Abstract
Despite widespread efforts at weight loss, the prevalence of obesity continues to rise. Restrained eating is a pattern of attempted weight control characterized by cognitive restriction of food intake that has paradoxically been linked with overeating and/or weight gain. It is not known whether restrained eating is associated with abnormalities in appetite-regulating hormones, independent of its effects on body weight. To address this question, we assessed cognitive restraint using the Three-Factor Eating Questionnaire and obtained fasting measurements of ghrelin, leptin and insulin from 24 healthy, nonobese (body mass index (BMI) 19.7 to 29.6 kg/m2) adult subjects who were at a stable, lifetime maximum weight. We chose to study subjects at stable maximum weight to avoid the secondary effects of weight reduction on body weight-regulating hormones. Subjects were classified by cognitive restraint scale score into Low, Indeterminate, and High Restraint groups. Higher ghrelin levels were significantly associated with restraint in an unadjusted model (P = 0.004) and after adjustment for BMI (P = 0.007). No relationships were found between restraint scores and either leptin (P = 0.75) or insulin (P = 0.36). These findings show an orexigenic hormonal profile in restrained eaters, independent of changes in body weight.
Keywords: restraint, ghrelin, leptin, insulin, obesity, body weight, eating disorders, appetite regulation
BACKGROUND
The prevalence of obesity [1] and obesity-related health complications [2] continues to rise relentlessly, even though many Americans report attempts to manage their body weight. In the 2000 Behavioral Risk Factor Surveillance System study, 70% of obese women and 60% of overweight women reported that they were currently trying to lose weight [3]. The reasons why people are often unsuccessful at maintaining healthy weights are complex but include genetics [4], physical inactivity, poor diet [5], and psychological [6] and socioeconomic factors [7]. In addition, some investigators have found that weight loss attempts themselves predict weight gain over time [8–12].
Restrained eating is one pattern of attempted weight regulation that has been prospectively associated with a higher risk of developing obesity among pre-adolescent and adolescent girls [13, 14], as well as greater weight gain in adults [10, 15]. Restrained eating is characterized by the exertion of cognitive control over food intake. Restrained eaters’ success in actually achieving weight loss varies [6, 16], which may be due to a propensity among some to overeat [17]. The mechanisms by which restrained eating and repeated attempts at dieting may encourage weight gain are uncertain, but two proposed possibilities are the promotion of binge eating [12, 18] and a genetic predisposition toward both weight gain and the behavioral response of recurrent dieting [8]. In addition, a potential mechanism for these observed associations is suggested by findings of low leptin levels in restrained eaters independent of BMI [19–21], reflecting a relatively appetite-stimulated state that could promote increased food consumption and weight gain.
Understanding the milieu of body-weight regulating hormones in restrained eaters is complicated by the need to differentiate effects of the exertion of cognitive control over eating from secondary effects of weight reduction. Weight loss from caloric restriction in obese or lean individuals elicits compensatory changes in body-weight regulatory hormones that promote weight regain. The weight-reduced state is characterized by low levels of the anorectic hormones leptin [22, 23] and insulin [23], and high levels of ghrelin [24]. Ghrelin is the only known circulating orexigenic (appetite-stimulating) hormone. It is implicated in both the short-term control of food intake at individual meals and in long-term body-weight regulation [25]. Ghrelin levels have repeatedly been shown to increase following weight loss resulting from multiple causes [26–29], and they are also responsive to nutritional status independent of body weight [30]. In response to weight reduction, leptin and insulin levels fall, whereas ghrelin rises, and these hormonal changes elicit commensurate alterations in central neural pathways [4, 31] that stimulate appetite and decrease metabolic rate, promoting weight regain. Consequently, prior findings in restrained eaters of low leptin [20, 21, 32, 33] and insulin levels [34] could be explained by the secondary effects of weight loss, as these studies did not control for whether or not subjects were weight-reduced. We are unaware of any studies that have investigated the relationships between restrained eating and body-weight regulating hormones in subjects known to be at a stable, lifetime maximum weight.
Therefore, we asked the question: is there an association between restrained eating and levels of leptin, insulin, or ghrelin among individuals who are currently not weight-reduced? To address this question, we assessed restrained eating using a modified Three-Factor Eating Questionnaire and obtained fasting measurements of ghrelin, leptin and insulin on three separate occasions from healthy, nonobese subjects who were at a stable lifetime maximum weight.
