.1 |
| Radiation therapy | ||||||||
| None | 8,128 | 47.0 | 7,790 | 95.8 | 185 | 2.3 | 153 | 1.9 |
| Any | 8,944 | 51.7 | 8,637 | 96.6 | 128 | 1.4 | 179 | 2.0 |
| Unknown | 214 | 1.2 | 201 | 93.9 | 1 | 0.5 | 12 | 5.6 |
| Adjuvant therapy | ||||||||
| Neither | 8,388 | 48.5 | 8,038 | 95.8 | 170 | 2.0 | 180 | 2.1 |
| Chemotherapy | 2,496 | 14.4 | 2,380 | 95.4 | 61 | 2.4 | 55 | 2.2 |
| Hormonal | 3,744 | 21.7 | 3,636 | 97.1 | 47 | 1.3 | 61 | 1.6 |
| Both | 1,649 | 9.5 | 1,592 | 96.5 | 27 | 1.6 | 30 | 1.8 |
| Unknown | 1,009 | 5.8 | 982 | 97.3 | 9 | 0.9 | 18 | 1.8 |
| Mode of detection of 1st cancer diagnosis | ||||||||
| Screen-detected (screen and positive) | 6,183 | 35.8 | 5,984 | 96.8 | 87 | 1.4 | 112 | 1.8 |
| Screen interval-detected (screen and negative) | 2,702 | 15.6 | 2,597 | 96.1 | 44 | 1.6 | 61 | 2.3 |
| Diagnostic detected (diagnostic and positive) | 3,389 | 19.6 | 3,242 | 95.7 | 77 | 2.3 | 70 | 2.1 |
| Diagnostic interval-detected (diagnostic and negative) | 953 | 5.5 | 912 | 95.7 | 19 | 2.0 | 22 | 2.3 |
| Unknown | 4,059 | 23.5 | 3,893 | 95.9 | 87 | 2.1 | 79 | 1.9 |
| Characteristics of women at 1st cancer diagnosis | ||||||||
| Age at 1st diagnosis | ||||||||
| 18–39 | 710 | 4.1 | 668 | 94.1 | 25 | 3.5 | 17 | 2.4 |
| 40–49 | 3,411 | 19.7 | 3,253 | 95.4 | 87 | 2.6 | 71 | 2.1 |
| 50–59 | 4,616 | 26.7 | 4,452 | 96.4 | 78 | 1.7 | 86 | 1.9 |
| 60–69 | 3,864 | 22.4 | 3,732 | 96.6 | 57 | 1.5 | 75 | 1.9 |
| 70–79 | 3,291 | 19.0 | 3,164 | 96.1 | 50 | 1.5 | 77 | 2.3 |
| ≥80 | 1,394 | 8.1 | 1,359 | 97.5 | 17 | 1.2 | 18 | 1.3 |
| Year of 1st diagnosis | ||||||||
| 1996–1997 | 3,322 | 19.2 | 3,165 | 95.3 | 73 | 2.2 | 84 | 2.5 |
| 1998–1999 | 3,789 | 21.9 | 3,598 | 95.0 | 88 | 2.3 | 103 | 2.7 |
| 2000–2001 | 3,784 | 21.9 | 3,607 | 95.3 | 83 | 2.2 | 94 | 2.5 |
| 2002–2003 | 3,458 | 20.0 | 3,364 | 97.3 | 46 | 1.3 | 48 | 1.4 |
| 2004–2006 | 2,933 | 17.0 | 2,894 | 98.7 | 24 | 0.8 | 15 | 0.5 |
| Race/ethnicity | ||||||||
| White | 13,178 | 76.2 | 12,686 | 96.3 | 227 | 1.7 | 265 | 2.0 |
| Black | 132 | 0.8 | 124 | 93.9 | 5 | 3.8 | 3 | 2.3 |
| Hispanic | 1,884 | 10.9 | 1,823 | 96.8 | 39 | 2.1 | 22 | 1.2 |
| Asian/Pacific Islander | 242 | 1.4 | 233 | 96.3 | 2 | 0.8 | 7 | 2.9 |
| Native American | 303 | 1.8 | 293 | 96.7 | 8 | 2.6 | 2 | 0.7 |
| Other | 195 | 1.1 | 184 | 94.4 | 7 | 3.6 | 4 | 2.1 |
| Unknown | 1,352 | 7.8 | 1,285 | 95.0 | 26 | 1.9 | 41 | 3.0 |
| Menopausal status (≤6 months of diagnosis) | ||||||||
| Pre-menopausal | 2,485 | 14.4 | 2,377 | 95.7 | 57 | 2.3 | 51 | 2.1 |
| Post-menopausal | 9,757 | 56.4 | 9,435 | 96.7 | 142 | 1.5 | 180 | 1.8 |
| Unknown | 5,044 | 29.2 | 4,816 | 95.5 | 115 | 2.3 | 113 | 2.2 |
| 1st degree family history of breast cancer (before 1st diagnosis) | ||||||||
| No | 11,247 | 65.1 | 10,855 | 96.5 | 195 | 1.7 | 197 | 1.8 |
| Yes | 2,881 | 16.7 | 2,764 | 95.9 | 46 | 1.6 | 71 | 2.5 |
| Unknown | 3,158 | 18.3 | 3,009 | 95.3 | 73 | 2.3 | 76 | 2.4 |
| Breast density (before 1st diagnosis and within 2 years of mammogram used for mode of detection) | ||||||||
| Almost entirely fatty | 493 | 2.9 | 480 | 97.4 | 11 | 2.2 | 2 | 0.4 |
| Scattered fibroglandular | 3,900 | 22.6 | 3,771 | 96.7 | 65 | 1.7 | 64 | 1.6 |
| Heterogeneously dense | 4,275 | 24.7 | 4,109 | 96.1 | 58 | 1.4 | 108 | 2.5 |
| Extremely dense | 930 | 5.4 | 885 | 95.2 | 21 | 2.3 | 24 | 2.6 |
| Unknown | 7,688 | 44.5 | 7,383 | 96.0 | 159 | 2.1 | 146 | 1.9 |
BCS breast conserving surgery, ER estrogen receptor, PR progesterone receptor
Recurrent cancer includes a 2nd cancer in the same breast and with the same histology as the initial cancer
At initial diagnosis, 35.8% of women were screen-detected, 15.6% screen interval-detected, 19.6% diagnostic detected, and 5.5% diagnostic interval-detected; 23.5% had an unknown initial diagnosis mode.
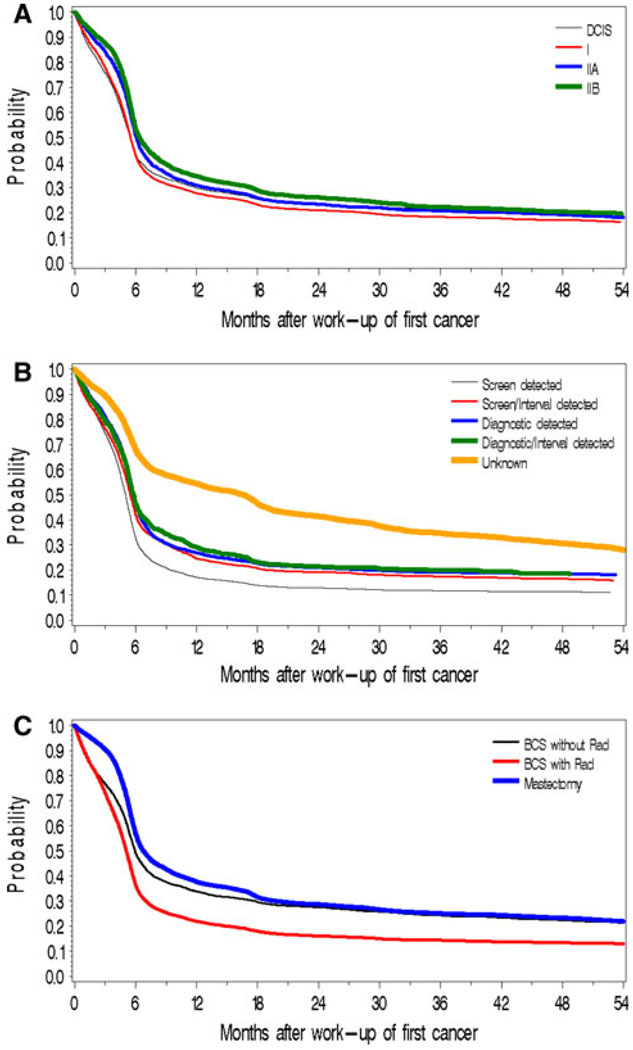
Time to first surveillance mammogram was rapid in the year following initial diagnosis; however, uptake did not markedly increase in the 18-months following diagnosis (Fig. 2a–c). Women diagnosed with stage IIB cancer had a lower and slower uptake (Fig. 2a). Women who were initially screen-detected were more likely to undergo surveillance than non screen-detected (67% by 6-months and 83% by 12-months vs. 54–58% by 6-months and 71–76% by 12-months, respectively). Women with unknown detection mode had the slowest uptake (32% by 6-months, 46% by 12-months) (Fig. 2b). Women receiving breast conserving surgery (BCS) with radiation had the most rapid and greatest uptake (64% by 6-months, 78% by 12-months) (Fig. 2c).
Fig. 2.
Time to first surveillance mammogram by characteristics of initial cancer. a By stage at first diagnosis. b By mode of detection for initial diagnosis. c By treatment for initial diagnosis
Overall second events rates/1,000 woman years were 5.37 for recurrence and 5.88 for second primaries. Among women without a second event, 53% were followed for 5 years (mean = 47 months). As a sensitivity analysis, we included women from the initial cohort with missing stage or surgery, multiple diagnoses in the initial 6-month period, bilateral cancer and missing laterality of the second event and observed slightly higher recurrence rates (6.56/1,000 woman years), but not of second primaries (6.03/1,000 woman years) (data not shown).
Initial stage was associated with recurrence and second primaries with the highest rates in women with DCIS (8.5 and 7.4, respectively) and stage IIB (7.1 and 7.8) vs. stages I (3.9 and 5.9) and IIA (5.3 and 3.8). Recurrence rates were higher for women with ER-negative tumors than those with ER+ tumors; however, second primary rates were higher only for women with ER−/PR− tumors. Second primary rates did not differ by primary therapy; however, recurrence rates were higher in women with BCS without radiation (Table 2). Recurrence rates decreased with increasing age at diagnosis, but rates of second primaries did not vary systematically except among women aged ≥80 years, whose rates were markedly lower. Higher breast density was associated with higher second primary rates (1.3 almost entirely fatty; 5.0 scattered fibroglandular; 7.7 heterogeneously and extremely dense), but there was no trend in recurrence rates.
Table 2.