METHODS
Participants
Subjects were healthy male and female volunteers recruited from the local community using posted advertisements. Potential subjects were invited to the General Clinical Research Center (GCRC) for an in-depth screening assessment if they were healthy, non-dieters, had a BMI within the normal to overweight range (between 18.5 and 29.9 kg/m2) based on self-reported weight and height, and were not pregnant. Screened subjects were also required to be habitual breakfast eaters due to the distinctive preprandial surge in ghrelin that has been shown to adapt to different meal patterns [35]. In addition, we have validated in habitual breakfast eaters that the fasting preprandial ghrelin level correlates extremely well with 24-hour area-under-the-curve for ghrelin (r = 0.89, P < 0.001) [36]. At the in-person visit, we measured height and weight to the nearest 0.5 cm and 0.1 kg, respectively, to determine BMI, and we obtained detailed medical and nutrition histories. Measured weight was required to be stable within 2 kg over the past 6 months, and all subjects were within 2.5 kg of their self-reported lifetime maximum weight. Other exclusionary conditions included chronic illness; prior gastrointestinal surgery; alcohol, tobacco, or illicit drug use; or current dieting or eating disorder. All participants provided voluntary, written informed consent. The University of Washington Institutional Review Board approved the experimental protocol and procedures.
Study Design
Participants came to the GCRC for 3 separate study sessions, separated by intervals of at least 1 week, that were preceded by a 12-hour overnight fast. A peripheral blood sample was collected between 0800 and 0900 on each of the 3 study days. Basal energy expenditure was estimated using the Harris-Benedict equation.
Assays
All assays were run in duplicate, and all samples from a given participant were run in the same assay, where applicable. Plasma total immunoreactive ghrelin was measured by radioimmunoassay (Linco, St Louis MO). Lower and upper limits of detectability were 40 and 2560 pg/ml, respectively. The intraassay coefficient of variation (CV) was 4%, and the interassay CV was 15%. Total immunoreactive insulin was determined using a double-antibody radioimmunoassay [37]. The lower and upper limits of detectability were 2.2 uU/ml and 300 uU/ml, respectively, with an intrassay CV of 6% and an interassay CV of 10%. Plasma leptin was measured by commercial radioimmunoassay (Linco, St. Louis MO). The lower limit of detectability was 0.5 ng/ml, while the upper limit was 100 ng/ml. The intraassay CV was 4% and interassay CV was 5%. Plasma glucose was determined using the hexokinase method.
Weight history and eating habits
Lifetime maximum weight and duration at the current weight were self-reported. Subjects completed a shortened Three-Factor Eating Questionnaire (TFEQ) [38] to assess eating behavior. The cognitive restraint subscale was chosen to assess restrained eating, as it appears to be one of the best available measures of a purely restrictive eating pattern [39]. Preliminary intercorrelation analyses demonstrate excellent correlation (r = 0.99) between the original TFEQ cognitive restraint scale containing 21 items, and the modified version that we used containing 15 items.
Statistical analyses
We calculated cognitive restraint scale scores from the TFEQ. We obtained hormone levels by averaging fasting values from 3 separate days. Descriptive variables are presented as arithmetic means with 95% confidence intervals (CI) or proportions. In order to be consistent with regression analyses results (see below), geometric means are presented for all hormonal outcome variables and for glucose. Spearman’s rank correlation coefficients were used to describe relationships among continuous covariates. Due to a non-normal distribution of restraint scale scores, these scores were grouped by tertiles. The resultant categories were named “Low Restraint” (score 0 to 1), “Indeterminate Restraint” (score 2 to 4) and “High Restraint” (score 5 to 11). We used Kruskal-Wallis and Fisher’s exact tests to assess group differences. We used linear regression to examine the association of the independent variable of cognitive restraint scale scores with hormonal outcome variables. Hormone levels were log-transformed due to non-normal distributions. A potential outlier was identified from simple observation and descriptive analyses. Model checking for analyses of ghrelin confirmed that this was an isolated highly influential data point. This individual was excluded, resulting in a final sample size of 24 subjects for all analyses. We used likelihood ratio tests to determine if the restraint scale score was best modeled as a dummy variable or as a linear categorical variable. The dummy variable model is the most flexible model because it does not assume a linear relationship between the dependent and independent variables. Following the unadjusted regression analysis, we used a multivariate model to adjust for body mass index, chosen a priori due to documented relationships with hormones of interest and restraint scale scores. Further exploratory modeling also looked for confounding due to age and sex by entering these into the multivariate model. Adjusted results are presented as least squared geometric means. All analyses were conducted using Stata 9.0 (Stata Corporation, College Station, TX).
RESULTS
Subject characteristics
Subjects were 10 men (42%) and 14 women (58%). BMI ranged from 19.7 to 29.6 kg/m2, and age ranged from 18 to 65 years. Modified restraint scale scores ranged from 0 to 12. Estimated basal energy expenditure was 1571 kcal/24 hours (95% CI 1488–1654). Subject characteristics are presented in Table 1.
Table 1.
Clinical characteristics for all subjects (n = 24)
| Mean* (95% CI) | |
|---|---|
| Age, years | 30.3 (24.7–35.9) |
| BMI, kg/m2 | 23.8 (22.8–24.8) |
| Cognitive restraint scale score# | 3.8 (2.5–5.1) |
| Ghrelin, pg/ml | 775 (667–899) |
| Leptin, ng/ml | 6.2 (4.2–9.1) |
| Insulin, μU/ml | 12.9 (11.4–14.7) |
| Glucose, mg/dl | 89 (86–91) |
Hormone data and glucose levels were obtained from 3 morning fasting baseline samples in each subject and are presented as geometric means.