Rates (per 1,000 woman years) of breast cancer recurrences and second primaries by characteristics at initial diagnosis
| Breast cancer recurrencesa | Second breast primaries | |||||
|---|---|---|---|---|---|---|
| N | Rates | 95% CI | N | Rates | 95% CI | |
| Characteristics of 1st cancer diagnosis and treatment | ||||||
| Stage at 1st diagnosis | ||||||
| 0 | 92 | 8.5 | (6.8, 10.4) | 80 | 7.4 | (5.8, 9.2) |
| I | 110 | 3.9 | (3.2, 4.7) | 167 | 5.9 | (5.0, 6.8) |
| IIA | 68 | 5.3 | (4.1, 6.7) | 49 | 3.8 | (2.8, 5.0) |
| IIB | 44 | 7.1 | (5.2, 9.5) | 48 | 7.8 | (5.7, 10.3) |
| Nodal status | ||||||
| No invasion | 157 | 4.4 | (3.7, 5.1) | 193 | 5.3 | (4.6, 6.2) |
| Invasion | 65 | 5.7 | (4.4, 7.2) | 71 | 6.2 | (4.8, 7.8) |
| Hormone receptor | ||||||
| ER−/PR− | 60 | 9.0 | (6.9, 11.6) | 43 | 6.4 | (4.7, 8.7) |
| ER−/PR+ | 8 | 10.8 | (4.7, 21.3) | 2 | 2.7 | (0.3, 9.8) |
| ER+/PR− | 24 | 5.5 | (3.5, 8.2) | 16 | 3.7 | (2.1, 6.0) |
| ER+/PR+ | 109 | 3.6 | (2.9, 4.3) | 161 | 5.3 | (4.5, 6.2) |
| Treatment | ||||||
| BCS without radiation therapy | 120 | 12.7 | (10.6, 15.2) | 59 | 6.3 | (4.8, 8.1) |
| BCS with radiation therapy | 118 | 4.2 | (3.5, 5.1) | 155 | 5.5 | (4.7, 6.5) |
| BCS with and without radiation therapy | 238 | 6.4 | (5.6, 7.2) | 214 | 5.7 | (5.0, 6.5) |
| Mastectomy | 76 | 3.6 | (2.8, 4.5) | 130 | 6.2 | (5.2, 7.3) |
| Adjuvant therapy | ||||||
| Neither | 170 | 6.1 | (5.3, 7.1) | 180 | 6.5 | (5.6, 7.5) |
| Chemotherapy | 61 | 7.2 | (5.5, 9.3) | 55 | 6.5 | (4.9, 8.5) |
| Hormonal | 47 | 3.5 | (2.5, 4.6) | 61 | 4.5 | (3.4, 5.8) |
| Both | 27 | 4.4 | (2.9, 6.4) | 30 | 4.9 | (3.3, 6.9) |
| Mode of 1st cancer diagnosis | ||||||
| Screen-detected | 87 | 4.1 | (3.3, 5.1) | 112 | 5.3 | (4.4, 6.4) |
| Screen interval | 44 | 4.8 | (3.5, 6.4) | 61 | 6.6 | (5.1, 8.5) |
| Diagnostic detected | 77 | 6.8 | (5.4, 8.6) | 70 | 6.2 | (4.8, 7.9) |
| Diagnostic interval | 19 | 5.9 | (3.6, 9.2) | 22 | 6.8 | (4.3, 10.3) |
| Unknown | 87 | 6.3 | (5.1, 7.8) | 79 | 5.8 | (4.6, 7.2) |
| Characteristics of women at 1st cancer diagnosis | ||||||
| Age at 1st diagnosis | ||||||
| 18–39 | 25 | 9.7 | (6.3, 14.4) | 17 | 6.6 | (3.9, 10.6) |
| 40–49 | 87 | 7.4 | (5.9, 9.1) | 71 | 6.0 | (4.7, 7.6) |
| 50–59 | 78 | 5.0 | (3.9, 6.2) | 86 | 5.5 | (4.4, 6.8) |
| 60–69 | 57 | 4.3 | (3.3, 5.6) | 75 | 5.7 | (4.5, 7.1) |
| 70–79 | 50 | 4.6 | (3.4, 6.0) | 77 | 7.0 | (5.5, 8.8) |
| ≥80 | 17 | 4.0 | (2.3, 6.4) | 18 | 4.2 | (2.5, 6.7) |
| Race/ethnicity | ||||||
| White | 227 | 5.1 | (4.5, 5.8) | 265 | 5.9 | (5.3, 6.7) |
| Black | 5 | 11.2 | (3.6, 26.1) | 3 | 6.7 | (1.4, 19.6) |
| Hispanic | 39 | 6.3 | (4.5, 8.6) | 22 | 3.6 | (2.2, 5.4) |
| Asian/Pacific Islander | 2 | 2.5 | (0.3, 9.1) | 7 | 8.8 | (3.5, 18.1) |
| Native American | 8 | 7.9 | (3.4, 15.6) | 2 | 2.0 | (0.2, 7.1) |
| Other | 7 | 10.9 | (4.4, 22.5) | 4 | 6.3 | (1.7, 16) |
| Menopausal status (≤6 months of diagnosis) | ||||||
| Pre-menopausal | 57 | 6.8 | (5.2, 8.9) | 51 | 6.1 | (4.6, 8.0) |
| Post-menopausal | 142 | 4.3 | (3.7, 5.1) | 180 | 5.5 | (4.7, 6.4) |
| 1st degree family history of breast cancer (before 1st diagnosis) | ||||||
| No | 195 | 5.2 | (4.5, 6.0) | 197 | 5.3 | (4.6, 6.1) |
| Yes | 46 | 4.9 | (3.6, 6.5) | 71 | 7.6 | (5.9, 9.5) |
| Breast density (before 1st diagnosis and within 2 years of mammogram used for mode of detection) | ||||||
| Almost entirely fatty | 11 | 7.0 | (3.5, 12.6) | 2 | 1.3 | (0.2, 4.6) |
| Scattered fibroglandular | 65 | 5.0 | (3.9, 6.4) | 64 | 5.0 | (3.8, 6.3) |
| Heterogeneously dense | 58 | 4.1 | (3.1, 5.4) | 108 | 7.7 | (6.3, 9.3) |
| Extremely dense | 21 | 6.8 | (4.2, 10.4) | 24 | 7.7 | (5.0, 11.5) |
| Self-reported lump before mammogram used for mode of detection | ||||||
| No | 165 | 4.5 | (3.8, 5.2) | 218 | 5.9 | (5.2, 6.8) |
| Yes | 66 | 7.1 | (5.5, 9.0) | 52 | 5.6 | (4.2, 7.3) |
BCS breast conserving surgery, ER estrogen receptor, PR progesterone receptor, CI confidence interval