On a modified TFEQ, where the range of possible scores for the parameter is 0–15.
Relationships between restraint scale group and covariates
There were no significant differences in BMI, age or sex distribution among restraint scale groups (all P = NS). Mean estimated basal energy expenditure was 1611 kcal/24 hours (95% CI 1346–1876) for the Low Restraint group, 1516 kcal/24 hours (95% CI 1402–1630) for the Indeterminate Restraint group, and 1609 (95% CI 1491–1727) for the High Restraint group.
Relationships between hormone levels and BMI, age, and sex
BMI exhibited expected relationships with hormone levels: associations were positive with insulin (r = 0.55, P = 0.006) and negative with ghrelin levels (r = −0.38, P = 0.07). BMI tended to be positively correlated with leptin (r = 0.31, P = 0.15). Age was not correlated with hormone levels (data not shown). Leptin levels were higher in females (female mean = 11.5, 95% CI = 8.2–16.2; male mean = 2.6, 95% CI = 1.9–3.4; P < 0.001), and glucose levels were lower (female mean = 87, 95% CI = 83–91; male mean = 91, 95% CI = 89–94; P = 0.01). There were no sex differences in ghrelin (P = 0.6) or insulin (P = 0.5).
Relationships between hormone levels and restrained eating
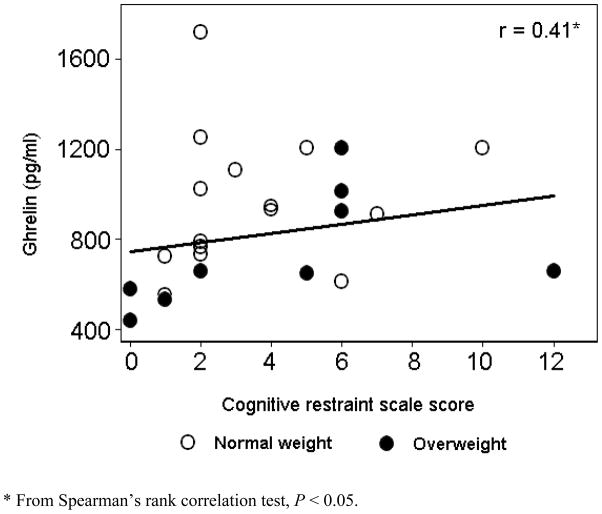
Simple correlations were calculated between hormone levels and restraint scale scores. Ghrelin correlated positively with restraint scale scores (r = 0.41, P < 0.05). In contrast, leptin (r = −0.04) and insulin (r = −0.09) levels appeared unrelated to the degree of restraint.
Data from the three groups defined by restraint scale scores were entered into regression models as dummy variables. Likelihood ratio tests confirmed that this was the better model fit than the linear model (P = 0.008). Table 2 shows results of both univariate regression models and multivariate models adjusted for BMI. Geometric means are presented for unadjusted analyses and calculated least squared geometric means are presented for hormonal outcomes of interest and for the nutrient glucose in adjusted analyses. Ghrelin levels were significantly higher in both the High and Indeterminate Restraint groups as compared to the Low Restraint group. The association of higher ghrelin levels with higher restraint scale scores persisted after controlling for BMI. When age and sex were added in an exploratory multivariate model, the relationship appeared to be independent of these covariates (P = .02). The relationship of ghrelin to restraint scale scores and BMI is illustrated in Figure 1. For insulin and leptin, there were no statistically significant differences across the 3 groups. However, a pattern was present for all hormonal outcomes when we focused on the means for the extremes of restraint scale scorers. Means for the High Restraint group (top tertile) as compared to the Low Restraint group (lowest tertile) showed higher ghrelin, lower leptin, and lower insulin levels. Each of these differences is in the direction of greater appetite stimulation among restrained eaters. Finally, glucose levels were higher in the High Restraint group, only after adjustment for BMI, and with borderline statistical significance (P = 0.05). However, this association was no longer significant in analyses that fully adjusted for the effects of BMI, age, and sex (P = 0.62).
Table 2.
Unadjusted and adjusted mean hormone and glucose levels with 95% CIs categorized by Restraint Scale Score
| Cognitive Restraint Scale Score Category | P Value* | ||||
|---|---|---|---|---|---|
| Low (n = 5) | Indeterminate (n = 10) | High (n = 9) | |||
| Ghrelin (pg/ml) | Unadjusted# | 503 (387–655) | 893 (742–1076) | 840 (690–1021) | 0.004 |
| Adjusted** | 521 (403–675) | 853 (706–1031) | 867 (713–1052) | 0.007 | |
| Leptin (ng/ml) | Unadjusted | 6.7 (2.8–16.1) | 7.0 (3.8–13.0) | 5.1 (2.7–9.8) | 0.75 |
| Adjusted | 5.8 (2.5–13.5) | 8.4(4.5–15.5) | 4.5 (2.4–8.5) | 0.38 | |
| Insulin (μU/ml) | Unadjusted | 14.3 (10.8–19.1) | 11.6 (9.5–14.2) | 13.8 (11.1–17.0) | 0.36 |
| Adjusted | 13.6 (10.5–17.7) | 12.4 (10.3–15.1) | 13.1 (10.8–16.0) | 0.85 | |
| Glucose (mg/dl) | Unadjusted | 87 (82–92) | 86 (83–90) | 92 (88–96) | 0.07 |
| Adjusted | 88 (83–93) | 86 (82–89) | 93 (89–97) | 0.05 | |
P value from global F tests (unadjusted) or multiple partial F tests (adjusted).
All unadjusted means are geometric means.
All adjusted means are least squared geometric means adjusted for BMI.
Figure 1.
The relationship of ghrelin levels, BMI, and cognitive restraint scale scores
DISCUSSION
These results provide evidence for physiological differences in body weight-regulating hormones based on a psychometrically defined pattern of eating behavior. In particular, we found a positive association between restrained eating, as measured by cognitive restraint scale scores, and levels of ghrelin in subjects at their stable, lifetime maximum weight. A positive correlation between ghrelin and cognitive restraint was also previously found in severely underweight anorexic patients [40]. However, in normal-weight young women, no such relationship was detected [41]. Our research is distinct from both of these studies because we controlled for the secondary effects of weight reduction by restricting our sample to individuals at a stable, lifetime maximum weight. Furthermore, our work is also novel in that it includes males and females with a range of ages, as well as overweight participants.
There are several potential mechanisms that could explain higher ghrelin levels in restrained eaters. Perhaps the most intriguing possibility is that high ghrelin levels could be biological correlates of restrained eating behavior. Restrained eaters might be intrinsically relatively appetite-stimulated and therefore counteract their ample appetite with increased cognitive control of their food intake. A second possibility is that restrained eaters might be maintaining their body weight below where it would be naturally, in the absence of cognitive inhibition. In other words, although they are at a stable, lifetime maximum weight, they might be essentially physiologically weight-reduced through prevention of weight gain by low-level food restriction. Due to the cross-sectional nature of our data, we cannot distinguish between these possibilities. Finally, high ghrelin levels may result from short-term fasting or sporadic food restriction in restrained eaters, behaviors that could acutely increase ghrelin levels even without changing body weight. Ghrelin levels surge during fasting and in the late part of intermeal intervals when nutrients are absent from the gut [25]. Food restriction by the restrained eaters in this study may not have been sufficient or persistent enough to result in reduction of body weight below their lifetime maximal weight, and therefore should not have stimulated the response of ghrelin in its role as a regulator of long-term energy balance. However, intermittent restriction or fasting might still trigger a rise in ghrelin as a short-term regulator of food intake by the gut [25]. In addition, abundant caloric intake in unrestrained eaters would tend to suppress ghrelin, possibly resulting in lower observed fasting levels.
These findings suggest that ephysiological mechanisms are important to consider when exploring the overeating and weight gain observed in some restrained eaters [13–15, 18]. If ghrelin levels are inherently elevated in restrained eaters, this hormone’s orexigenic properties would pose a challenge to cognitive attempts at restricting food intake. Although we selected individuals who were weight-stable, there may be others who are unable to maintain restraint in the face of physiological appetite stimulation, and they succumb to the overeating, weight cycling, or weight gain that characterize some populations of restrained eaters [6]. In identical twin pairs, a restrictive/overeating eating style predicted excess weight even when genetic background was controlled for [42]. Interestingly, restrained eaters are not consistently found to be in energy deficit [43], as documented by stable lifetime maximum weight in this study and resting energy expenditure in other studies [44–46]. However, alterations in hormones such as ghrelin suggest a biological impetus for weight gain despite energy balance — a loophole of sorts in the body weight regulatory system. For example, there is mounting evidence that ghrelin may enhance the hedonic appeal of foods through its action on dopaminergic reward pathways in the brain [47, 48]. This would potentially enhance preferences for preferred foods [49] or highly palatable foods among restrained eaters. One study has documented a tendency for restrained eaters to increase fat intake when given fructose-sweetened beverages — a predilection that was not present among unrestrained eaters [50].
Our results contradict prior reports of low leptin [20, 21, 32, 33, 40] and insulin [34] levels in restrained eaters, in that we found no such association, but we corroborate the general concept that restrained eaters have a relatively appetite-stimulating hormonal profile. Leptin levels were previously negatively correlated with restrained eating in underweight women [20], normal-weight controls [40], obese women [32], and obese women with frequent binge eating [33]. In a prospective study, restrained eating at baseline was associated with lower leptin levels 6 months later [21]. There was no difference in leptin in the one study [19] of obese women with binge eating disorder who had been weight-stable for at least 6 months. Leptin is most closely correlated with body adiposity [51], but it is also responsive acutely to fasting [52]. Our finding that leptin levels were unrelated to cognitive restraint scale scores could be explained by our having limited the study to non-dieting individuals at a stable, lifetime maximum weight. In addition, our study included male restrained eaters. Males have lower mean leptin levels than females do [22], potentially resulting in a floor effect that may have lessened our ability to detect further suppression due to restrained eating among men. Moreover, we cannot exclude that clinically significant relationships with leptin or insulin would be present if a larger sample were studied. In summary, despite some contradictory findings, a growing body of evidence suggests that restrained eating behavior is associated with an appetite-stimulating hormonal milieu.