Recurrent cancer includes a 2nd cancer in the same breast as the 1st cancer with the same histology for the 2nd breast cancer
Interval cancers accounted for 37.6% of recurrences and 36.3% of second primaries (data not shown). We could not define diagnosis mode for 28.1% (32.2% for recurrences and 24.4% for second primaries). Just over one-third of second events were diagnosed following a screening (19.7% recurrence, 24.7% second primaries) or diagnostic mammogram (10.5, 14.5%, respectively), and the rest were interval-detected following a screening (21.0, 21.2%) or a diagnostic exam (16.6, 15.1%). Few women reported a lump before the mammogram leading to their second cancer diagnosis: 8.0% of recurrences and 4.9% second primaries.
Some subgroups experienced higher recurrence rates, with no difference in second primaries, including pre-menopausal women, node positive women, women who were initially detected following a diagnostic mammogram, and women who initially had a self-reported lump. Women with a family history had higher rates of second primaries but not of recurrence. Notably, lower rates of second events were observed in women aged ≥80 years and adjuvant hormonal therapy users.
Mode of initial diagnosis was the only variable that was significantly associated with risk of a second cancer diagnosis after adjustment for other prognostic factors; women whose first cancer was diagnostic detected had significantly higher risk of a second cancer compared to women whose 1st cancer was screen-detected (Table 3). We conducted sensitivity analyses that included all prognostic factors and varied the combination of risk factors; hazard estimates did not vary in direction or significance in these sensitivity analyses and detection mode was the only significant risk factor (data not shown).
Table 3.
Multivariable global hazard of having a second breast cancer event diagnosed adjusted for all variables in the table
| Model 4a (N = 8,662) | |||
|---|---|---|---|
| HR | 95% CI | P value | |
| Breast density (before 1st diagnosis and within 2 years of mammogram used for mode of detection) | |||
| Almost entirely fatty | Ref | 0.6859 | |
| Scattered fibroglandular | 1.07 | (0.60, 1.90) | |
| Heterogeneously dense | 1.20 | (0.67, 2.13) | |
| Extremely dense | 1.30 | (0.68, 2.48) | |
| Mode of 1st cancer diagnosis | |||
| Screen-detected | Ref | 0.0008 | |
| Screen interval-detected | 1.38 | (1.02, 1.86) | |
| Diagnostic detected | 1.78 | (1.35, 2.35) | |
| Diagnostic interval-detected | 1.32 | (0.86, 2.04) | |
| 1st degree family history of breast cancer (before 1st diagnosis) | |||
| No | Ref | 0.9442 | |
| Yes | 1.01 | (0.77, 1.32) | |
| Age at 1st diagnosis | |||
| 18–39 | 1.06 | (0.61–1.83) | 0.6809 |
| 40–49 | 0.99 | (0.72–1.35) | |
| 50–59 | Ref | ||
| 60–69 | 1.08 | (0.79–1.49) | |
| 70–79 | 1.17 | (0.84–1.65) | |
| 80+ | 0.76 | (0.44–1.30) | |
| Stage at 1st diagnosis | |||
| 0 | 1.66 | (1.25–2.21) | 0.003 |
| I | Ref | ||
| IIA | 0.91 | (0.66–1.26) | |
| IIB | 1.12 | (0.75–1.66) | |
| Adjuvant therapy | |||
| Neither | Ref | 0.0067 | |
| Chemotherapy | 1.22 | (0.86–1.75) | |
| Hormonal | 0.68 | (0.51–0.93) | |
| Both | 0.75 | (0.47–1.18) | |
P values are based on likelihood ratio tests and are also adjusted for mammography registry
P ≤ 0.05
Discussion
This study provides important information on timing and detection of second breast cancer events after an initial diagnosis of early breast cancer. We identified certain subgroups with higher second breast cancer event rates. Surveillance may be particularly important for women <50 years or with initial DCIS, stage IIB, or ER – cancers as these subgroups had the highest 5-year cumulative hazards of recurrence and second primaries. Women with a family history of breast cancer had higher rates of second primaries but not recurrences. The only subgroups with a distinctly lower risk for either second event were women aged ≥80 years and those who received adjuvant hormonal therapy.
Randomized trials [19–22] provide critical information on prognostic factors. However, initial detection mode and breast density are less well understood as risk factors for second breast events. It is important to understand whether detection mode and characteristics of patients and tumors can be used to tailor surveillance recommendations to improve long-term outcomes, particularly if women could benefit from more or less surveillance during certain times following initial diagnosis. Our study was not designed to address the evidence gaps around recommendations on when to stop surveillance mammography since we followed women for only 5 years after initial diagnosis. It was designed to examine whether any characteristics of women or their initial tumors might be useful to guide recommendations around initial 5-year surveillance after early stage breast cancer diagnosis.
Several studies and expert reviews have demonstrated surveillance mammography is effective for improving long-term outcomes (e.g., breast cancer mortality) for women with early stage breast cancer [3–5], including at least one study demonstrating decreased breast cancer mortality [6]. Scrutiny of over-diagnosis from screening mammography has increased [23]. The concern is that mammography identifies breast lesions with limited potential for malignancy and will trigger workup and treatment that may have no long-term impact on mortality [23]. Inappropriate screening harms women and healthcare systems by increasing anxiety and adding to unnecessary healthcare expenditures [24]. We know of no research that has attempted to quantify over-diagnosis from surveillance mammograms. This is important for breast cancer survivors, who may undergo additional treatment for a second cancer that may not influence long-term outcomes.
We hypothesized that the mode of initial detection might affect second event rates and when and how often women receive surveillance mammography. Women who were initially screen-detected were more likely than others to return for their first surveillance mammogram [4]. Some mammography facilities routinely perform diagnostic examinations for anyone—even asymptomatic patients—with a personal history of breast cancer, so it may be most appropriate to compare detection mode of second events by combining screen- and diagnostic-detected vs. interval-detected cancers. There are evidence gaps around how second breast cancer events are detected. A systematic review [7] found extensive variation across 10 studies (N = 102–7,000 patients) that reported on detection mode for recurrent breast cancers. In three studies, 73–88% of recurrences were detected from physical exam; this ranged from 12 to 54% in the remaining seven studies [7]. The range was also large for the proportion of recurrences detected by mammography (8–50%) [7]. Not surprisingly, Grunfeld’s review [7] found that recurrent cancers detected by mammography were smaller and less invasive than were those detected through physical exam. Isolating the effect of surveillance mammography on long-term outcomes—such as mortality—remains challenging because of lead and length biases that impact the timing of when second events are detected. However, recent studies have attempted to isolate the influence of these biases, and findings keep suggesting that detection mode remains an independent prognostic factor [25–27]. Consistent with recent literature [25–27], women who were initially diagnosed following a diagnostic mammogram had significantly higher risks of second events even after adjusting for prognostic factors; this further suggests that mode of initial detection is an important prognostic factor for second cancer events.
Our finding that approximately 37% of second cancers were interval cancers serves as an important reminder—women, radiologists, oncologists, and primary care providers need to continue to be aware of newly arising symptoms and breast changes in both breasts (and chest wall) even after a recent negative screening or diagnostic mammogram. Houssami et al. [27] reported that 14% of second cancers were found by clinical breast exam alone. The absence of a breast lump at presentation should not reassure clinicians since lumps were present in only 8% of recurrences and 5% of second primaries at the mammogram leading to the second diagnosis; approximately 5% of women with no history of breast cancer report a breast lump at the time of a screening mammogram [9].
The 5-year relative survival rates for DCIS have remained unchanged at around 100% [28]. Many studies examining surveillance mammography have not included women with DCIS [6, 26, 29], who comprise ~20% of all breast cancer cases [30]. Importantly, in this study, women with initial DCIS have substantially higher second cancer rates regardless of initial treatment. This could reflect residual breast disease from initial diagnosis [31]. Clinically, this identifies an important sub-group of women who may benefit from increased surveillance mammography since second breast cancer event rates in this population mirrored women with stage IIB tumors.
Although our findings confirmed that the vast majority of women receive a first surveillance mammogram within 1-year of initial diagnosis, there are still opportunities to increase outreach to populations who do not return for a mammogram within 18-months of initial diagnosis. Examining surveillance mammography receipt is important for understanding timing and detection mode of second breast events. In this study, we could not determine what type of provider recommended surveillance or how women were reminded to receive mammography, both of which affect uptake and adherence [32, 33]. Continuity and coordination across health care providers may help ensure high adherence to mammography surveillance [3, 33, 34].
Identifying and correctly distinguishing between recurrences and second primaries is challenging and can be influenced by physician judgment, pathologists and available data from initial diagnosis. Our definition of recurrence was stricter than others [35–38] because we required the same laterality and histology. Previous studies have reported recurrence rates of 3%/year after DCIS, which is substantially higher than the 1%/year we observed. However, our observed second primary rates (0.6%/year invasive and 0.8%/year DCIS) are consistent with other cohorts [39, 40]. Using pathology databases to identify second events has additional limitations since some regional and distant recurrences may not have pathologic confirmation, which leads to under-ascertainment of distant recurrences. This should not affect our second breast cancer rates because they were ascertained through cancer registries. Distinguishing between recurrences and second primaries may be less critical when considering surveillance recommendations.
We limited our follow-up to 5 years because BCSC is not a closed cohort. We could not examine second events by comorbidities. Also, even with our large sample, we lacked enough numbers of women with stage IIIA (N = 581; 19 went on to have a recurrence (89% of these having a regional recurrence) and 10 a second primary), so they were excluded. These women had a significantly shorter time to second breast cancer, and most of them received chemotherapy.
Strengths of our study include: studying outcomes from community-based mammography facilities in geographically diverse populations; including younger and older women; and including second events that did not rely exclusively on tumor registries. We know of only one other study that has included initial DCIS cases [27] and none that has included DCIS as a second event. Approximately 20% of diagnoses of incident breast cancer are DCIS, so DCIS is important for understanding the risk of second events.