Our conclusions are limited by several factors. The small sample size might have caused us to miss statistically significant associations that would have been found in a larger sample. In addition, unmeasured confounders may have been present that could explain our findings. Also, our weight stability and lifetime maximum weight criteria were self-reported. Our estimates of basal energy expenditure are insufficient to confirm that our subjects were not in energy deficit. Further studies should directly measure resting metabolic rate. Although the TFEQ cognitive restraint scale has been successfully used in several modified formats [53, 54], our version has not been subjected to rigorous validity and reliability testing in large samples. Finally, our sample is highly restricted in that it contains only healthy, nonobese adults at stable, maximum weight. Moreover, the individuals who scored high in cognitive restraint also denied that they were currently dieting for weight loss. Although this unique population is ideal for answering the very specific questions of whether a relationship between hormonal profile and cognitive restraint is present independent of effects on weight, our results may not generalize beyond this select population. In addition, these criteria may have identified an atypical group of restrained eaters. Although differences between groups were not significant, there was a tendency for males and older persons to be over-represented in the High Restraint group, perhaps because females are more likely to self-report our exclusionary criteria of current dieting. However, we do not believe that confounding by the effects of gender or age fully explain our results for several reasons. For one, our finding regarding ghrelin persisted after statistical adjustment for gender and age. Secondly, age is inconsistently associated with ghrelin levels [36, 56, 57]. Finally, when gender differences in ghrelin levels are present in the literature, females tend to have higher ghrelin levels [55, 56], and therefore our inclusion of restrained eating males would, if anything, bias us against the finding of high ghrelin levels in the high restraint group. In summary, despite these limitations, our research provides an initial assessment of the hormonal profile associated with restrained eating in weight-stable individuals who are not weight-reduced.
There are some interesting implications for future research based on these preliminary data. First, our findings should be confirmed with larger samples. Second, studies of body-weight regulatory hormones and eating behavior that do not restrict subjects to their lifetime maximum weight should document the extent that their subjects are weight-reduced. This will add confidence to any conclusions that appetite stimulation is due to eating behaviors as opposed to the secondary effects of weight reduction. Third, prospective data are needed to determine whether individuals who are inherently predisposed to greater appetite stimulation engage in restrained eating behavior for weight control or whether behavioral changes elicit compensatory responses by the appetite and body-weight regulatory system. Fourth, considerable individual variability in ghrelin levels has been observed [36], and this was true of both the high and low cognitive restraint scorers in our sample. Behavioral contributions to this observed variability remain essentially unexplored. Finally, investigations of hormonal rhythmicity in restrained eaters are suggested by evidence for alterations in diurnal patterns of leptin in anorexia nervosa [58] and blunting of the short-term response of ghrelin to food intake with both anorexia [59] and bulimia nervosa [60]. Given the rise in obesity and the propensity of normal-weight individuals to engage in weight-control practices to improve body image [3], a thorough understanding of the physiological underpinnings of and responses to restrictive eating behavior is needed.
Acknowledgments
This research was supported by National Institutes of Health, National Institute of Diabetes, Digestive and Kidney Diseases grants RO1 DK61516 (Dr. Cummings) and Career Development Award K23 DK070826 (Dr. Schur). Dr. Foster-Schubert is supported by the National Institutes of Health Roadmap Multidisciplinary Clinical Research Career Development Award Grant 5K12RR023265. A portion of this work was supported by the General Clinical Research Center at the University of Washington (National Institutes of Health, Grant M01RR-00037) and the Diabetes Endocrinology Research Center (National Institutes of Health, Grant DK-35816).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. Jama. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. Jama. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 3.Bish CL, Blanck HM, Serdula MK, Marcus M, Kohl HW, 3rd, Khan LK. Diet and physical activity behaviors among Americans trying to lose weight: 2000 Behavioral Risk Factor Surveillance System. Obes Res. 2005;13:596–607. doi: 10.1038/oby.2005.64. [DOI] [PubMed] [Google Scholar]
- 4.Cummings DE, Schwartz MW. Genetics and pathophysiology of human obesity. Annu Rev Med. 2003;54:453–471. doi: 10.1146/annurev.med.54.101601.152403. [DOI] [PubMed] [Google Scholar]
- 5.National Heart Lung and Blood Institute. The Evidence Report. Bethesda, MD: National Institutes of Health, Public Health Service, U.S. Department of Health and Human Services; 1998. Clinical Guidelines on the Identification, Evaluation and Treatment of Overweight and Obesity in Adults. Report No.: NIH Publication No. 98–4083. [Google Scholar]
- 6.Ogden J. The psychology of eating. Malden, MA: Blackwell Publishers, Ltd; 2003. pp. 102–131. [Google Scholar]
- 7.Sobal J, Stunkard AJ. Socioeconomic status and obesity: a review of the literature. Psychol Bull. 1989;105:260–275. doi: 10.1037/0033-2909.105.2.260. [DOI] [PubMed] [Google Scholar]
- 8.Korkeila M, Rissanen A, Kaprio J, Sorensen TI, Koskenvuo M. Weight-loss attempts and risk of major weight gain: a prospective study in Finnish adults. Am J Clin Nutr. 1999;70:965–975. doi: 10.1093/ajcn/70.6.965. [DOI] [PubMed] [Google Scholar]
- 9.Kroke A, Liese AD, Schulz M, Bergmann MM, Klipstein-Grobusch K, Hoffmann K, Boeing H. Recent weight changes and weight cycling as predictors of subsequent two year weight change in a middle-aged cohort. Int J Obes Relat Metab Disord. 2002;26:403–409. doi: 10.1038/sj.ijo.0801920. [DOI] [PubMed] [Google Scholar]
- 10.French SA, Jeffery RW, Forster JL, McGovern PG, Kelder SH, Baxter JE. Predictors of weight change over two years among a population of working adults: the Healthy Worker Project. Int J Obes Relat Metab Disord. 1994;18:145–154. [PubMed] [Google Scholar]
- 11.Coakley EH, Rimm EB, Colditz G, Kawachi I, Willett W. Predictors of weight change in men: results from the Health Professionals Follow-up Study. Int J Obes Relat Metab Disord. 1998;22:89–96. doi: 10.1038/sj.ijo.0800549. [DOI] [PubMed] [Google Scholar]
- 12.Neumark-Sztainer D, Wall M, Guo J, Story M, Haines J, Eisenberg M. Obesity, disordered eating, and eating disorders in a longitudinal study of adolescents: how do dieters fare 5 years later? J Am Diet Assoc. 2006;106:559–568. doi: 10.1016/j.jada.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Stice E, Cameron RP, Killen JD, Hayward C, Taylor CB. Naturalistic weight-reduction efforts prospectively predict growth in relative weight and onset of obesity among female adolescents. J Consult Clin Psychol. 1999;67:967–974. doi: 10.1037//0022-006x.67.6.967. [DOI] [PubMed] [Google Scholar]
- 14.Stice E, Presnell K, Shaw H, Rohde P. Psychological and behavioral risk factors for obesity onset in adolescent girls: a prospective study. J Consult Clin Psychol. 2005;73:195–202. doi: 10.1037/0022-006X.73.2.195. [DOI] [PubMed] [Google Scholar]
- 15.Klesges RC, Isbell TR, Klesges LM. Relationship between dietary restraint, energy intake, physical activity, and body weight: a prospective analysis. J Abnorm Psychol. 1992;101:668–674. doi: 10.1037//0021-843x.101.4.668. [DOI] [PubMed] [Google Scholar]
- 16.Tuschl RJ. From dietary restraint to binge eating: some theoretical considerations. Appetite. 1990;14:105–109. doi: 10.1016/0195-6663(90)90004-r. [DOI] [PubMed] [Google Scholar]
- 17.Lowe MR. The effects of dieting on eating behavior: a three-factor model. Psychol Bull. 1993;114:100–121. doi: 10.1037/0033-2909.114.1.100. [DOI] [PubMed] [Google Scholar]
- 18.Polivy J, Herman CP. Dieting and binging. A causal analysis. Am Psychol. 1985;40:193–201. doi: 10.1037//0003-066x.40.2.193. [DOI] [PubMed] [Google Scholar]