Evidence to aid initial treatment decisions by using molecular markers will likely continue to improve breast cancer outcomes [21, 22]. However, we should not lose sight of our need to continue to consider tailored surveillance recommendations. This study does not provide evidence to support changing surveillance intervals for different subgroups. However, it does suggest that certain subgroups may benefit from increased surveillance: women who are young or have ER-negative tumors or DCIS or stage IIB disease. Surveillance may be less important for older women (≥80 years) and for women using adjuvant hormonal therapy since rates of second cancers were low in these women. These data can also be used to help counsel women on detection mode for second cancers to seek care diligently for any symptoms that arise following a negative mammogram.
Acknowledgments
The National Cancer Institute-sponsored Breast Cancer Surveillance Consortium supported this work: U01CA63740, U01CA86076, U01CA86082, U01CA63736, U01CA70013, U01CA69976, U01CA63731, and U01CA70040). The authors had full responsibility in designing the study, collecting, analyzing and interpreting the data, deciding to submit the manuscript for publication, and writing the manuscript. We thank the BCSC participating mammography facilities, and radiologists for the data they have provided for this study. A list of the BCSC investigators and procedures for requesting BCSC data for research purposes are available at http://breastscreening.cancer.gov/. Data collection for this work was supported by NCI-funded Breast Cancer Surveillance Consortium co-operative agreements (63740, 86076, 86082, 63736, 70013, 69976, 63731, 70040). The collection of cancer incidence data used in this study was supported in part by several state public health departments and cancer registries throughout the United States. For a full description of these sources, please see http://breastscreening.cancer.gov/work/acknowledgement.html. We also thank Melissa Rabelhofer for her assistance with manuscript preparation and Rebecca Hughes for her editorial assistance.
Contributor Information
Diana S. M. Buist, Email: buist.d@ghc.org, Group Health Research Institute, Group Health Cooperative, 1730 Minor Avenue, Suite 1600, Seattle, WA 98101, USA.
Linn A. Abraham, Group Health Research Institute, Group Health Cooperative, 1730 Minor Avenue, Suite 1600, Seattle, WA 98101, USA
William E. Barlow, Group Health Research Institute, Group Health Cooperative, 1730 Minor Avenue, Suite 1600, Seattle, WA 98101, USA Cancer Research and Biostatistics, 1730 Minor Ave., Ste 1700, Seattle, WA 98101, USA.
Arun Krishnaraj, Department of Radiology, University of North Carolina, 2006, Old Clinic, CB#7510, Chapel Hill, NC 27599, USA.
Regan C. Holdridge, Comprehensive Cancer Centers of Nevada, 3730 S. Eastern Ave., Las Vegas, NV 89169, USA
Edward A. Sickles, Department of Radiology, University of California, 505 Parnassus Ave., San Francisco, CA 94143, USA
Patricia A. Carney, Departments of Family Medicine and Public Health and Preventive Medicine, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd., Mail Code CB 669, Portland, OR 97239, USA
Karla Kerlikowske, Department of Medicine and Epidemiology and Biostatistics, University of California, 185 Berry Street, Lobby 5, Suite 5700, San Francisco, CA 94107, USA.
Berta M. Geller, Health Promotion Research, University of Vermont, College of Medicine, 1 South Prospect Street, 429AR4, Burlington, VT 05401, USA
References
- 1.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, Godwin J, Gray R, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Peto R, Taylor C, Wang Y. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 3.Rojas MP, Russo A, Moschetti I, Coe L, Fossati R, Palli D, del Roselli TM, Liberati A. Follow-up strategies for women treated for early breast cancer. Cochrane Database Syst Rev. 2005;25(1) doi: 10.1002/14651858.CD001768.pub2. CD001768. doi: 10.1002/14651858.CD001768.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Khatcheressian JL, Wolff AC, Smith TJ, Grunfeld E, Muss HB, Vogel VG, Halberg F, Somerfield MR, Davidson NE. American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol. 2006;24:5091–5097. doi: 10.1200/JCO.2006.08.8575. [DOI] [PubMed] [Google Scholar]
- 5.Schootman M, Jeffe DB, Lian M, Aft R, Gillanders WE. Surveillance mammography and the risk of death among elderly breast cancer patients. Breast Cancer Res Treat. 2008;111:489–496. doi: 10.1007/s10549-007-9795-1. doi: 10.1007/s10549-007-9795-1. [DOI] [PubMed] [Google Scholar]