- 19.Adami GF, Campostano A, Cella F, Scopinaro N. Serum leptin concentration in obese patients with binge eating disorder. Int J Obes Relat Metab Disord. 2002;26:1125–1128. doi: 10.1038/sj.ijo.0802010. [DOI] [PubMed] [Google Scholar]
- 20.von Prittwitz S, Blum WF, Ziegler A, Scharmann S, Remschmidt H, Hebebrand J. Restrained eating is associated with low leptin levels in underweight females. Mol Psychiatry. 1997;2:420–422. doi: 10.1038/sj.mp.4000300. [DOI] [PubMed] [Google Scholar]
- 21.Laessle RG, Wurmser H, Pirke KM. Restrained eating and leptin levels in overweight preadolescent girls. Physiol Behav. 2000;70:45–47. doi: 10.1016/s0031-9384(00)00243-2. [DOI] [PubMed] [Google Scholar]
- 22.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz MW, Seeley RJ. Seminars in medicine of the Beth Israel Deaconess Medical Center. Neuroendocrine responses to starvation and weight loss. N Engl J Med. 1997;336:1802–1811. doi: 10.1056/NEJM199706193362507. [DOI] [PubMed] [Google Scholar]
- 24.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 25.Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006;89:71–84. doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 26.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753–4758. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 27.Wisse BE, Frayo RS, Schwartz MW, Cummings DE. Reversal of cancer anorexia by blockade of central melanocortin receptors in rats. Endocrinology. 2001;142:3292–3301. doi: 10.1210/endo.142.8.8324. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu Y, Nagaya N, Isobe T, Imazu M, Okumura H, Hosoda H, Kojima M, Kangawa K, Kohno N. Increased plasma ghrelin level in lung cancer cachexia. Clin Cancer Res. 2003;9:774–778. [PubMed] [Google Scholar]
- 29.Foster-Schubert KE, McTiernan A, Frayo RS, Schwartz RS, Rajan KB, Yasui Y, Tworoger SS, Cummings DE. Human plasma ghrelin levels increase during a one-year exercise program. J Clin Endocrinol Metab. 2005;90:820–825. doi: 10.1210/jc.2004-2081. [DOI] [PubMed] [Google Scholar]
- 30.Tolle V, Kadem M, Bluet-Pajot MT, Frere D, Foulon C, Bossu C, Dardennes R, Mounier C, Zizzari P, Lang F, Epelbaum J, Estour B. Balance in ghrelin and leptin plasma levels in anorexia nervosa patients and constitutionally thin women. J Clin Endocrinol Metab. 2003;88:109–116. doi: 10.1210/jc.2002-020645. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 32.Adami G, Campostano A, Cella F, Ferrandes G. Serum leptin level and restrained eating: study with the Eating Disorder Examination. Physiol Behav. 2002;75:189–192. doi: 10.1016/s0031-9384(01)00639-4. [DOI] [PubMed] [Google Scholar]
- 33.d’Amore A, Massignan C, Montera P, Moles A, De Lorenzo A, Scucchi S. Relationship between dietary restraint, binge eating, and leptin in obese women. Int J Obes Relat Metab Disord. 2001;25:373–377. doi: 10.1038/sj.ijo.0801565. [DOI] [PubMed] [Google Scholar]
- 34.Pirke KM, Tuschl RJ, Spyra B, Laessle RG, Schweiger U, Broocks A, Sambauer S, Zitzelsberger G. Endocrine findings in restrained eaters. Physiol Behav. 1990;47:903–906. doi: 10.1016/0031-9384(90)90017-x. [DOI] [PubMed] [Google Scholar]
- 35.Drazen DL, Vahl TP, D’Alessio DA, Seeley RJ, Woods SC. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology. 2006;147:23–30. doi: 10.1210/en.2005-0973. [DOI] [PubMed] [Google Scholar]
- 36.Purnell JQ, Weigle DS, Breen P, Cummings DE. Ghrelin levels correlate with insulin levels, insulin resistance, and high-density lipoprotein cholesterol, but not with gender, menopausal status, or cortisol levels in humans. J Clin Endocrinol Metab. 2003;88:5747–5752. doi: 10.1210/jc.2003-030513. [DOI] [PubMed] [Google Scholar]
- 37.Morgan CR, Lazarow A. Immunoassay of insulin: two antibody system. Diabetes. 1963;12:115–126. [Google Scholar]
- 38.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 39.Laessle RG, Tuschl RJ, Kotthaus BC, Pirke KM. A comparison of the validity of three scales for the assessment of dietary restraint. J Abnorm Psychol. 1989;98:504–507. doi: 10.1037//0021-843x.98.4.504. [DOI] [PubMed] [Google Scholar]
- 40.Haas V, Onur S, Paul T, Nutzinger DO, Bosy-Westphal A, Hauer M, Brabant G, Klein H, Muller MJ. Leptin and body weight regulation in patients with anorexia nervosa before and during weight recovery. Am J Clin Nutr. 2005;81:889–896. doi: 10.1093/ajcn/81.4.889. [DOI] [PubMed] [Google Scholar]
- 41.St-Pierre DH, Karelis AD, Cianflone K, Conus F, Mignault D, Rabasa-Lhoret R, St-Onge M, Tremblay-Lebeau A, Poehlman ET. Relationship between ghrelin and energy expenditure in healthy young women. J Clin Endocrinol Metab. 2004;89:5993–5997. doi: 10.1210/jc.2004-0613. [DOI] [PubMed] [Google Scholar]