- 6.Lash TL, Fox MP, Buist DS, Wei F, Field TS, Frost FJ, Geiger AM, Quinn VP, Yood MU, Silliman RA. Mammography surveillance and mortality in older breast cancer survivors. J Clin Oncol. 2007;25:3001–3006. doi: 10.1200/JCO.2006.09.9572. [DOI] [PubMed] [Google Scholar]
- 7.Grunfeld E, Noorani H, McGahan L, Paszat L, Coyle D, van Walraven C, Joyce J, Sawka C. Surveillance mammography after treatment of primary breast cancer: a systematic review. Breast. 2002;11:228–235. doi: 10.1054/brst.2001.0404. [DOI] [PubMed] [Google Scholar]
- 8.Ballard-Barbash R, Taplin SH, Yankaskas B, Ernster V, Rosenberg RD, Carney P, Barlow WE, Geller B, Kerlikowske K, Edwards BK, Lynch C, Urban N, Chrvala CA, Key CR, Poplack S, Worden JK, Kessler L. Breast Cancer Surveillance Consortium: a national mammography screening and outcomes database. Am J Roentgenol. 1997;169:1001–1008. doi: 10.2214/ajr.169.4.9308451. [DOI] [PubMed] [Google Scholar]
- 9. [Accessed 5 March 2010];National Cancer Institute Breast Cancer Surveillance Consortium Homepage. http://breastscreening.cancer.gov/
- 10.Carney PA, Geller BM, Moffett H, Ganger M, Sewell M, Barlow WE, Stalnaker N, Taplin SH, Sisk C, Ernster VL, Wilkie HA, Yankaskas B, Poplack SP, Urban N, West MM, Rosenberg RD, Michael S, Mercurio TD, Ballard-Barbash R. Current medicolegal and confidentiality issues in large, multicenter research programs. Am J Epidemiol. 2000;152:371–378. doi: 10.1093/aje/152.4.371. [DOI] [PubMed] [Google Scholar]
- 11.Greene F, Page D, Fleming I, Fritz A, Balch C, Haller D, Morrow M, editors. AJCC cancer staging manual. Philadelphia: Lippincott Raven; 2001. [Google Scholar]
- 12.American College of Radiology. Breast imaging reporting and data system (BI-RADS™) Reston, VA: American College of Radiology; 1998. [Google Scholar]
- 13.Rosenberg RD, Yankaskas BC, Hunt WC, Ballard-Barbash R, Urban N, Ernster VL, Kerlikowske K, Geller B, Carney PA, Taplin S. Effect of variations in operational definitions on performance estimates for screening mammography. Acad Radiol. 2000;7:1058–1068. doi: 10.1016/s1076-6332(00)80057-4. [DOI] [PubMed] [Google Scholar]
- 14.Yankaskas BC, Taplin SH, Ichikawa L, Geller BM, Rosenberg RD, Carney PA, Kerlikowske K, Ballard-Barbash R, Cutter GR, Barlow WE. Association between mammography timing and measures of screening performance in the United States. Radiology. 2005;234:363–373. doi: 10.1148/radiol.2342040048. doi: 10.1148/radiol.2342040048. [DOI] [PubMed] [Google Scholar]
- 15.Breast Cancer Surveillance Consortium. [Accessed 25 Feb 2009];National Cancer Institute; Performance measures for 3,603,832 screening mammography examinations from 1996 to 2006 by age and time (months) since previous mammography. 2008 http://breastscreening.cancer.gov/data/performance/screening/perf_age_time.html.
- 16.Sickles EA, Miglioretti DL, Ballard-Barbash R, Geller BM, Leung JW, Rosenberg RD, Smith-Bindman R, Yankaskas BC. Performance benchmarks for diagnostic mammography. Radiology. 2005;235:775–790. doi: 10.1148/radiol.2353040738. [DOI] [PubMed] [Google Scholar]
- 17.Geller BM, Kerlikowske K, Carney PA, Abraham LA, Yankaskas BC, Taplin SH, Ballard-Barbash R, Dignan MB, Rosenberg R, Urban N, Barlow WE. Mammography surveillance following breast cancer. Breast Cancer Res Treat. 2003;81:107–115. doi: 10.1023/A:1025794629878. [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute BCSC. [Accessed 25 Aug 2008];Abnormal interpretations for 4,032,556 screening mammography examinations from 1996–2005. 2007 http://breastscreening.cancer.gov/data/benchmarks/screening/table3.html.
- 19.Isaacs C, Stearns V, Hayes DF. New prognostic factors for breast cancer recurrence. Semin Oncol. 2001;28:53–67. doi: 10.1016/s0093-7754(01)90045-4. [DOI] [PubMed] [Google Scholar]
- 20.Fisher ER, Anderson S, Tan-Chiu E, Fisher B, Eaton L, Wolmark N. Fifteen-year prognostic discriminants for invasive breast carcinoma: National Surgical Adjuvant Breast and Bowel Project Protocol-06. Cancer. 2001;91:1679–1687. [PubMed] [Google Scholar]
- 21.Cianfrocca M, Goldstein LJ. Prognostic and predictive factors in early-stage breast cancer. Oncologist. 2004;9:606–616. doi: 10.1634/theoncologist.9-6-606. doi: 10.1634/theoncologist.9-6-606. [DOI] [PubMed] [Google Scholar]
- 22.Colozza M, Azambuja E, Cardoso F, Sotiriou C, Larsimont D, Piccart MJ. Proliferative markers as prognostic and predictive tools in early breast cancer: where are we now? Ann Oncol. 2005;16:1723–1739. doi: 10.1093/annonc/mdi352. doi: 10.1093/annonc/mdi352. [DOI] [PubMed] [Google Scholar]
- 23.Zahl PH, Maehlen J, Welch HG. The natural history of invasive breast cancers detected by screening mammography. Arch Intern Med. 2008;168:2311–2316. doi: 10.1001/archinte.168.21.2311. doi: 10.1001/archinte.168.21.2311. [DOI] [PubMed] [Google Scholar]