- 42.Keski-Rahkonen A, Bulik CM, Pietilainen KH, Rose RJ, Kaprio J, Rissanen A. Eating styles, overweight and obesity in young adult twins. Eur J Clin Nutr. 2007;61:822–829. doi: 10.1038/sj.ejcn.1602601. [DOI] [PubMed] [Google Scholar]
- 43.Lowe MR, Kral TV. Stress-induced eating in restrained eaters may not be caused by stress or restraint. Appetite. 2006;46:16–21. doi: 10.1016/j.appet.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 44.Keim NL, Horn WF. Restrained eating behavior and the metabolic response to dietary energy restriction in women. Obes Res. 2004;12:141–149. doi: 10.1038/oby.2004.19. [DOI] [PubMed] [Google Scholar]
- 45.Beiseigel JM, Nickols-Richardson SM. Cognitive eating restraint scores are associated with body fatness but not with other measures of dieting in women. Appetite. 2004;43:47–53. doi: 10.1016/j.appet.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Bathalon GP, Hays NP, McCrory MA, Vinken AG, Tucker KL, Greenberg AS, Castaneda C, Roberts SB. The energy expenditure of postmenopausal women classified as restrained or unrestrained eaters. Eur J Clin Nutr. 2001;55:1059–1067. doi: 10.1038/sj.ejcn.1601268. [DOI] [PubMed] [Google Scholar]
- 47.Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26:2274–2279. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 49.Schmid DA, Held K, Ising M, Uhr M, Weikel JC, Steiger A. Ghrelin stimulates appetite, imagination of food, GH, ACTH, and cortisol, but does not affect leptin in normal controls. Neuropsychopharmacology. 2005;30:1187–1192. doi: 10.1038/sj.npp.1300670. [DOI] [PubMed] [Google Scholar]
- 50.Teff KL, Elliott SS, Tschop M, Kieffer TJ, Rader D, Heiman M, Townsend RR, Keim NL, D’Alessio D, Havel PJ. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. 2004;89:2963–2972. doi: 10.1210/jc.2003-031855. [DOI] [PubMed] [Google Scholar]
- 51.Weigle DS, Ganter SL, Kuijper JL, Leonetti DL, Boyko EJ, Fujimoto WY. Effect of regional fat distribution and Prader-Willi syndrome on plasma leptin levels. J Clin Endocrinol Metab. 1997;82:566–570. doi: 10.1210/jcem.82.2.3761. [DOI] [PubMed] [Google Scholar]
- 52.Boden G, Chen X, Mozzoli M, Ryan I. Effect of fasting on serum leptin in normal human subjects. J Clin Endocrinol Metab. 1996;81:3419–3423. doi: 10.1210/jcem.81.9.8784108. [DOI] [PubMed] [Google Scholar]
- 53.Mazzeo SE, Aggen SH, Anderson C, Tozzi F, Bulik CM. Investigating the structure of the eating inventory (three-factor eating questionnaire): a confirmatory approach. Int J Eat Disord. 2003;34:255–264. doi: 10.1002/eat.10180. [DOI] [PubMed] [Google Scholar]
- 54.de Lauzon B, Romon M, Deschamps V, Lafay L, Borys JM, Karlsson J, Ducimetiere P, Charles MA. The Three-Factor Eating Questionnaire-R18 is able to distinguish among different eating patterns in a general population. J Nutr. 2004;134:2372–2380. doi: 10.1093/jn/134.9.2372. [DOI] [PubMed] [Google Scholar]
- 55.Greenman Y, Golani N, Gilad S, Yaron M, Limor R, Stern N. Ghrelin secretion is modulated in a nutrient- and gender-specific manner. Clin Endocrinol (Oxf) 2004;60:382–388. doi: 10.1111/j.1365-2265.2004.01993.x. [DOI] [PubMed] [Google Scholar]
- 56.Makovey J, Naganathan V, Seibel M, Sambrook P. Gender differences in plasma ghrelin and its relations to body composition and bone - an opposite-sex twin study. Clin Endocrinol (Oxf) 2007;66:530–537. doi: 10.1111/j.1365-2265.2007.02768.x. [DOI] [PubMed] [Google Scholar]
- 57.Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, Nozoe S, Hosoda H, Kangawa K, Matsukura S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab. 2002;87:240–244. doi: 10.1210/jcem.87.1.8129. [DOI] [PubMed] [Google Scholar]
- 58.Stoving RK, Vinten J, Handberg A, Ebbesen EN, Hangaard J, Hansen-Nord M, Kristiansen J, Hagen C. Diurnal variation of the serum leptin concentration in patients with anorexia nervosa. Clin Endocrinol (Oxf) 1998;48:761–768. doi: 10.1046/j.1365-2265.1998.00434.x. [DOI] [PubMed] [Google Scholar]
- 59.Nedvidkova J, Krykorkova I, Bartak V, Papezova H, Gold PW, Alesci S, Pacak K. Loss of meal-induced decrease in plasma ghrelin levels in patients with anorexia nervosa. J Clin Endocrinol Metab. 2003;88:1678–1682. doi: 10.1210/jc.2002-021669. [DOI] [PubMed] [Google Scholar]
- 60.Monteleone P, Martiadis V, Fabrazzo M, Serritella C, Maj M. Ghrelin and leptin responses to food ingestion in bulimia nervosa: implications for binge-eating and compensatory behaviours. Psychol Med. 2003;33:1387–1394. doi: 10.1017/s0033291703008316. [DOI] [PubMed] [Google Scholar]