- 24.Mille D, Roy T, Carrere MO, Ray I, Ferdjaoui N, Spath HM, Chauvin F, Philip T. Economic impact of harmonizing medical practices: compliance with clinical practice guidelines in the follow-up of breast cancer in a French Comprehensive Cancer Center. J Clin Oncol. 2000;18:1718–1724. doi: 10.1200/JCO.2000.18.8.1718. [DOI] [PubMed] [Google Scholar]
- 25.Joensuu H, Lehtimaki T, Holli K, Elomaa L, Turpeenniemi-Hujanen T, Kataja V, Anttila A, Lundin M, Isola J, Lundin J. Risk for distant recurrence of breast cancer detected by mammography screening or other methods. JAMA. 2004;292:1064–1073. doi: 10.1001/jama.292.9.1064. doi: 10.1001/jama.292.9.1064. [DOI] [PubMed] [Google Scholar]
- 26.Shen Y, Yang Y, Inoue LY, Munsell MF, Miller AB, Berry DA. Role of detection method in predicting breast cancer survival: analysis of randomized screening trials. J Natl Cancer Inst. 2005;97:1195–1203. doi: 10.1093/jnci/dji239. doi: 10.1093/jnci/dji239. [DOI] [PubMed] [Google Scholar]
- 27.Houssami N, Ciatto S, Martinelli F, Bonardi R, Duffy SW. Early detection of second breast cancers improves prognosis in breast cancer survivors. Ann Oncol. 2009;20:1505–1510. doi: 10.1093/annonc/mdp037. doi: 10.1093/annonc/mdp037. [DOI] [PubMed] [Google Scholar]
- 28. [Accessed 25 Feb 2009];National Cancer Institute; Surveillance Epidemiology and End Results Fast Stats. http://seer.cancer.gov/faststats/
- 29.Immonen-Raiha P, Kauhava L, Parvinen I, Holli K, Kronqvist P, Pylkkanen L, Helenius H, Kaljonen A, Rasanen O, Klemi PJ. Mammographic screening reduces risk of breast carcinoma recurrence. Cancer. 2005;103:474–482. doi: 10.1002/cncr.20793. doi: 10.1002/cncr.20793. [DOI] [PubMed] [Google Scholar]
- 30.American Cancer Society. Cancer facts & figures 2009. Atlanta: American Cancer Society; 2009. [Google Scholar]
- 31.Lehman CD, Gatsonis C, Kuhl CK, Hendrick RE, Pisano ED, Hanna L, Peacock S, Smazal SF, Maki DD, Julian TB, DePeri ER, Bluemke DA, Schnall MD. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med. 2007;356:1295–1303. doi: 10.1056/NEJMoa065447. doi: 10.1056/NEJMoa065447. [DOI] [PubMed] [Google Scholar]
- 32.Andersen MR, Urban N. The use of mammography by survivors of breast cancer. Am J Public Health. 1998;88:1713–1714. doi: 10.2105/ajph.88.11.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keating NL, Landrum MB, Guadagnoli E, Winer EP, Ayanian JZ. Surveillance testing among survivors of early-stage breast cancer. J Clin Oncol. 2007;25:1074–1081. doi: 10.1200/JCO.2006.08.6876. doi: 10.1200/JCO.2006.08.6876. [DOI] [PubMed] [Google Scholar]
- 34.Hewitt M, Greenfield S, Stovall E. From cancer patient to cancer survivor: lost in transition. Washington, DC: The National Academies Press; 2006. [Google Scholar]
- 35.Geiger AM, Thwin SS, Lash TL, Buist DS, Prout MN, Wei F, Field TS, Ulcickas Yood M, Frost FJ, Enger SM, Silliman RA. Recurrences and second primary breast cancers in older women with initial early-stage disease. Cancer. 2007;109:966–974. doi: 10.1002/cncr.22472. [DOI] [PubMed] [Google Scholar]
- 36.Huang E, Buchholz TA, Meric F, Krishnamurthy S, Mirza NQ, Ames FC, Feig BW, Kuerer HM, Ross MI, Singletary SE, McNeese MD, Strom EA, Hunt KK. Classifying local disease recurrences after breast conservation therapy based on location and histology: new primary tumors have more favorable outcomes than true local disease recurrences. Cancer. 2002;95:2059–2067. doi: 10.1002/cncr.10952. doi: 10.1002/cncr.10952. [DOI] [PubMed] [Google Scholar]
- 37.Janschek E, Kandioler-Eckersberger D, Ludwig C, Kappel S, Wolf B, Taucher S, Rudas M, Gnant M, Jakesz R. Contralateral breast cancer: molecular differentiation between metastasis and second primary cancer. Breast Cancer Res Treat. 2001;67:1–8. doi: 10.1023/a:1010661514306. [DOI] [PubMed] [Google Scholar]
- 38.Smith TE, Lee D, Turner BC, Carter D, Haffty BG. True recurrence vs. new primary ipsilateral breast tumor relapse: an analysis of clinical and pathologic differences and their implications in natural history, prognoses, and therapeutic management. Int J Radiat Oncol Biol Phys. 2000;48:1281–1289. doi: 10.1016/s0360-3016(00)01378-x. [DOI] [PubMed] [Google Scholar]
- 39.Fisher B, Costantino J, Redmond C, Fisher E, Margolese R, Dimitrov N, Wolmark N, Wickerham DL, Deutsch M, Ore L, et al. Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. N Engl J Med. 1993;328:1581–1586. doi: 10.1056/NEJM199306033282201. [DOI] [PubMed] [Google Scholar]
- 40.Kerlikowske K, Molinaro A, Cha I, Ljung BM, Ernster VL, Stewart K, Chew K, Moore DH, 2nd, Waldman F. Characteristics associated with recurrence among women with ductal carcinoma in situ treated by lumpectomy. J Natl Cancer Inst. 2003;95:1692–1702. doi: 10.1093/jnci/djg097. [DOI] [PubMed] [Google Scholar]